Abstract
One hundred ninety-eight children and adolescents were entered in the Associazione Italiana di Ematologia ed Oncologia Pediatrica (AIEOP)-ALL95 study for high-risk acute lymphoblastic leukemia (ALL). Inclusion criteria were poor response to initial prednisone/intrathecal methotrexate (prednisone-poor response [PPR]), resistance to induction therapy, translocation t(9;22), infants with the t(4;11), or CD10− ALL. The event-free survival (EFS) rate at 4 years was 56.5% (SE, 3.9%) for the entire group. The overall EFS rate in the current study was significantly better (P = .002) than that obtained in a comparable group of patients treated in the early 1990s in the AIEOP-ALL91 study. In particular, patients with PPR had a 4-year EFS of 61.1% (SE, 4.4%) versus 42.8% (SE, 5.4%) in the ALL 91 study (P = .008). Among PPR patients, those who were PPR-only (60.1%)—that is, they achieved CR and were negative for t(9;22) and t(4;11) translocations—had the best outcomes with this intensive treatment, even when additional adverse features (hyperleukocytosis, T phenotype) were present (4-year EFS, 70.1%; SE, 4.7%). We attribute this improvement to the replacement of 6 alternating blocks of non–cross-resistant drugs with an 8-drug reinduction regimen (Berlin-Frankfurt-Muenster [BFM] protocol II), repeated twice, in the context of a standard BFM-type intensive chemotherapy for high-risk ALL. This modified therapy may lead to high cure rates for patients defined as at high risk for intrinsic resistance to corticosteroids only.
Introduction
The definition and treatment of high-risk childhood acute lymphoblastic leukemia (ALL) remain controversial. Consensus criteria of the Rome/National Cancer Institute Workshop1 have been useful in promoting greater uniformity among international leukemia study groups, but they are limited by failure to identify subgroups with poor prognosis defined by genetic alterations or poor blast cell sensitivity to chemotherapy. In the last decade, the Associazione Italiana di Ematologia ed Oncologia Pediatrica (AIEOP)2 and the Berlin-Frankfurt-Muenster (BFM) study groups have emphasized the role of corticosteroid sensitivity of leukemic blasts at the time of diagnosis, a risk feature first described and subsequently assessed by the BFM group.3,4Patients with at least 1000 blast cells/μL peripheral blood after 7 days of prednisone monotherapy and one injection of intrathecal methotrexate (IT-MTX) were considered to have a poor response to prednisone (PPR) and no more than a 35% chance of becoming long-term disease-free survivors on standard therapeutic protocols.3-5 Other high-risk features included failure to achieve complete remission (CR) after the first 6 weeks of induction therapy, presence of the clonal translocation t(9;22), or age younger than 1 year with either t(4;11) or CD10− leukemic blasts.2-5 Collectively, these criteria were intended to identify a subset of patients (approximately 15% of the total study population) whose event-free survival rate was expected not to exceed 35%.
Initial attempts to improve outcome in this high-risk group using a modified version of the intensive BFM-ALL REZ protocol for relapsed ALL6 yielded unsatisfactory results in the BFM-ALL-905 and the AIEOP-ALL91 studies.2 We attributed this outcome to insufficient leukemia control, despite the use of a series of 9 alternating blocks of non–cross-resistant drugs. In these 2 studies, the second part of the traditional BFM induction phase (protocol Ib) was omitted as was the traditional reinduction phase. Designated protocol II, the latter treatment is generally considered an integral part of BFM-type therapy4 and has been used by the Children's Cancer Group (CCG) to improve leukemia control in patients with delayed responses to induction therapy.7 8 Therefore, in the subsequent AIEOP-ALL95 study started in May 1995, we replaced most of the former consolidation phase (ie, 6 of the 9 multidrug blocks) with a reinduction phase consisting of protocol II, repeating it twice in the context of an otherwise conventional BFM treatment for high-risk ALL. Reported here are the results of our modified therapy administered to the series of patients at high risk as diagnosed from May 1995 to December 1999.
Patients and methods
Patients
From May 1995 to December 1999, 1599 previously untreated patients younger than 18 years with newly diagnosed non-B ALL, were registered from 40 AIEOP institutions of the AIEOP-ALL95 study. Forty patients were centrally notified but lacked essential information; 16 were not evaluable because of unknown immunophenotype (n = 12), cytochemical staining results (n = 3), or leukocyte count at diagnosis (n = 1); and 58 were ineligible according to the study protocol because of Down syndrome (n = 31), acute undifferentiated leukemia (n = 2), acute myeloid leukemia (n = 5), B-cell ALL (n = 1), secondary leukemia (n = 4), or antiblastic drug pretreatment (n = 15). Thus, 1485 patients were eligible and evaluable. Of those, 198 (13.3%) patients were assigned to the high-risk group, based on at least one of the following criteria: PPR, failure to achieve CR after the first 6 weeks (day 42) of induction therapy (protocol Ia), evidence of translocation t(9;22), or age younger than 1 year with either t(4;11) or B-lineage CD10−ALL upon prospective screening. Seven additional patients who, though eligible for the intermediate-risk group were treated according to the high-risk protocol because of clinical decision, were excluded from this analysis.
Diagnostic studies
The diagnosis of ALL was based on morphologic, cytochemical, and immunophenotypic criteria. All patients had less than 3% blast cells positive for myeloperoxidase or Sudan black and were negative for nonspecific esterase according to French-American-British criteria.9
Immunophenotyping was performed at the AIEOP reference laboratory by flow cytometry with a large panel of commercial monoclonal antibodies directed against the following surface and intracellular antigens: CD1a, CD3, CD4, CD5, CD7, CD10, CD13, CD14, CD15, CD19, CD20, CD24, CD33, CD34, CDw65, HLA DR, IgM, and terminal desoxyribonucleotidyl transferase. Threshold positivities were set at 20% for surface antigens and 10% for intracellular markers, according to the BFM family criteria.10
CR was defined as no physical signs of leukemia, no detectable leukemic cells on the blood smears, bone marrow with active hemopoiesis, cerebrospinal fluid with fewer than 5 cells/μL, and no blasts on cytospin. Treatment in patients who did not achieve CR after the first 6 weeks of therapy (protocol Ia) was continued with phase Ib and consolidation (see below). Only patients who did not obtain CR by the end of consolidation phase were defined as resistant to this protocol.
