Abstract
There has not been a reported series of children with therapy-induced myelodysplastic syndrome/acute myeloid leukemia (tMDS/tAML) who were treated systematically. This paper describes 24 children with tMDS/tAML who were assigned randomly to standard- or intensive-timing induction on protocol CCG 2891. Presenting features and outcomes of those children were compared with those of 960 patients with de novo MDS (62 patients) or AML (898 patients). Children with tMDS/tAML were older at presentation (P = .015), had lower white blood cell counts (P = .01), and were more likely to have MDS (21% vs 7%) (P = .02) and trisomy 8 (P = .06). Fewer had hepatomegaly (P = .02), splenomegaly (P = .03), hepatosplenomegaly (P = .02), or classic AML translocations [t(8;21), t(15;17), 16q22; P = .02]. They had a poorer induction rate (50% vs 72%,P = .016), overall survival (26% vs 47% at 3 years,P = .007), and event-free survival (21% vs 39% at 3 years, P =.023). Disease-free survival after achieving remission was similar (45% vs 53%, P = .868). Children with tMDS/tAML who received intensive-timing induction had better outcomes than those who received standard-timing induction (overall survival 32% vs 0%, P = .54). In this study, the latency period to development of tMDS/tAML was the same for presumed alkylator-induced as for topoisomerase-induced myeloid leukemia. The findings of this study confirm that most children with tMDS/tAML have disease resistant to current therapies. Standard-timing induction appears less effective for this population.
Introduction
Treatment-induced acute myeloid leukemia (tAML) or myelodysplastic syndrome (tMDS) presents, at a minimum, in 2 distinctive patterns. In adults, the first manner of presentation, after therapy with alkylating agents and radiation,1,2 is associated with structural deletional aberrations of chromosome 5 or 7 or, more uncommonly, 1, 3, 4, 8, 11, 12, 14, 17, 18, 20, or 21.1,3-5 The risk of developing tMDS/tAML appears to be proportional to the cumulative dose given and potentiated when different alkylators are used together.6 After a latency period of approximately 5 years, patients present with a preleukemic syndrome that often evolves into AML. In contrast, the second pattern of tMDS/tAML involves patients who receive epipodophyllotoxins or other topoisomerase II–inhibiting drugs or DNA-intercalating agents (amsacrine, mitoxantrone, anthracyclines) in whom tMDS or tAML develops 2 to 3 years after their initial cancer therapies, frequently without a myelodysplastic phase.7-9 The translocations 11q23, 21q22, or 3q26 and French-American-British (FAB) types M4 or M5 are common.10-16 Treatment regimens that include alkylators and topoisomerase II inhibitors can increase the risk relative to those agents given individually.6 17
It has been estimated that tMDS/tAML affects at least 1% of childhood cancer patients, with a higher incidence in children treated for Hodgkin disease (4%).18,19 Winick et al9estimated the risk of tMDS/tAML after multidrug treatment (including etoposide) for acute lymphocytic leukemia as high as 5.9%. In a Pediatric Oncology Group study, 14 (2.2%) of 646 children with primary T-cell acute lymphoblastic leukemia (ALL) or T-cell non-Hodgkin lymphoma developed tMDS/tAML.20 In a similar population treated on Uninted Kingdom Children's Cancer Study Group regimens, 6 (2.3%) of 261 children developed tMDS/tAML.21 The incidence of tMDS/tAML in children treated aggressively for Ewing sarcoma, particularly those who received granulocyte colony-stimulating factor (G-CSF), was reported to be 6.7%, and it was higher for children who received the most intensive treatment.22
There are few reported studies of treatment of childhood tMDS/tAML. Studies describe patients diagnosed over long periods or who received differing therapies. The purpose of this study was to compare clinical presentation, bone marrow characteristics, and treatment response of 24 children and adolescents with tMDS/tAML with those of 960 children who presented with primary or de novo MDS/AML enrolled on the same clinical trial.