Treatment
The treatment plan is summarized in Table1. Briefly, patients underwent 7 days of prephase steroid therapy (at increasing doses up to 60 mg/m2) together with one dose of IT-MTX. Induction therapy consisted of protocol I (Ia: vincristine, prednisone, daunomycin, L-asparaginase; Ib: cyclophosphamide, 6-mercaptopurine, cytarabine),4 followed by consolidation therapy with 3 blocks of non–cross-resistant drugs, including either high-dose methotrexate (HD-MTX; 5 g/m2) or high-dose cytarabine (HD-ARAC; 2 + 2 g/m2).5 6 Reinduction therapy consisted of protocol II, followed by interim maintenance therapy during which cranial irradiation (dosing based on age and central nervous system [CNS] status) was delivered, and by a second protocol II without IT chemotherapy. Maintenance therapy consisted of 28-day cycles of oral 6-mercaptopurine (50 mg/m2, days 1-21), weekly intramuscular methotrexate (20 mg/m2, days 1, 8, 15), pulses of prednisone (40 mg/m2, days 22-26), and vincristine (1.5 mg/m2, day 22). An interval of 2 weeks between each part of therapy (induction, consolidation, reinduction, and maintenance) was scheduled to allow marrow reconstitution. Total duration of treatment was 24 months. Bone marrow transplantation (BMT) from a matched related donor was advised for patients in first CR with the following characteristics: failure to achieve CR after the first 6 weeks of induction therapy (protocol Ia); infants with the t(4;11) translocation or a CD10− B-lineage immunophenotype; t(9;22) translocation; PPR; and either a T-cell immunophenotype or a leukocyte count of 100 000/μL or the t(4;11) translocation. BMT from an alternative donor, though not recommended, was performed in 12 patients considered at particularly high risk for treatment failure; one patient underwent autologous BMT.
Treatment schedule for high-risk patients
| . | mg/m2 . | Day . |
|---|---|---|
| Induction (protocol I) | ||
| Protocol Ia | ||
| Vincristine | 1.5 | 8, 15, 22, 29 |
| Prednisone | 60 | 1-28 |
| Daunorubicin | 30 | 8, 15, 22, 29 |
| L-Asparaginase | 10 000† | 19, 22, 26, 28, 31, 34, 37, 40 |
| Protocol Ib | ||
| Cyclophosphamide | 1 000 | 43, 71 |
| 6-Mercaptopurine | 60 | 43-70 |
| Cytarabine | 75 | 45-48, 52-55, 59-62, 66-69 |
| Intrathecal methotrexate | By age‡ | 1 |
| Triple intrathecal therapy | By age‡ | 15, 29, 45, 591-154 |
| Consolidation | ||
| Block 1 | ||
| Vincristine | 1.5 | 1, 8 |
| Dexamethasone | 20 | 1-5 |
| 6-Mercaptopurine | 100 | 1-5 |
| Methotrexate | 5 000 | 1 |
| Leucovorin (levo) | 7.5 | + 36, 42, 48 h after methotrexate infusion start |
| Cytarabine | 2 000 q12h | 5 |
| L-Asparaginase | 25 000† | 6 |
| Triple intrathecal therapy | By age‡ | 1 |
| Block 2 | See legend | |
| Vindesine | 3 000 | 1 |
| Dexamethasone | 10 | 1-5 |
| 6-Thioguanine | 100 | 1-5 |
| Methotrexate | 5 000 | 1 |
| Leucovorin (levo) | 7.5 | + 36, 42, 48 h after methotrexate infusion start |
| Daunorubicin | 50 | 5 |
| L-Asparaginase | 25 000† | 5 |
| Cyclophosphamide | 150 | 5 |
| Triple intrathecal therapy | By age‡ | 1 |
| Block 3 | ||
| Dexamethasone | 20 | 1-5 |
| Cytarabine | 2 000 q12h | 1-2 |
| L-Asparaginase | 25 000† | 6 |
| Etoposide | 150 | 3-5 |
| Triple intrathecal therapy | By age‡ | 5 |
| Reinduction (protocol II)* | ||
| Dexamethasone | 10 | 1-21 |
| Vincristine | 1.5 | 8, 15, 22, 29 |
| Doxorubicin | 30 | 8, 15, 22, 29 |
| L-Asparaginase | Random | |
| 6-Thioguanine | 60 | 36-49 |
| Cyclophosphamide | 1 000 | 36 |
| Cytarabine | 75 | 38-41, 45-48 |
| Triple intrathecal therapy | By age‡ | 38, 45 (only during first reinduction) |
| Cranial radiotherapy | Based on age and CNS status1-153 | |
| Maintenance | ||
| 6-Mercaptopurine | 50 | 1-21 |
| Methotrexate (intramuscular) | 20 | 1, 8, 15 |
| Vincristine | 1.5 | 22 |
| Prednisone | 40 | 22-26 |
| . | mg/m2 . | Day . |
|---|---|---|
| Induction (protocol I) | ||
| Protocol Ia | ||
| Vincristine | 1.5 | 8, 15, 22, 29 |
| Prednisone | 60 | 1-28 |
| Daunorubicin | 30 | 8, 15, 22, 29 |
| L-Asparaginase | 10 000† | 19, 22, 26, 28, 31, 34, 37, 40 |
| Protocol Ib | ||
| Cyclophosphamide | 1 000 | 43, 71 |
| 6-Mercaptopurine | 60 | 43-70 |
| Cytarabine | 75 | 45-48, 52-55, 59-62, 66-69 |
| Intrathecal methotrexate | By age‡ | 1 |
| Triple intrathecal therapy | By age‡ | 15, 29, 45, 591-154 |
| Consolidation | ||
| Block 1 | ||
| Vincristine | 1.5 | 1, 8 |
| Dexamethasone | 20 | 1-5 |
| 6-Mercaptopurine | 100 | 1-5 |
| Methotrexate | 5 000 | 1 |
| Leucovorin (levo) | 7.5 | + 36, 42, 48 h after methotrexate infusion start |
| Cytarabine | 2 000 q12h | 5 |
| L-Asparaginase | 25 000† | 6 |
| Triple intrathecal therapy | By age‡ | 1 |
| Block 2 | See legend | |
| Vindesine | 3 000 | 1 |
| Dexamethasone | 10 | 1-5 |
| 6-Thioguanine | 100 | 1-5 |
| Methotrexate | 5 000 | 1 |
| Leucovorin (levo) | 7.5 | + 36, 42, 48 h after methotrexate infusion start |
| Daunorubicin | 50 | 5 |
| L-Asparaginase | 25 000† | 5 |
| Cyclophosphamide | 150 | 5 |
| Triple intrathecal therapy | By age‡ | 1 |
| Block 3 | ||
| Dexamethasone | 20 | 1-5 |
| Cytarabine | 2 000 q12h | 1-2 |
| L-Asparaginase | 25 000† | 6 |
| Etoposide | 150 | 3-5 |
| Triple intrathecal therapy | By age‡ | 5 |
| Reinduction (protocol II)* | ||
| Dexamethasone | 10 | 1-21 |
| Vincristine | 1.5 | 8, 15, 22, 29 |
| Doxorubicin | 30 | 8, 15, 22, 29 |
| L-Asparaginase | Random | |
| 6-Thioguanine | 60 | 36-49 |
| Cyclophosphamide | 1 000 | 36 |
| Cytarabine | 75 | 38-41, 45-48 |
| Triple intrathecal therapy | By age‡ | 38, 45 (only during first reinduction) |
| Cranial radiotherapy | Based on age and CNS status1-153 | |
| Maintenance | ||
| 6-Mercaptopurine | 50 | 1-21 |
| Methotrexate (intramuscular) | 20 | 1, 8, 15 |
| Vincristine | 1.5 | 22 |
| Prednisone | 40 | 22-26 |
Protocol II was repeated after a 6-week interim maintenance phase during which the patients received 6-mercaptopurine and methotrexate as in the maintenance described below (§).
IU/m2.
Age-adjusted doses of triple intrathecal therapy were for methotrexate, cytarabine, and prednisolone, respectively, as follows: younger than 1 year, 6/16/4 mg; 1 or older but younger than 2 years, 8/20/6 mg; 2 or older but younger than 3 years, 10/26/8 mg; 3 years or older, 12/30/10 mg.
Cranial radiotherapy was administered once during interim maintenance at the following doses: age 1-2 years, 12 Gy (18 Gy if CNS+ at diagnosis); age 2 years and older, 18 Gy (24 if CNS+); for high-risk patients younger than 1 year, extended triple intrathecal therapy during maintenance was substituted for cranial radiotherapy. Then tapered.
Patients with CNS leukemia had additional triple intrathecal therapy on days 8 and 22.
Treatment burden
Treatment burden in the AIEOP-ALL95 high-risk study was evaluated on the basis of need for blood derivatives (packed red blood cells, platelets) and need for and duration of intravenous antibiotic therapy, central venous line, and hospitalization.
Statistical analysis
Event-free survival (EFS) and survival curves were constructed by the Kaplan-Meier method. The log-rank test was used for univariate comparisons, and the Cox regression model was used to adjust the main comparisons by other prognostic features (leukocyte count, cutoff 100 000/μL; immunophenotype, T vs non-T; age, cutoff 10 years; and sex).11 The starting point for the observation time was the date of diagnosis. Death in induction, resistance, relapse, death in continuous CR (CCR), and secondary malignancy were considered adverse events in the calculation of EFS rates (induction death and resistance were considered events at time zero), whereas death from any cause was the sole event in determining overall survival. In both analyses, the observation time was censored at the last follow-up date if no event was noted. Follow-up was updated as of December 31, 2000; 1 (0.5%) patient was lost to follow-up. The primary analyses describe the outcome of the therapeutic approach for high-risk (HR) ALL, including the option of BMT, but secondary analysis was also performed after censoring the observation times on the date of transplantation. To gauge the efficacy of our modified therapy, we compared the outcome of high-risk patients in study AIEOP-ALL95 with that of a subgroup of patients from AIEOP-ALL912 selected according to the eligibility criteria of the AIEOP-ALL95 study.
Results
Laboratory and clinical characteristics of the 198 patients treated in the HR protocol are shown in Table2. Eligibility criteria are presented in hierarchical order; thus, column (1) counts all patients who were resistant to protocol Ia of the induction therapy, regardless of the remaining features, and so on for the subsequent columns. Consequently, column (4) identifies the patients who were defined as HR only because of their poor response to prednisone (ie, they indeed achieved CR and were negative for t(9;22) or t(4;11) translocations; PPR-only). Seventy-six percent (151 of 198) had PPR; in 119 (60.1%) patients, this was the sole high-risk feature. The next most frequent high-risk factor was resistance to induction therapy on protocol Ia (42 of 198, 21.2%), followed by the t(9;22) translocation (26 of 198, 13.1%), and infant age with either t(4;11) or CD10− ALL (11 of 198, 5.6%).