Patients, materials, and methods
Patients
The 24 patients with tMDS/tAML were compared with 960 of 1201 patients registered on protocol CCG (Children's Cancer Group) 2891 (we excluded 191 children with Down syndrome, 8 with isolated chloroma, 24 with tMDS/tAML, and 18 who were ineligible for the study, mainly because of incorrect diagnoses). Eligible patients for CCG 2891 have been described.23 24 Information related to initial presentation and treatment of primary tumors in patients with tMDS/tAML was collected from data entry forms and treating institutions.
Treatment protocol
Patients enrolled in CCG 2891, including those with tMDS/tAML, were assigned randomly to receive intensive-timing DCTER (a 5-drug chemotherapy with dexamethasone, cytosine arabinoside, 6-thioguanine, etoposide, and daunorubicin, given over 4 days and, after a 6-day rest, a second 4-day cycle regardless of bone marrow or hematologic status) or standard-timing DCTER (the second 4-day cycle was given at the time of peripheral count recovery or determination of residual leukemia at day 14). The last cohort of patients in CCG 2891 received intensively timed DCTER with G-CSF. When interim analyses showed that standard timing conferred an inferior event-free survival (EFS), the standard timing arm was replaced.24 A second randomization was done for patients who achieved remission after 4 cycles of induction therapy. Patients with compatible family donors were assigned nonrandomly to bone marrow transplantation. The remaining patients were assigned randomly to receive autologous marrow transplantation or intensified chemotherapy.23
Bone marrow examination
The study hematopathologist (D.R.B.) reviewed representative pretreatment bone marrow smears to confirm eligibility and assign FAB classifications,25-27 as described before.23,28 A panel of CCG cytogeneticists reviewed photographs of institutional karyotype preparations. Local institutions performed immunophenotyping. Initial diagnostic slides of patients in this study whose primary disease was ALL were reviewed centrally. Seven patients were tested for mixed lineage leukemia (MLL) gene rearrangement locus as detected by Southern blot analysis.29
Statistical analysis
Data from CCG 2891 through August 11, 2000, were used to compare patients with tMDS/tAML with patients who presented with de novo MDS/AML. Significance of observed differences in proportion was tested using the χ2 and Fisher exact tests when data were sparse. For continuous data, the Mann-Whitney test was used to compare medians of distributions.30
Patients lost to follow-up were censored at their last known point of study, with a cutoff of February 11, 2000. The Kaplan-Meier method was used to calculate actuarial estimates of overall survival (OS) from on-study date; EFS, time from on-study date to induction failure, marrow relapse, or death; and disease-free survival (DFS), the time from achieving remission to marrow relapse or death.31Differences in survival, EFS, and DFS were tested for significance using the log-rank statistic.32
Results
Among 1201 patients registered on CCG 2891, 26 (2.2%) had tMDS/tAML. Among those, 2 were not included in this analysis; 1 was ineligible (primary diagnosis of chloroma rather than lymphoma, as originally thought), and 1 had Down syndrome (primary tumor neuroblastoma). Table 1 lists the presenting features of patients with tMDS/tAML. Five children had ALL, 4 had central nervous system (CNS) tumors, 4 had Hodgkin disease, 3 had germ cell tumors, 3 had Ewing sarcoma, 2 had other sarcomas, 2 had non-Hodgkin lymphoma, and 1 had neuroblastoma. The median time to development of second malignancy was 37 months (range, 5-116 months). Among 5 children in this study who had primary ALL, only 1 had received epipodophyllotoxin therapy. Most children reported in this study who had primary solid tumors or lymphomas received treatment with alkylating agents or anthracyclines; 3 received epipodophyllotoxins; the exact treatment is unknown for 1 child.
Table 2 gives details of presentation at diagnosis, showing that children with tMDS/tAML were older (P = .02) and had lower white blood cell counts (P = .01) and less pronounced hepatosplenomegaly (P = .02) than children with de novo MDS/AML. When the 5 children with tMDS were compared with the 60 children with primary MDS, no significant differences in clinical presentation were noted although one third of the children with primary MDS had hepatomegaly and/or splenomegaly at presentation. The tMDS/tAML patients did not have CNS involvement (P = .26) or chloroma (P = .26) at presentation. Children with tMDS/tAML were more likely to have MDS at presentation (21% vs 7%, P = .02). Five of the 24 children were Hispanic (vs 13% of children presenting with primary MDS/AML).