Characteristics of 198 patients with high-risk childhood acute lymphoblastic leukemia according to their eligibility criteria
| . | (1) Resistant to protocol Ia . | (2) t(9,22) . | (3) Infants t(4;11) or CD10− . | (4) PPR only . | Total . | % . |
|---|---|---|---|---|---|---|
| Total (%) | 42* (21.2) | 26 (13.1) | 11 (5.6) | 119 (60.1) | 198 | — |
| Sex | ||||||
| Male | 30 | 18 | 4 | 69 | 121 | 61.1 |
| Female | 12 | 8 | 7 | 50 | 77 | 38.9 |
| Age, y | ||||||
| Younger than 1 | 3 | 0 | 11 | 5 | 19 | 9.6 |
| 1 to 5 | 19 | 17 | 0 | 77 | 113 | 57.1 |
| 6 to 9 | 9 | 7 | 0 | 18 | 34 | 17.2 |
| 10 to 17 | 11 | 2 | 0 | 19 | 32 | 16.1 |
| WBC count, /μL | ||||||
| Less than 20 000 | 15 | 9 | 2 | 38 | 64 | 32.3 |
| 20 000 to 99 999 | 14 | 15 | 3 | 51 | 83 | 41.9 |
| 100 000 or more | 13 | 2 | 6 | 30 | 51 | 25.8 |
| Immunophenotype | ||||||
| Non-T | 32 | 26 | 11 | 85 | 154 | 77.8 |
| T | 10 | 0 | 0 | 34 | 44 | 22.2 |
| Prednisone response | ||||||
| Good | 16 | 23 | 6 | 0 | 45 | 22.7 |
| Poor | 24 | 3 | 5 | 119 | 151 | 76.3 |
| Unknown | 2 | 0 | 0 | 0 | 2 | 1.0 |
| CNS involvement | ||||||
| Negative | 40 | 26 | 11 | 113 | 190 | 96.0 |
| Positive | 0 | 0 | 0 | 6 | 6 | 3.0 |
| Unknown | 2 | 0 | 0 | 0 | 2 | 1.0 |
| t(9,22) | ||||||
| Nonpositive | 38 | 0 | 11 | 119 | 168 | 84.8 |
| Positive | 4 | 26 | 0 | 0 | 30 | 15.2 |
| t(4,11) | ||||||
| Nonpositive | 41 | 26 | 4 | 118 | 189 | 95.5 |
| Positive | 1 | 0 | 7 | 1 | 9 | 4.5 |
| . | (1) Resistant to protocol Ia . | (2) t(9,22) . | (3) Infants t(4;11) or CD10− . | (4) PPR only . | Total . | % . |
|---|---|---|---|---|---|---|
| Total (%) | 42* (21.2) | 26 (13.1) | 11 (5.6) | 119 (60.1) | 198 | — |
| Sex | ||||||
| Male | 30 | 18 | 4 | 69 | 121 | 61.1 |
| Female | 12 | 8 | 7 | 50 | 77 | 38.9 |
| Age, y | ||||||
| Younger than 1 | 3 | 0 | 11 | 5 | 19 | 9.6 |
| 1 to 5 | 19 | 17 | 0 | 77 | 113 | 57.1 |
| 6 to 9 | 9 | 7 | 0 | 18 | 34 | 17.2 |
| 10 to 17 | 11 | 2 | 0 | 19 | 32 | 16.1 |
| WBC count, /μL | ||||||
| Less than 20 000 | 15 | 9 | 2 | 38 | 64 | 32.3 |
| 20 000 to 99 999 | 14 | 15 | 3 | 51 | 83 | 41.9 |
| 100 000 or more | 13 | 2 | 6 | 30 | 51 | 25.8 |
| Immunophenotype | ||||||
| Non-T | 32 | 26 | 11 | 85 | 154 | 77.8 |
| T | 10 | 0 | 0 | 34 | 44 | 22.2 |
| Prednisone response | ||||||
| Good | 16 | 23 | 6 | 0 | 45 | 22.7 |
| Poor | 24 | 3 | 5 | 119 | 151 | 76.3 |
| Unknown | 2 | 0 | 0 | 0 | 2 | 1.0 |
| CNS involvement | ||||||
| Negative | 40 | 26 | 11 | 113 | 190 | 96.0 |
| Positive | 0 | 0 | 0 | 6 | 6 | 3.0 |
| Unknown | 2 | 0 | 0 | 0 | 2 | 1.0 |
| t(9,22) | ||||||
| Nonpositive | 38 | 0 | 11 | 119 | 168 | 84.8 |
| Positive | 4 | 26 | 0 | 0 | 30 | 15.2 |
| t(4,11) | ||||||
| Nonpositive | 41 | 26 | 4 | 118 | 189 | 95.5 |
| Positive | 1 | 0 | 7 | 1 | 9 | 4.5 |
Eligibility criteria are in hierarchical order. Thus, column (1) counts all patients who were resistant to induction (protocol Ia) regardless of the remaining features and so on for the subsequent columns. Consequently, column (4) identifies the patients who were defined as HR only because of their poor response to prednisone (they achieved CR and were negative for t(9;22) or t(4;11) translocation, PPR-only).
Of these 42 patients (2.8% of the entire AIEOP-ALL95 study population) who were at high risk because of failure to achieve CR after protocol Ia, 2 presented as standard risk, 14 as intermediate risk, and 26 already as high risk according to other criteria.
Three (1.5%) patients died on induction therapy (due to mediastinal syndrome, brain hemorrhage, or septicemia) (Table 3), and 11 (5.6%) failed to achieve CR by the end of consolidation therapy; thus, the CR rate in this high-risk group was 92.9% (184 of 198).
Treatment results and status of the patients according to their eligibility criteria in hierarchical order
| . | (1) Resistant to protocol Ia . | (2) t(9,22) . | (3) Infants t(4;11) or CD10− . | (4) PPR-only . | Total . | % . |
|---|---|---|---|---|---|---|
| Total | 42 | 26 | 11 | 119 | 198 | |
| Deaths in protocol Ia | 0 | 0 | 1 | 2 | 3 | 1.5 |
| Resistant | 113-150 | 0 | 0 | 0 | 11 | 5.6 |
| CR | 313-151 | 26 | 10 | 117 | 184 | 92.9 |
| Relapses | 16 | 11 | 3 | 25 | 553-152 | 27.8 |
| Marrow | 13 | 9 | 3 | 21 | 46 | |
| Marrow + CNS | 0 | 1 | 0 | 0 | 1 | |
| Marrow + Testis | 2 | 1 | 0 | 1 | 4 | |
| CNS | 1 | 0 | 0 | 3 | 4 | |
| Deaths in CCR | 3 | 1 | 1 | 5 | 103-153 | 5.0 |
| CCR | 12 | 14 | 6 | 87 | 119 | 60.1 |
| 4-year EFS | 25.6 (7.1) | 46.0 (11.6) | 54.6 (15.0) | 70.1 (4.7) | 56.5 (3.9) | |
| 4-year survival | 36.0 (8.1) | 50.7 (13.3) | 54.6 (15.0) | 75.9 (4.3) | 62.8 (3.9) | |
| 4-year EFS (censoring BMT first CR) | 21.1 (7.7) | 47.6 (12.9) | 58.4 (16.3) | 70.2 (5.1) | 57.2 (4.2) |
| . | (1) Resistant to protocol Ia . | (2) t(9,22) . | (3) Infants t(4;11) or CD10− . | (4) PPR-only . | Total . | % . |
|---|---|---|---|---|---|---|
| Total | 42 | 26 | 11 | 119 | 198 | |
| Deaths in protocol Ia | 0 | 0 | 1 | 2 | 3 | 1.5 |
| Resistant | 113-150 | 0 | 0 | 0 | 11 | 5.6 |
| CR | 313-151 | 26 | 10 | 117 | 184 | 92.9 |
| Relapses | 16 | 11 | 3 | 25 | 553-152 | 27.8 |
| Marrow | 13 | 9 | 3 | 21 | 46 | |
| Marrow + CNS | 0 | 1 | 0 | 0 | 1 | |
| Marrow + Testis | 2 | 1 | 0 | 1 | 4 | |
| CNS | 1 | 0 | 0 | 3 | 4 | |
| Deaths in CCR | 3 | 1 | 1 | 5 | 103-153 | 5.0 |
| CCR | 12 | 14 | 6 | 87 | 119 | 60.1 |
| 4-year EFS | 25.6 (7.1) | 46.0 (11.6) | 54.6 (15.0) | 70.1 (4.7) | 56.5 (3.9) | |
| 4-year survival | 36.0 (8.1) | 50.7 (13.3) | 54.6 (15.0) | 75.9 (4.3) | 62.8 (3.9) | |
| 4-year EFS (censoring BMT first CR) | 21.1 (7.7) | 47.6 (12.9) | 58.4 (16.3) | 70.2 (5.1) | 57.2 (4.2) |
Eligibility criteria are in hierarchical order. Thus, column (1) counts all patients who were resistant to induction (protocol Ia), regardless of the remaining features and so on for the subsequent columns. Consequently, column (4) identifies the patients who were defined as HR only because of their poor response to prednisone (they achieved CR and were negative for t(9;22) or t(4;11) translocation, PPR-only).
These 11 patients did not achieve remission by the end of consolidation therapy.
These 31 patients, who were not in CR after protocol Ia, achieved remission by the end of consolidation (block) therapy.
Two of these relapses occurred after BMT in first CR.
Six of these deaths were attributed to transplantation-related events.
Relapse was the most common cause of treatment failure and occurred in 55 (27.8%) children at a median time of 12 months after remission induction (range, 1-63 months). Most of the relapses were in the bone marrow (46 isolated and 5 combined); 4 patients had an isolated relapse in the CNS. Ten patients died during continuous CR—3 of septicemia, 1 of pneumonia during chemotherapy, and 6 of BMT-related complications.
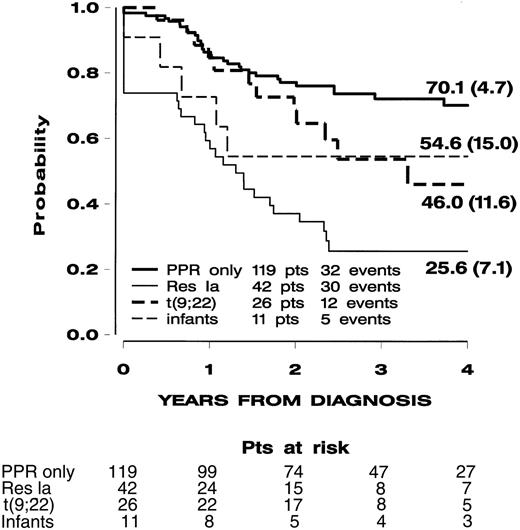
With a median follow-up of 3.2 years, the estimated 4-year probabilities of EFS and overall survival were 56.5% (SE, 3.9%) and 62.8% (SE, 3.9%), respectively. Figure1 shows the EFS curves for the 4 subgroups defined in Table 2. Their outcomes were significantly heterogeneous according to the log-rank test (P = .0001), and this result was maintained after adjusting the comparison (for leukocyte count, immunophenotype, age) in a Cox model.
Event-free survival (SE) of the 198 children with high-risk non-B ALL treated in the AIEOP-ALL95 study according to subgroups defined by eligibility criteria in hierarchical order.
See Table 2. Patients (pts) resistant to protocol Ia (Res Ia), regardless of the remaining features, and so on for the subsequent subgroups (ie, patients with t(9;22) translocation, infants with t(4;11) or CD10−, PPR-only patients (these were poor responders to prednisone but free of all previous features).