Marrow aspirates from children with tMDS/tAML showed trilineage dyspoietic and megaloblastoid changes, but they were not prominent. Table 3 lists cytogenetic changes of patients with tMDS/tAML compared with those of patients with primary MDS/AML. None of the 6 children whose archival bone marrow slides were analyzed showed the MLL gene rearrangement (although 3 other patients had an 11q23 translocation).
Half of the 24 patients with tMDS/tAML achieved remission (Table4). Of the 12 who did not achieve remission, 1 withdrew, 1 refused treatment, and 4 died (day 2, day 3, day 15, day 49). Among the 5 patients assigned randomly to standard induction, 1 died on day 15 of disease progression, 1 refused treatment, 1 did not attain remission, and 1 achieved remission (Table4). The fifth patient was assigned to standard timing but was changed to intensive-timing induction when the standard arm was closed. That child achieved remission but died of infection at 11 months. All 4 patients who received standard-timing induction died—3 of progressive disease and 1 of graft-versus-host disease.
Nineteen patients were assigned randomly to receive intensive-timing induction with (10 patients) or without (9 patients) G-CSF. Among children who received intensive-timing induction, 3 died early (1 on day 2 from hemorrhage, 1 on day 3 of progressive disease, 1 at 7 weeks of infection [before starting cycle 2 of induction therapy]), 5 did not achieve remission, and 1 withdrew from the study at 1 month without achieving remission.
Ten children (53%) were in remission at the end of induction. Of these 10 children, 2 died from progressive disease and 2 died from infection. Among 6 long-term survivors, 2 received allogeneic transplantations, 2 received chemotherapy consolidation, 1 received autologous transplantation with stem cells collected before treatment for his primary malignancy, and 1 received nonprotocol treatment.
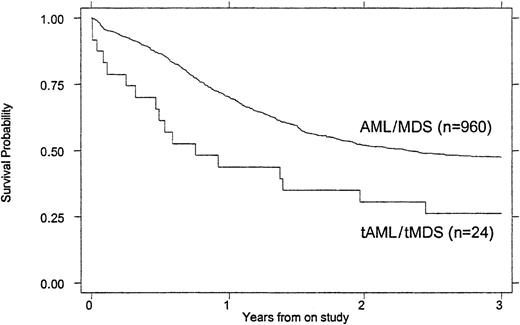
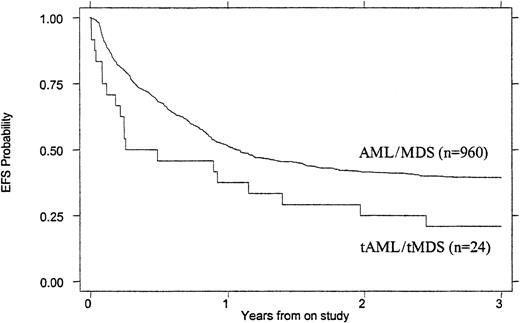
Treatment outcomes are summarized in Tables 4 and5. Children with tMDS/tAML had a poorer induction rate (50% vs 72%, P = .016), OS (26% vs 47% at 3 years, P = .007), and EFS (21% vs 39% at 3 years, P = .023) than children with de novo AML/MDS (Figures 1 and2). When the remission rate was calculated excluding withdrawals before remission status could be determined, the rate for children with tMDS/tAML was 55% versus 76% (P = .24) for children with de novo MDS/AML. DFS after achieving remission was similar (45% vs 53%, P = .868) (Figure 3). Actuarial estimates of OS and EFS at 3 years were approximately 50% lower for tMDS/tAML patients. The induction success rate (P = .005), OS (P = .002), EFS (P < .0001), and DFS (P < .001) were significantly better for children with de novo MDS/AML on protocol CCG 2891 assigned to intensive-timing induction rather than standard-timing induction.23 24Although none of the 4 children with tMDS/tAML who received standard-timing induction survived compared with 6 of 20 who received (19 assigned to receive) intensive-timing induction were long-term survivors (6 of the 10 children who achieved remission), this was not statistically different (P = .54).