Event-free survival (SE) of the 198 children with high-risk non-B ALL treated in the AIEOP-ALL95 study according to subgroups defined by eligibility criteria in hierarchical order.
See Table 2. Patients (pts) resistant to protocol Ia (Res Ia), regardless of the remaining features, and so on for the subsequent subgroups (ie, patients with t(9;22) translocation, infants with t(4;11) or CD10−, PPR-only patients (these were poor responders to prednisone but free of all previous features).
The PPR-only subgroup had a 4-year EFS of 70.1% (SE, 4.7%). Patients who were PPR-only but also had T immunophenotype (n = 34) or leukocyte counts exceeding 100 000/μL (n = 30) had 4-year EFS of 66.6% (SE, 8.2%) and 60.1% (SE, 9.6%), respectively (Figure2). Noticeably, PPR-only patients who had both unfavorable features (n = 21) had a 4-year EFS of 61.2% (SE, 10.8%).
Event-free-survival (SE) of children with high-risk non–B ALL.
Children were grouped as PPR-only and T-ALL (n = 34) or PPR-only and leukocyte count of 100 000/μL (n = 30), treated in the AIEOP-ALL95 study. PPR-only is the subgroup of the PPR patients who achieved CR and were negative for t(9;22) and t(4;11) translocations. By definition, this subgroup does not include resistant patients; thus, EFS resembles a disease-free survival type of analysis.
Event-free-survival (SE) of children with high-risk non–B ALL.
Children were grouped as PPR-only and T-ALL (n = 34) or PPR-only and leukocyte count of 100 000/μL (n = 30), treated in the AIEOP-ALL95 study. PPR-only is the subgroup of the PPR patients who achieved CR and were negative for t(9;22) and t(4;11) translocations. By definition, this subgroup does not include resistant patients; thus, EFS resembles a disease-free survival type of analysis.
The subgroup of patients who were resistant to protocol Ia had the worst prognosis, with a 4-year EFS of 25.6% (SE, 7.1%); by definition, this group included all 11 resistant patients.
The t(9;22) and infant t(4;11)/CD10− subgroups had an intermediate outcome, but their relatively small sizes precluded additional meaningful statistical analysis. Translocation t(9,22) was found overall in 30 patients (4 of them were also resistant to protocol Ia); of these 30 patients, 5 had PPR (2 were also resistant to protocol Ia). Of the 5 t(9,22) and PPR patients, none are in CCR; this finding is consistent with previous reports.12 Of the 11 infants with t(4;11)/CD10−, 5 were also PPR and of these 2 are in CCR.
According to study design, a subset of 15 patients underwent BMT from a matched-related donor. Twelve other patients underwent BMT from alternative donors (9 matched unrelated donor, 2 mismatched donors, 1 fetal liver). One patient underwent autologous BMT. Of the patients who underwent transplantation, 18 remained in CCR for a median of 33 months (range, 1-54 months) after BMT; 6 patients died in remission and 4 had relapses. When EFS curves were calculated censoring the observation time at the date of BMT, results in the 4 subgroups did not change (Table 3).
The overall 4-year EFS estimate was significantly better than that obtained in the group of 114 patients selected with the same high-risk criteria and treated in study AIEOP-ALL91: 56.5% (SE, 3.9%) versus 40.2% (SE, 4.6%) (P = .002). The distribution of patient characteristics in the 2 groups was similar, and the advantage in overall EFS was maintained when the test for comparison was stratified by the 4 subgroups in a Cox model (P = .0005) and when the observation time was censored at the date of BMT. (Four-year EFS rates in AIEOP-ALL95 versus AIEOP-ALL91 were 57.2% [SE, 4.2%] versus 39.8% [SE, 5.1%] [P = .0007]).
Although the relatively small size precluded meaningful comparison of the remaining subgroups, the PPR-only subgroup was also analyzed separately. In protocols 95 and 91, the estimated 4-year EFS was 70.1% (SE, 4.7%) versus 48.9% (SE, 6.2%), respectively, and the difference was significant (P = .01) independently of other prognostic factors (white blood cell [WBC] count, immunophenotype, age, and sex, which were included in a Cox model). Noticeably, in the PPR-only subgroups, by definition, the EFS curves do not include resistance as an event (Table3). However, an advantage was still observed in the AIEOP-ALL95 study compared with the AIEOP-ALL91 study when all patients with PPR were considered: 4-year EFS was 61.1% (SE, 4.4%) versus 42.8% (SE, 5.4%) (P = .008), respectively (Figure 3).
Event-free survival (SE) of children with high-risk ALL who were PPR or PPR-only, treated in the AIEOP-ALL95 and AIEOP-ALL91 studies.
PPR-only is the subgroup of PPR patients who achieved CR and were negative for t(9;22) and t(4;11) translocations. By definition, this subgroup does not include resistant patients; thus, EFS resembles a disease-free survival type of analysis.
Event-free survival (SE) of children with high-risk ALL who were PPR or PPR-only, treated in the AIEOP-ALL95 and AIEOP-ALL91 studies.
PPR-only is the subgroup of PPR patients who achieved CR and were negative for t(9;22) and t(4;11) translocations. By definition, this subgroup does not include resistant patients; thus, EFS resembles a disease-free survival type of analysis.
Analysis of the requirements for supportive care throughout the various phases of treatment is shown in Table4 for patients with available data. Red blood cell transfusions were necessary for approximately 90% of the patients during protocols Ia and Ib, and consolidation with blocks were necessary for approximately 65% of the patients during protocol II. Platelet support was mainly restricted to the induction and consolidation phases. Intravenous antibiotics were also required for a substantial proportion of patients during intensive chemotherapy phases; the peak frequency was 82% during consolidation block therapy (mean duration of treatment, 14 days; range, 2-40 days). During the same phase, 83% of patients required a central venous line, and the mean hospital stay was 32 days (range, 6-65 days) compared with 22 days (3-58 days) during protocol Ia. No cases of cardiac failure were reported, and cardiac function in patients in complete remission, off therapy, and without transplantation, measured as ejection or shortening fraction (in 80% of the patients), was in the normal range.