OS of children with tMDS/tAML compared with that of children with de novo MDS/AML.
P = .007.
OS of children with tMDS/tAML compared with that of children with de novo MDS/AML.
P = .007.
EFS of children with tMDS/tAML compared with that of children with de novo MDS/AML.
P = .023.
EFS of children with tMDS/tAML compared with that of children with de novo MDS/AML.
P = .023.
DFS of children with tMDS/tAML compared with that of children with de novo MDS/AML.
P = .868.
DFS of children with tMDS/tAML compared with that of children with de novo MDS/AML.
P = .868.
Discussion
This study confirms that children and adolescents develop tMDS/tAML subsequent to chemotherapy for many childhood malignancies, shown by the patients in this study (Table 1) and reported in the literature.14 33-39 Our patients (Table 1) show the reported increased incidence of second malignancies found in those with Hodgkin disease and with Ewing sarcoma who receive modern, aggressive therapy.
Adults with tMDS/tAML after alkylating chemotherapy frequently experience a preleukemic phase beginning 26 to 94 months after initial treatment40 and lasting 2 to 18 months. In adults, those who progress to AML usually do so within 33 to 98 months after initial therapy of their primary disease.3 40-42 Most children with tMDS/tAML follow the more recently described clinical pattern typified by exposure to epipodophyllotoxins. The mean time to onset of tMDS/tAML after diagnosis of first malignancy for the children in this study was approximately 42 months (range 5-116 months; median 37 months), regardless of the chemotherapeutic agent(s) suspected of inducing the tMDS/tAML. The 3 children with suspected “alkylator-induced” (A type) tMDS/tAML had a mean time to onset of 74 months (median, 73 months). The 7 children with “topoisomerase-induced” (E type) tMDS/tAML had a mean time to onset of 41 months, median 31 months. The 13 children exposed to both alkylator and topoisomerase agents (A/E type) had a mean time to onset of 35 months, median of 37 months. In contrast to adult tMDS/tAML, this suggests that the onset and clinical course of tMDS/tAML in children is accelerated, despite the etiologic agent.
Clinical presentations of children in this study are outlined in Table2. The apparent increased incidence of tMDS/tAML in Hispanic children noted by Winick et al43 was noted in our study (5 of 24 children were Hispanic; 21% vs 13% for primary MDS/AML), but this ratio was not statistically significant (P = .233). No child in this study with tMDS/tAML had neurofibromatosis44or other congenital disorder associated with preleukemia.
Morphologic changes found in bone marrow aspirates of the children in this study at presentation included more dyspoietic and megaloblastoid changes than those of children with primary MDS/AML, but those features were not prominent. The erythroid series was affected more often than the myeloid or megakaryocytic series, but anisopoikilocytosis and other erythrocyte abnormalities were uncommon. Precise morphologic classification is reported to be difficult in adult patients with tMDS/tAML; however, it was possible to classify all the patients in this study with tMDS/tAML according to FAB classification (Table2): 50% had FAB M4/5, 21% had FAB M1/2, and 21% had MDS. These results are similar to those of Pui et al8 (M4/5 54%, M1/2 20%, MDS 9% of 35 children). When compared with de novo childhood MDS/AML, presentation with MDS was more common in childhood tMDS/tAML (Table 2).
The 11q23 rearrangement was common in our population (Table 3). ALL patients treated on CCG protocols did not receive topoisomerase agents as part of therapy. In the present study, among 3 patients with the 11q23 gene rearrangement, 2 had FAB M5 and 1 refractory anemia with excess blasts (RAEB). In pediatric tMDS/tAML, fewer patients compared with adults have abnormalities of chromosomes 5 or 7 or the 21q22 translocation. Deletions of chromosome 7 were found in 2 children in the present study, in keeping with the postulation that monosomy 7 is an opportunistic cytogenetic event.45 Trisomy 8 was more common in children with tAML/tMDS (P = .06); however, the available cytogenetic results were limited. Classical AML translocations (t(8;21, t(15;17), 16q22) were more common in children with de novo MDS/AML (24% vs 0%, P = .05).
The relatively few patients in this study made it impossible to determine favorable prognostic factors at diagnosis for children with tMDS/tAML. However, children who received intensive-timing induction had better OS (Table 4). Resistance to conventional treatment has been noted before3 and was summarized more recently by Kantarjian et al.46 When similar complete remission rates for adults with primary or secondary AML are reported, studies generally include patients with preleukemic phases before development of “secondary” or overt leukemia. For the adult patients who achieve complete remissions, long-term survival is unusual. Most long-term survivors have bone marrow transplantations; differentiating agents, hormones, and hematopoietic growth factors have been disappointing.47 Patients with secondary acute promyelocytic leukemia have responded to all-trans-retinoic acid.48 Children who develop secondary acute promyelocytic leukemia after treatment for Langerhans cell histiocytosis appear to have better prognoses than other children who develop tMDS/tAML.49
In the present study, treatment with standard- or intensive-timing induction with or without G-CSF induced remission in only half of tMDS/tAML patients. Among 6 long-term survivors, all received intensive-timing induction (Table 4). These results are in keeping with the benefits of intensive-timing induction for long-term outcomes of children with de novo MDS/AML: OS (49% vs 34% at 8 years,P = .0021), EFS (45% vs 28% at 8 years,P < .0001), and DFS (56% vs 36% at 8 years after achieving remission, P < .0001). The outcomes of children with tMDS/tAML treated on CCG 2891 were improved over results reported in the literature (as outlined below): a long-term survival for 32% of children who received intensive-timing induction.
Geller et al50 reported successful treatment with bone marrow transplantation in 3 of 5 children and young adults with tMDS/tAML after Hodgkin disease. The variable responses to bone marrow transplantation of adult patients with tMDS/tAML are summarized by Kantarjian et al.46 Five of 5 adult patients with tMDS/tAML achieved remissions after induction treatment with idarubicin, high-dose cytarabine, and etoposide.51 More recent experience with hematopoietic stem cell transplantation resulted in a 5-year DFS of 30% for adult patients (7 of 22) receiving transplantation preparation with BUCY-t (dose-tailored busulfan and cyclophosphamide).52 In a study of the French Society of Bone Marrow Transplantation of adults with tMDS/tAML, the 2-year EFS was 28% following bone marrow transplantation.53
Nine of 13 children with epipodophyllotoxin-associated tMDS/tAML reported by Pui et al,12 using various regimens, achieved remission, but median survival was only 13 months and OS was less than 20%. In their more recent study,54 again using differing regimens, the remission-induction rate was 92%. Only 1 child of 17 treated with chemotherapy and 3 of 16 who received marrow transplantation were long-term survivors. Comparable results were found by Sandler et al55 in their study of a similar population (13 of 16 successful remission inductions, 2 of 10 long-term survivors after bone marrow transplantation, and 1 of 4 after chemotherapy alone). In general, pediatric patients have received more aggressive treatment prior to developing tMDS/tAML than adult patients. This intense treatment may make achievement of long-term remission more difficult than for less intensely treated adult patients.
The results of our study emphasize the need for prevention and improved methods of treatment for tMDS/tAML in children. Potential tests that might identify children at increased risk of secondary MDS/AML are being examined.6 Improved long-term outcomes of primary malignancies have come with the cost of secondary cancers. Overall, the balance appears to be in favor of improved long-term survival. The outcome of primary cancer is still the major determinant of a childhood cancer patient's ultimate success.
A complete list of the participating Children's Cancer Group investigators, institutions, and grant numbers is given in the “.”
Supported by the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dorothy R. Barnard, Children's Oncology Group, PO Box 60012, Arcadia, CA 91066-6012; e-mail:dorothy.barnard@iwk.nshealth.ca andsmason@childrensoncologygroup.org.