Treatment burden according to treatment phase
| . | Protocol Ia . | Protocol Ib . | Consolidation blocks . | Protocol II, 1st . | Protocol II, 2nd . |
|---|---|---|---|---|---|
| No. patients analyzed | 182 | 157 | 124 | 109 | 31 |
| Red blood cells | |||||
| Patients receiving at least 1 U, % | 85 | 96 | 90 | 69 | 65 |
| No. units, mean | 2.9 | 3.1 | 3.7 | 2.4 | 2.1 |
| Platelets | |||||
| Patients receiving at least 1 U, % | 51 | 36 | 70 | 16 | 23 |
| No. units, mean | 4.3 | 3.6 | 5.1 | 3.6 | 3.4 |
| IV antibiotic therapy | |||||
| Patients, % | 46 | 37 | 82 | 46 | 74 |
| Duration in days, mean | 11 | 7 | 14 | 9 | 14 |
| Central venous line | |||||
| Patients, % | 67 | 75 | 83 | 80 | 74 |
| No. days, mean | 35 | 50 | 68 | 74 | 78 |
| Hospitalization | |||||
| Patients, % | 97 | 85 | 99 | 85 | 87 |
| No. days, mean | 22 | 14 | 32 | 11 | 19 |
| Phase duration, days | |||||
| Scheduled by protocol | 42 | 42 | 63 | 64 | 64 |
| Observed, mean | 46 | 59 | 73 | 81 | 89 |
| . | Protocol Ia . | Protocol Ib . | Consolidation blocks . | Protocol II, 1st . | Protocol II, 2nd . |
|---|---|---|---|---|---|
| No. patients analyzed | 182 | 157 | 124 | 109 | 31 |
| Red blood cells | |||||
| Patients receiving at least 1 U, % | 85 | 96 | 90 | 69 | 65 |
| No. units, mean | 2.9 | 3.1 | 3.7 | 2.4 | 2.1 |
| Platelets | |||||
| Patients receiving at least 1 U, % | 51 | 36 | 70 | 16 | 23 |
| No. units, mean | 4.3 | 3.6 | 5.1 | 3.6 | 3.4 |
| IV antibiotic therapy | |||||
| Patients, % | 46 | 37 | 82 | 46 | 74 |
| Duration in days, mean | 11 | 7 | 14 | 9 | 14 |
| Central venous line | |||||
| Patients, % | 67 | 75 | 83 | 80 | 74 |
| No. days, mean | 35 | 50 | 68 | 74 | 78 |
| Hospitalization | |||||
| Patients, % | 97 | 85 | 99 | 85 | 87 |
| No. days, mean | 22 | 14 | 32 | 11 | 19 |
| Phase duration, days | |||||
| Scheduled by protocol | 42 | 42 | 63 | 64 | 64 |
| Observed, mean | 46 | 59 | 73 | 81 | 89 |
Mean values were estimated including only patients who actually required the specific support.
Consolidation blocks data presented pertain to the entire phase (3 blocks).
Central venous line data are cumulative.
Discussion
Traditionally, age and leukocyte count, as well as DNA content and immunophenotype, have been considered the most useful prognostic factors in the clinical management of childhood ALL, though they often fail to accommodate patients who do not respond to induction/consolidation therapy or who have relapses on postremission regimens. The BFM experience has clearly demonstrated that in vivo response to 7 days of prephase steroid therapy is a powerful predictor of treatment outcome.3,4,12-14 In the AIEOP-ALL91 study, the high-risk group had a 5-year EFS rate of only 40% (SE, 4%),2 which was not remarkably different from the results achieved in ALL-BFM90, 34% (3%) at 6 years of follow-up.5 In this last study, PPR was recognized as the most powerful independent prognostic factor in a large unselected cohort of patients with childhood ALL who achieved remission on protocol Ia. In the AIEOP and the BFM studies, rotational administration of high-dose chemotherapy blocks did not provide significantly better leukemia control than standard BFM chemotherapy.4
The present data suggest an improvement in the prognosis of childhood ALL patients with PPR as their only high-risk feature. Despite the retrospective nature of the analysis, the 2 study populations (AIEOP-ALL95 and AIEOP-ALL91) were comparable with regard to eligibility requirements, age, leukocyte count, and immunophenotype so that the improvement in 4-year event-free-survival is most likely mainly related to differences in therapy. In the AIEOP-ALL95 study, treatment intensification was achieved with the use of a more traditional BFM-type backbone chemotherapy, consolidation with only 3 blocks, and repeated use of protocol II. This approach resulted in a significant improvement for the subgroup of high-risk patients defined as PPR-only. Interestingly, this advantage extended also to patients showing the association of adverse prognostic factors such as T phenotype, suggesting that this modified BFM-type therapy, developed also according to the experience of the CCG group,8 is particularly effective for such patients. This improvement, as shown in Figure 3, may account for the improvement obtained in the outcome of the whole group of PPR patients. It should be noted, however, that rotational intensive block chemotherapy, as already reported,2,5 provided results inferior to those of conventional BFM-type chemotherapy.4 15
Unfortunately, this improvement does not extend to all high-risk subgroups. Patients who did not achieve CR by the completion of protocol Ia, with or without detectable clonal translocations, remain the subgroup at highest risk for leukemic relapse with a 4-year EFS rate of 25.6% (SE, 7.1%). Patients with clonal translocations had a 4-year EFS of approximately 50%; nonetheless, the simultaneous presence of PPR with these features confers a poor prognosis.
As with most programs of intensive chemotherapy, the modified treatment devised for AIEOP-ALL95 requires careful monitoring to prevent fatal or life-threatening complications. On average, these patients spent approximately 100 days in the hospital during the 5 intensive treatment phases covering the first 9 months from diagnosis. However, the death rate in first CR was only 2%, justifying wider application of this therapy in patients with high-risk ALL. Although this treatment regimen included a high cumulative dose of anthracycline (daunomycin and doxorubicin) of 410 mg/m2, cardiac function was not reported as a clinical problem after treatment completion in this study. These results underscore the treatment dependence of high-risk prognostic features in children with ALL. The ability to rescue high-risk patients from the toxic effects of intensified chemotherapy would appear to justify further attempts to identify occult high-risk features, such as polymerase chain reaction–detectable minimal residual disease, as a means to refine treatment and ultimately to increase the cure rate in childhood ALL.
We thank Dr Daniela Silvestri for her excellent contribution to this study.
The following institutions enrolled patients in the AIEOP-ALL 95 study: Ancona, Clinica Pediatrica (Dr L. Felici, Dr P. Pierani); Bari, Clinica Pediatrica I (Prof F. Schettini, Dr N. Santoro); Bari, Clinica Pediatrica II (Prof N. Rigillo, Dr ssa S. Bagnulo); Bergamo, Div. Pediatria (Prof F. Bergonzi, Dr P. E. Cornelli), Ematologia (Prof T. Barbui); Bologna, Clinica Pediatrica (Prof G. Paolucci, Dr A. Pession, Dr R. Rondelli); Brescia, Clinica Pediatrica (Prof A. G. Ugazio, Dr A. Arrighini); Cagliari, Servizio di Oncoematologia Pediatrica (Prof P. F. Biddau, Dr ssa R. Mura); Catania, Divisione di Onco-Ematologia Pediatrica (Prof G. Schilirò, Dr L. Lo Nigro); Catanzaro, Div. di Ematologia (Prof S. Magro, Dr ssa C. Consarino); Firenze, Ospedale Meyer, Dipartimento di Pediatria, U. O. Oncoematologia Pediatrica (Profssa G. Bernini, Dr ssa A. Lippi); Genova, Ist. “G. Gaslini” (Prof G. Dini, Dr ssa C. Micalizzi); Milano Osp. Niguarda (Dr Fedeli); Milano Clinica Pediatrica (Dr Portaleone); Modena, Clinica Pediatrica (Profssa F. Massolo, Dr ssa M. Cellini); Monza, Clinica Pediatrica (Prof G. Masera, Dr V. Conter, Dr C. Rizzari, Dr M. Jankovic); Napoli, Ospedale Pausilipon (Prof V. Poggi, Dr ssa M. F. Pintà Boccalatte, Dr ssa C. De Fusco); Napoli, II Università, Dipartimento di Pediatrica, Servizio Autonomo di Oncologia Pediatrica, (Profssa M. T. Di Tullio, Dr ssa F. Casale, Dr ssa A. Murano); Napoli, Clinica Pediatrica II (Prof S. Auricchio, Dr A. Fiorillo, Dr ssa R. Migliorati); Napoli, Ospedale SS. Annunziata (Prof F. Tancredi, Dr A. Correra); Padova, Clinica Pediatrica II (Prof L. Zanesco, Prof G. Basso, Dr ssa C. Messina); Palermo, Clinica Pediatrica I (Profssa M. Lo Curto, Dr ssa G. Fugardi); Parma, Clinica Pediatrica (Dr G. Izzi, Dr ssa P. Bertolini); Pavia, Clinica Pediatrica (Profssa F. Severi, Dr M. Aricò, Dr F. Locatelli); Perugia, Divisione di Oncoematologia Pediatrica, Ospedale Silvestrini (Dr A. Amici, Dr P. Zucchetti); Pescara, Divisione di Ematologia (Dr A. Di Marzio, Dr R. Di Lorenzo, Prof G. Torlontano); Pisa, Clinica Pediatrica III (Prof P. Macchia, Dr C. Favre); Reggio Calabria, Divisione di Ematologia, Ospedali Riuniti (Prof F. Nobile, Dr ssa M. Comis); Roma, Divisione di Ematologia Pediatrica, Ospedale “Bambino Gesù”-(Prof G. De Rossi, Dr C. Miano); Roma, Cattedra di Ematologia (Prof F. Mandelli, Dr ssa am Testi); Roma, Clinica Pediatrica (Prof G. Multari, Dr ssa B. Werner); Roma, Clinica Pediatrica (Prof Castello); S. Giovanni Rotondo, Ospedale “Casa Sollievo della Sofferenza,” Divisione di Pediatria, Sezione di Ematologia ed Oncologia Pediatrica (Prof P. Paolucci, Dr S. Ladogana); Sassari, Clinica Pediatrica (Prof D. Gallisai, Dr C. Cosmi); Siena, Clinica Pediatrica (Prof G. Morgese, Dr A. Acquaviva, Dr A. D'Ambrosio); Torino, Clinica Pediatrica (Prof E. Madon, Prof R. Miniero, Dr ssa E. Barisone); Trieste, Clinica Pediatrica (Prof P. Tamaro, Dr G. A. Zanazzo); Varese, Clinica Pediatrica (Prof L. Nespoli, Dr ssa S. Binda); Verona, Clinica Pediatrica (Prof L. Tatò, Dr Marradi).
Supported by MURST (grant 2001068982-007), Fondazione Tettamanti, Città della Speranza, and other charities and parent associations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maurizio Aricò, Onco-Ematologia Pediatrica, Ospedale dei Bambini G. Di Cristina, Via Benedettini 1, Palermo, Italy; e-mail: arico@ospedalecivicopa.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal