Abstract
Donor lymphocyte infusion (DLI) can produce durable remissions in patients with chronic myelogenous leukemia (CML) who have a relapse after an allogeneic stem cell transplantation. However, the best modality to administer DLI is still unclear. The effect of the initial cell dose (ICD; ie, mononuclear cells × 108/kg received in the first instance) on outcome was retrospectively analyzed in 298 of 344 patients treated with DLI at 51 centers. Patients were classified into 3 groups according to the ICD: 98 in group A (≤ 0.20), 107 in group B (0.21-2.0), and 93 in group C (> 2.0). Additional infusions were given to 62%, 20%, and 5% of patients in groups A, B, and C, respectively. A lower ICD was associated with less graft-versus-host disease (GVHD; A, 26%; B, 53%; C, 62%;P < .001), less myelosuppression (A, 10%; B, 23%; C, 24%; P = .01), and similar response rate (A, 78%; B, 73%; C, 70%; P = .48). Nonadjusted estimates of 3-year survival, failure-free survival, and DLI-related mortality were 84%, 66%, and 5% respectively, in group A; 63%, 57%, and 20% in group B; and 58%, 45%, and 22% in group C. Outcome analysis was adjusted for patient age, donor type, sex of donor, sex mismatch, disease phase at transplantation, T-cell depletion, interval from transplantation to DLI, GVHD prior to relapse, relapse type, and date of DLI. After adjustment, lower ICD was associated with less GVHD, less myelosuppression, same response rate, better survival, better failure-free survival, and less DLI-related mortality. Our results suggest that the first DLI dose should not exceed 0.2 × 108 mononuclear cells/kg.
Introduction
Salvage therapy with donor lymphocyte infusion (DLI) can restore remission in many patients with chronic myelogenous leukemia (CML) who have a relapse after allogeneic stem cell transplantation (SCT).1-6 Because the probability of response decreases with the disease progression, molecular/cytogenetic monitoring of the disease after transplantation and prompt therapy with DLI before the development of hematologic relapse may represent the optimal management of patients after transplantation.7 The efficacy of DLI in CML has been limited to some extent by the morbidity and mortality associated with graft-versus-host disease (GVHD) and myelosuppression, which are frequently observed in responding patients.1-7 The overall probability of mortality in remission was reported to be as high as 18% at 2 years in 2 separate surveys conducted in Europe and North America.8 9
From retrospective data, it might be anticipated that giving DLI to patients in cytogenetic relapse rather than at later stages would significantly reduce the risk of developing bone marrow aplasia, but would not affect the risk of acute GVHD.9 However, response to DLI is not always associated with GVHD, suggesting that the graft-versus-leukemia (GVL) effect may be independent of the clinical development of GVHD.8,9 Starting the transfusion of donor cells at low cell numbers followed by escalating doses as required (ie, until achievement of response or development of GVHD) may reduce the incidence and severity of GVHD, while preserving the GVL effect.10,11 When a previous survey on DLI was conducted by the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group (EBMT) very few patients having a relapse after allografting for CML were treated with escalating doses of DLI before 1994.8 As the escalating dose regimen has become more frequently adopted in the following years, we wished to evaluate on a large scale the impact of the dose schedule of DLI on patient outcome. Therefore we have analyzed a total of 344 patients by updating 81 patients included in our previous survey8 and by collecting data from 263 new cases primarily treated from 1994 to 1998. This analysis has identified a significant correlation of the dose schedule with both safety and efficacy of DLI in relapsed CML.
Patients and methods
Data collection
On September 2000, EBMT member centers were asked to report and update their experience with patients treated with unmanipulated DLI for recurrent CML after the first allogeneic SCT from an HLA-identical sibling or an HLA-matched volunteer unrelated donor (VUD). The study was approved by the review board of the Chronic Leukemia Working Party. Patients were included regardless of disease stage. The reports included the following data: age and sex of the patients and their donors, histocompatibility and relationship with the donor, date of initial diagnosis, date of the first SCT, phase of the disease at SCT, conditioning regimen, GVHD prophylaxis, grade of acute and chronic GVHD after SCT, date of relapse, relapse type at the time of DLI, dates of lymphocyte transfusions, number of mononuclear cells (MNCs) transfused (at first infusion and cumulative), occurrence and severity of GVHD (acute and chronic) or myelosuppression (ie, neutropenia, thrombocytopenia) following DLI, response to DLI, type of remission, date of leukemia recurrence after DLI-induced remission, date of myeloablative conditioning and second SCT, date and disease status at last visit, and date and cause of death.
As of March 2001, data on 370 patients were reported: 344 patients treated with DLI before 1999 were included in this study, 26 patients treated after 1998 were excluded.
Definitions
DLI.
Lymphocytes were collected from the donors by leukaphereses on one or more occasions. The infusions given on multiple days at less than 7 days apart were counted as one infusion. None of the patients treated with DLI had GVHD at the time of infusion or was on immunosuppressive therapy.
The method of administration of DLI was not uniform, therefore we defined the following objective parameters:
1. The initial cell dose (ICD) indicating the number of MNC/kg transfused at the first infusion
2. The number of additional infusions (NAIs) given after the first infusion
3. The total cell dose (TCD) as the cumulative MNC dose/kg transfused in all consecutive infusions
4. The treatment duration (TD) as the period (in days) between the first and the last infusion
5. The average interval between infusions (AII) was calculated in patients treated with multiple infusions according to the formula: AII = TD/NAI.
6. The additional cell dose (ACD) was calculated in patients treated with multiple infusions with the formula: ACD = TCD − ICD.
Relapse.
Relapse was classified into molecular relapse (ie, BCR/ABL transcripts detected by quantitative reverse transcription–polymerase chain reaction [RT-PCR] in 2 consecutive tests performed over a minimum of 4 weeks), cytogenetic relapse (ie, presence of one or more Philadelphia chromosome–positive [Ph+] metaphases at bone marrow cytogenetics), and hematologic relapse (ie, presence of peripheral blood leukocytosis accompanied by a hypercellular bone marrow with Ph chromosome positivity on cytogenetic analysis). In our study, all patients in molecular relapse at time of DLI had increasing levels of BCR/ABL transcripts detected by quantitative RT-PCR.
The phase of CML was classified in accordance with criteria proposed by the International Blood and Marrow Transplantation Registry.12 However, the appearance of complex cytogenetic abnormalities at hematologic relapse was not sufficient alone to define accelerated phase at relapse. Accelerated or blastic phase was categorized as “transformed.”
Outcome.
Acute and chronic GVHD occurring either prior to relapse or after DLI were graded according to the standard clinical criteria.13
Myelosuppression after DLI was defined as a cytopenia (neutrophils < 0.5 × 109/L or platelets < 20 × 109/L) unrelated to disease or chemotherapy.
Response required the absence of Philadelphia chromosome by standard cytogenetics or negativity for BCR/ABL transcripts by RT-PCR if standard cytogenetics prior to DLI was not performed or negative.
Survival was calculated from the date of the first infusion of donor cells until death or last follow-up evaluation.
Failure-free survival (FFS) was calculated from the date of the first infusion of donor cells until death, last follow-up evaluation, or occurrence of an event such as unresponsiveness to DLI or relapse after response to DLI. Because of a selection criteria (ie, DLI given before 1999) and period of data collection (ie, from September 2000 until March 2001), most patients had a follow-up longer than 18 months from DLI and in our opinion this period was adequate to observe the great majority of the events considered by the FFS curve.
The DLI-related mortality (DLI-RM) was calculated from the date of the first infusion of donor cells until nonleukemic death or last follow-up evaluation. Censoring at the date of relapse after DLI, at the date of second SCT (ie, reinfusion of donor stem cells following a myeloablative conditioning regimen), or at the date of leukemic death was applied in the calculation of DLI-RM.
Statistical analysis
The χ2, Mann-Whitney U, and Kruskal-Wallis H tests were used to compare groups as appropriate. The log-rank test was used to compare survival curves. Survival curves were calculated according to the method of Kaplan and Meier.14 Logistic regression analysis was used with GVHD, myelosuppression, and response as the dependent variables; a proportional hazard regression model (Cox model) was used for survival, FFS, and DLI-RM.14 In each situation the proportionality assumption was verified both graphically and by introducing time as a time-dependent covariate and testing for a significant interaction with the risk factors under study. Due to severe nonproportionality it is impossible to grasp the influence of ICD in one Cox model allowing for estimation of all effects of interest. We used a landmark analysis approach by dividing the follow-up time into 2 periods of interest. Patient survival is described with the standard techniques conditional on the patient being alive without relapse at the start of the second interval. To evaluate a possibly changing effect of ICD 2 separate sets of analyses were performed, one for each period: first period, from the first day with an infusion of donor lymphocytes to 9 months; second period, from 9 months until last follow-up (around 12 years). The median interval to death (ie, 9 months) was chosen as cutoff and all models fulfilled the proportionality assumption.
The reader should bear in mind that the interpretation of the effect of the various risk factors in the second period is not the usual one; the analysis is conditional on being alive at the start of the period and hence adequately describes the influence of, for example, ICD during that period. However, the associated hazard ratios (HRs) do not have any reasonable interpretation at, for example, the moment of starting therapy with DLI itself because by then it is still unknown who will actually survive the first period.
The following possible risk factors were evaluated for their relationship with the outcome measures: donor type, sex of donor, sex mismatch with the donor, phase at SCT, GVHD prophylaxis with T-cell depletion for SCT, interval from SCT to DLI, occurrence of GVHD after SCT, date of DLI, stage of relapse at time of DLI, and ICD. Moreover, the effect of ICD was studied in particular using the other risk factors as (potential) confounders. The effect of ICD on the various outcome measures was thus estimated in 3 ways: crude (univariate: not adjusting for any of the risk factors), adjusted for all risk factors regardless of their significance (full regression model), and adjusted for all risk factors with a multivariately significant effect on the outcome (the result of a backward stepwise elimination carried out starting with the full regression model). HRs were estimated with 95% CI (95CI). Values of P < .05 were considered statistically significant.
Results
Method of administration of DLI
Table 1 outlines the method of administration of DLI. The ICD was available for 298 of 344 patients (87%); the median value was 1 × 108/kg (range, 0.002-24.4 × 108/kg). The ICD was less than 0.02 × 108 in 7 patients, between 0.02 × 108 and 0.20 × 108 in 91, between 0.21 × 108 and 2.0 × 108 in 107, between 2.1 × 108 and 20 × 108 in 93, and above 20 × 108 in 1. Therefore we divided patients into 3 groups, according to the ICD: group A, less than 0.21 × 108 (98 patients, 33%); group B, 0.21 to 2.0 × 108 (107 patients, 36%); and group C, more than 2.0 × 108 (93 patients, 31%).
Treatment with DLI included only one infusion for 241 patients (70%), whereas it included additional consecutive infusions for the remaining 103 patients (30%). Additional infusions were given to 61 group A patients (61%), 24 group B patients (22%), 5 group C patients (5%), and 13 with unavailable ICD (28%). The NAIs, the AII, and the ICD were all available for 68 of 103 patients treated with multiple infusions: 43 of 61 in group A, 20 of 24 in group B, and 5 of 5 in group C. Forty-four patients received 1 or 2 additional infusions (27 group A, 13 group B, and 4 group C patients), and 24 had 3 or more additional infusions (16 group A, 7 group B, and 1 group C patients). Additional infusions were given at a median interval of 44 days (range, 7-489 days). Median AIIs were 48, 43, and 44 days in groups A, B, and C, respectively. Median ACD (× 108/kg) was 2 (range, 0.1-79 × 108/kg): 1.7, 2.5, and 2.4 in groups A, B, and C, respectively. Median TCD (× 108/kg) was 2 (range, 0.1-80 × 108/kg): 0.5, 1.5, and 3.8 in groups A, B, and C, respectively.
Pre-DLI features and ICD
The frequencies of pre-DLI features both in the entire population and in ICD groups are reported in Table2. The ICD groups were comparable in terms of pre-DLI features except for donor type, T-cell depletion, GVHD after SCT, stage of relapse at time of DLI, and period of DLI. Lower ICD was associated with a higher incidence of VUD, T-cell depletion, GVHD prior to relapse, molecular/cytogenetic relapse, and DLI in the years 1988 to 1994. The median interval from SCT to the last day with an infusion of donor cells was 32 months (range, 8-145 months): 33, 32, and 33 months in groups A, B, and C, respectively (not shown).
The ICD was assessed in 85 of 96 patients who were given DLI for molecular/cytogenetic relapse: 35 of 41 treated in the years 1988 to 1995 (group A in 17%, group B in 46%, and group C in 37%) and 58 of 63 treated in the years 1996 to 1998 (group A in 60%, group B in 31%, and group C in 9%; P < .001).
Outcome
GVHD.
Of the 333 evaluable patients, 159 (48%) developed acute GVHD (51 grade I, 64 grade II, 29 grade III, 13 grade IV, and 2 more than grade I not otherwise specified) and 58 of 301 assessable patients (19%) developed chronic GVHD (24 limited, 34 extensive). GVHD according to ICD is shown in Table 3. Acute GVHD was absent or grade I in 81 of 97 assessable group A patients (84%), 65 of 105 group B patients (62%), and 46 of 90 group C patients (50%;P < .001). Grade III to IV acute GVHD was observed in 6 group A patients (6%), 17 group B patients (16%), and 16 group C patients (18%; P = .04). Chronic GVHD was observed in 19 of 91 group A patients (21%), 25 of 84 group B patients (30%), and 12 of 61 group C patients (20%; P = 0.27).
Univariate analysis shows that GVHD (ie, acute GVHD grades II-IV or all chronic GVHD) is related to sex of donor, sex mismatch with the donor, phase at SCT, period of DLI, stage of relapse at time of DLI, and ICD (Table 4). The incidence of GVHD was 26%, 53%, and 62% in the A, B, and C groups, respectively (P < .001). The crude HR of GVHD was 3.1 in group B and 4.6 in group C, as compared to group A patients (Table5, univariate). The risk, adjusted on all variables, increases by a factor of 2.2 (95CI: 1.0-4.8,P = .047) in group B, and by a factor of 4.0 (95CI: 1.6-9.9, P = .002) in group C, as compared to group A patients (Table 5, full model). Sex mismatch with the donor, first chronic phase at SCT, DLI in the years 1988 to 1995, and ICD higher than 0.2 × 108 MNC/kg (ie, group B or group C) were factors associated with a higher risk of acute GVHD grades II to IV or chronic GVHD after DLI (Table 5, reduced model).
Myelosuppression.
Myelosuppression was observed in 63 patients (18%): neutropenia and thrombocytopenia in 46, neutropenia alone in 6, and thrombocytopenia alone in 11. Univariate analysis shows that myelosuppression is significantly related to stage of relapse at time of DLI (P = .009) and to ICD (P = .01; Table 4). The incidence of myelosuppression was 10%, 23%, and 24% in groups A, B, and C, respectively. The crude HR of myelosuppression was 2.7 in group B and 2.8 in group C, as compared to group A patients (Table 5, univariate). The adjusted risk increased by a factor of 2.7 (95CI, 1.1-7.0, P = .03) in group B and by a factor of 2.4 (95CI, 0.8-6.6, P = .10) in group C, as compared to group A patients (Table 5, full model). T-cell depletion at SCT, transformed relapse, and ICD higher than 0.2 × 108 MNC/kg (ie, group B or group C) were factors associated with a higher risk of myelosuppression after DLI (Table 5, reduced model).
Response.
A complete response with DLI was achieved in 239 patients (69.5%). Response rates were 78%, 73%, and 70% in groups A, B, and C, respectively (P = .48). The number of responders treated with a single infusion versus the number of responders treated with multiple infusions was 35 versus 41 in group A, 63 versus 15 in group B, and 63 versus 2 in group C. There was no significant effect of the ICD on response rate (Table 4 and Table 5, univariate and full model). VUD, more advanced phase at SCT, T-cell–replete SCT, clinical evidence of the disease at relapse (ie, hematologic relapse or transformed relapse) were factors associated with a lower probability of response to DLI (Table 5, reduced model).
Survival.
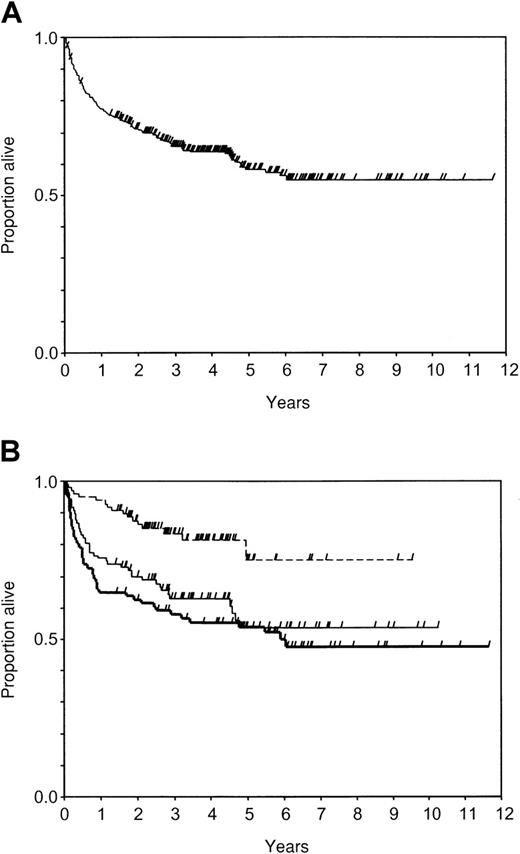
Two hundred sixteen patients were alive at a median of 48 months from the first infusion (range, 1-142 months): 177 in continuous remission after DLI, 11 relapsed after response to DLI, and 28 nonresponsive to DLI. Death occurred in 128 patients (77 nonresponsive to DLI, 38 responsive to DLI, and 13 relapsed after DLI) at a median of 9 months from the first infusion (range, 0.5-73 months); 62 died of progressive disease, 15 died of complications related to a second SCT performed after DLI, 49 died of complications related to DLI, and 2 died of other causes. The actuarial probability of survival was 0.77 (95CI, 0.73-0.82) at 1 year, 0.66 (95CI, 0.60-0.71) at 3 years, 0.58 (95CI: 0.52-0.64) at 5 years, and 0.55 (95CI: 0.48-0.62) at 10 years (Figure 1A).
Probability of survival after DLI.
Check marks indicate censored patients. (A) In all patients (n = 344); (B) in groups of initial cell dose: group A, less than or equal to 0.2 × 108 MNC/kg (n = 98, dotted line); group B, 0.21 to 2.0 × 108 MNC/kg (n = 107, thin line); group C, more than 2 × 108 MNC/kg (n = 93, thick line).
Probability of survival after DLI.
Check marks indicate censored patients. (A) In all patients (n = 344); (B) in groups of initial cell dose: group A, less than or equal to 0.2 × 108 MNC/kg (n = 98, dotted line); group B, 0.21 to 2.0 × 108 MNC/kg (n = 107, thin line); group C, more than 2 × 108 MNC/kg (n = 93, thick line).
In univariate analysis male donor, SCT in first chronic phase, interval from SCT to DLI longer than 2 years, period of DLI 1996 to 1998, less advanced stage of relapse at time of DLI, and lower ICD were significant favorable factors for survival after DLI (Table 4). Nonadjusted estimates of survival at 1 to 3 years were 0.94 to 0.84, 0.75 to 0.63, and 0.65 to 0.58 in groups A, B, and C, respectively (P < .001; Figure 1B). The crude HRs in the first period were 4.7 in group B and 6.1 in group C, as compared to group A patients (Table 5, univariate).
Multivariate analysis indicated that the rapidity of death in the first period increases by a factor of 3.2 (95CI, 1.0-10.3,P = .038) in group B and by a factor of 6.6 (95CI, 2.0-21.4, P = 0.002) in group C, as compared to patients in group A (Table 5, full model).
The VUD, female donor, sex mismatch with the donor, clinical evidence of relapse at time of DLI (ie, hematologic relapse or transformed relapse), and ICD higher than 0.2 × 108 MNC/kg (ie, group B or group C) were unfavorable prognostic factors in the first period (Table 5, reduced model).
The effect of the ICD on the rapidity of death in the second period was not significant in all analyses (Table 5): HRs of groups B and C were 1.2 (95CI, 0.5-2.8, P = .628) and 1.3 (95CI: 0.5-3.1,P = .582), respectively (Table 5, full model). More advanced phase at SCT and date of DLI in the years 1988 to 1994 were unfavorable prognostic factors in the second period (Table 5, reduced model).
FFS.
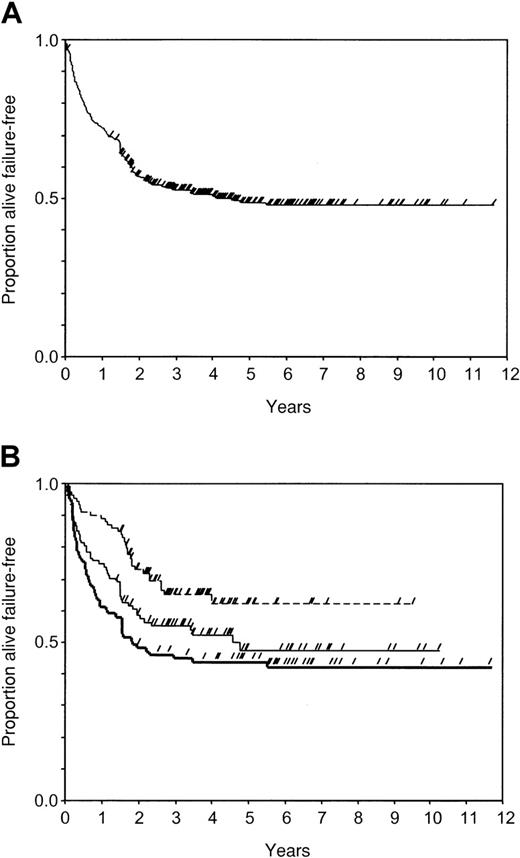
A total of 178 patients (52%) were alive and in continuous remission at a median of 1493 days (range, 32-4257 days) from the first infusion of donor cells, and 166 patients (48%) had failure of DLI at a median of 265 days (range, 15-1996 days) from the first infusion. The type of failure was no response to DLI in 92 patients, toxic death in 49, relapse after response to DLI in 23, and death from other causes in 2. The actuarial probability of FFS was 0.73 (95CI, 0.68-0.77) at 1 year, 0.53 (95CI, 0.47-0.58) at 3 years, 0.49 (95CI, 0.43-0.54) at 5 years, and 0.48 (95CI, 0.42-0.54) at 10 years (Figure 2A).
Probability of FFS after DLI.
Check marks censored patients. (A) In all patients (n = 344); (B) in groups of initial cell dose: group A, less than or equal to 0.2 × 108 MNC/kg (n = 98, dotted line); group B, 0.21-2.0 × 108 MNC/kg (n = 107, thin line); group C, more than 2 × 108 MNC/kg (n = 93, thick line).
Probability of FFS after DLI.
Check marks censored patients. (A) In all patients (n = 344); (B) in groups of initial cell dose: group A, less than or equal to 0.2 × 108 MNC/kg (n = 98, dotted line); group B, 0.21-2.0 × 108 MNC/kg (n = 107, thin line); group C, more than 2 × 108 MNC/kg (n = 93, thick line).
In the univariate analysis, FFS was significantly related with phase at SCT, T-cell depletion, interval from SCT to DLI, stage of relapse at time of DLI, and ICD (Table 4). Nonadjusted estimates at 1 to 3 years were 0.86 to 0.66, 0.67 to 0.57, and 0.54 to 0.45 in groups A, B, and C, respectively (P = .002; Figure3B). The crude HRs in the first period were 2.7 in group B and 4.1 in group C, as compared to group A patients (Table 5, univariate).
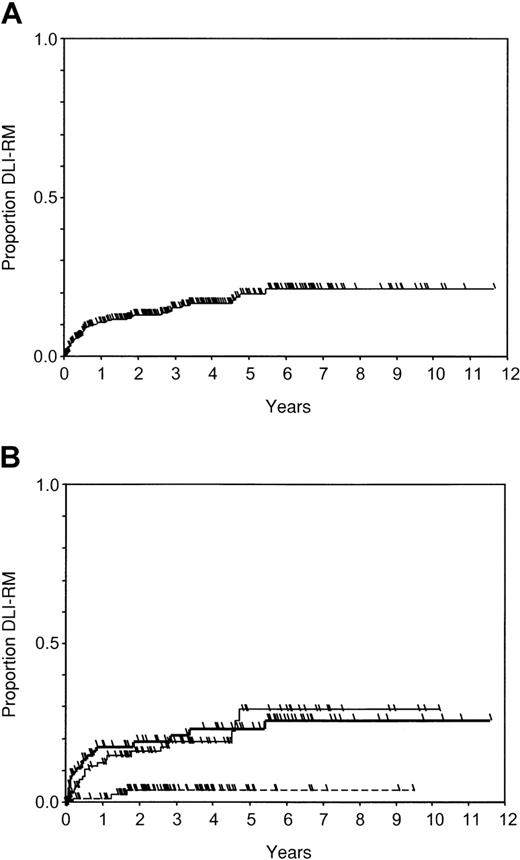
Probability of DLI-RM after DLI.
Check marks indicate censored patients. (A) In all patients (n = 344); (B) in groups of initial cell dose: group A, less than or equal to 0.2 × 108 MNC/kg (n = 98, dotted line); group B, 0.21-2.0 × 108 MNC/kg (n = 107, thin line); group C, more than 2 × 108 MNC/kg (n = 93, thick line).
Probability of DLI-RM after DLI.
Check marks indicate censored patients. (A) In all patients (n = 344); (B) in groups of initial cell dose: group A, less than or equal to 0.2 × 108 MNC/kg (n = 98, dotted line); group B, 0.21-2.0 × 108 MNC/kg (n = 107, thin line); group C, more than 2 × 108 MNC/kg (n = 93, thick line).
The adjusted risk of failure or death in the first period increases by a factor of 2.0 (95CI: 0.8-5.0, P = .124) in group B and by a factor of 3.6 (95CI, 1.4-9.0, P = .006) in group C, as compared to patients in group A (Table 5, full model). An interval from transplantation to first infusion shorter than 2 years, clinical evidence of relapse at time of DLI (ie, hematologic relapse or transformed relapse), and ICD higher than 0.2 × 108MNC/kg (ie, group B or group C) were unfavorable prognostic factors in the first period (Table 5, reduced model). The ICD did not influence FFS significantly in the second period: HRs of groups B and C were 1.1 (95CI, 0.6-2.2, P = .701) and 1.2 (95CI, 0.6-2.6,P = .582), respectively (Table 5, full model). More advanced phase at SCT was the only unfavorable prognostic factor in the second period (Table 5, reduced model).
DLI-RM.
Forty-nine patients died of GVHD or infections following therapy with DLI at a median interval of 189 days (range, 23-1996 days) from the first infusion. Thirty-seven patients were either in cytogenetic or molecular remission at the time of DLI-related death: 1 was given DLI at molecular relapse, 8 at cytogenetic relapse, 21 at hematologic relapse, and 7 at transformed relapse. The other 12 patients died of DLI-related complications either in marrow aplasia or in hematologic remission: 1 was given DLI at cytogenetic relapse, 8 at hematologic relapse, and 3 at transformed relapse.
Forty-eight patients were censored before the last follow-up: 23 because of relapse occurring at a median of 385 days (range, 55-1499 days) from DLI and 25 because of a second SCT performed at a median of 477 days (range, 83-1249 days) from DLI. Sixty-four patients were censored at time of death, which was caused by the disease in 62 at a median of 124 days (range, 15-4257 days), and by other causes in another 2 patients at 247 and 993 days, respectively. The actuarial probability of DLI-related death was 0.10 (95CI, 0.07-0.14) at 1 year, 0.16 (95CI, 0.12-0.20) at 3 years, 0.21 (95CI, 0.15-0.26) at 5 years, and 0.22 (95CI: 0.16-0.28) at 10 years (Figure 3A).
The DLI-RM was significantly related with sex of donor, with stage of relapse at time of DLI, and with ICD in the univariate analysis (Table4). Nonadjusted estimates at 1 to 3 years were 0.02 to 0.05, 0.13 to 0.20, and 0.18 to 0.22 in groups A, B, and C, respectively (P = .002; Figure 2B). The crude HRs in the first period were 5.9 in group B and 8.0 in group C as compared to group A patients (Table 5, univariate).
The adjusted risk in the first period increased by a factor of 9 (95CI, 1.1-77, P = .043) in group B and by a factor of 17.8 (95CI, 2-160, P = .010) in group C as compared to patients in group A (Table 5, full model). In the same period, female donor, sex mismatch with the donor, an interval from transplantation to first infusion shorter than 2 years, and ICD higher than 0.2 × 108 MNC/kg (ie, group B or group C) were unfavorable prognostic factors (Table 5, reduced model).
The crude HRs in the second period were 4.2 in group B and 3.2 in group C, as compared to group A patients (Table 5, univariate). The adjusted risk in the second period increased by a factor of 6.1 (95CI, 1.1-33.5,P = .036) in group B and by a factor of 4.2 (95CI: 0.6-27.8, P = .139) in group C, as compared to group A patients (Table 5, full model). GVHD prophylaxis with T-cell depletion and ICD higher than 0.2 × 108 MNC/kg (ie, group B or group C) were unfavorable prognostic factors in the second period (Table 5, reduced model).
Subsets analyses.
The significant prognostic effect of the ICD on GVHD, myelosuppression, survival, FFS, and DLI-RM and the absence of a significant influence of ICD on response to DLI were all confirmed when the statistical analysis was conducted on HLA-identical sibling and VUD transplants, separately.
Discussion
Previous studies of DLI in CML relapse showed that the major factor for response is relapse type.1-9 The response to DLI is often associated with the development of GVHD, but the incidence of severe GVHD is reduced when an escalating dose rather than a bulk dose regimen is used.11 13 However, it is not clear if an escalating dose regimen can induce durable remissions or is suitable for all patients.
This retrospective analysis of 344 patients from 51 centers affiliated with the EBMT confirms that adoptive immunotherapy with donor lymphocytes is an effective treatment of patients with CML who have a relapse after allogeneic SCT and suggests that the method of administration of DLI can influence the outcome independently of other relevant factors.
The administration of DLI was heterogeneous with respect to the number of infusions and mononuclear cell dose given at each infusion. Our data suggest that, irrespective of the initial cell dose, the treatment was stopped or continued with additional infusions according to disease response or the effect on the patient. Responses were achieved with multiple infusions irrespective of the initial cell dose. This fact would bias a comparison of patients treated with a “single bulk dose” with patients treated with “escalating doses.” Similarly, the prognostic effect of the number, dose, and frequency of additional infusions could not be correctly measured. Therefore, assuming that any patient who started therapy with donor lymphocytes was deemed to proceed with further infusions as appropriate, we focused our analysis on the prognostic effect of the ICD.
The ICD was related to several other features, suggesting that the strategy of treatment with donor lymphocytes was frequently chosen according to donor type, T-cell depletion, GVHD prior to relapse, relapse type, and year. Therefore, in our multivariate analysis, the effect of the ICD on outcome was also adjusted for these 5 factors, independently of their individual prognostic effect.
According to our analysis, the ICD did not influence response to DLI; however, the lower the starting dose, the higher the number of infusions given to achieve a response. The incidence and severity of GVHD as well as the incidence of myelosuppression after DLI increased with a higher ICD. GVHD and myelosuppression induced by DLI were frequently associated with nonleukemic death; thus, the ICD affected both survival and FFS as a consequence of its effect on DLI-related mortality. Noteworthy, ICD similarly affected survival, DLI mortality, and FFS.
Success of DLI was limited in the early 1990s by the significant toxicity and mortality associated with this form of treatment, as opposed to therapy with interferon-α (IFN-α).15-18Cytogenetic complete response to IFN-α alone can be achieved both in cytogenetic and in hematologic relapse, but the achievement of molecular remissions remains anecdotal. Therefore, most of these patients will be exposed to an increased risk of relapse and transformation as opposed to patients treated with DLI.19
A significant change in the approach to relapse and in the modality of giving DLI was registered in EBMT centers during the 1990s. In the early period of 1988 to 1995, very few patients were treated with DLI at molecular/cytogenetic relapse and the cell dose administered was usually more than 0.2 × 108 MNC/kg. Lower doses and treatment before the onset of the hematologic relapse became more frequent thereafter. These changes in the approach to treating relapse may well explain the improvement in survival after relapse which was observed in the EBMT registry data in the late 1990s.20
Our results demonstrate that patients with relapsed CML who start DLI, with a dose higher than 0.2 × 108 MNC/kg, are exposed to significant morbidity and mortality. The incidence of major untoward effects can be markedly reduced by starting with a cell dose below or equal to 0.2 × 108 MNC/kg. Starting with a low dose is therefore important to reduce toxicity of DLI, but our data suggest that, with a low starting dose, dose escalation is often necessary to achieve a response.
Furthermore, factors such as donor type, sex of donor, disease phase at transplantation, T-cell depletion, interval from transplantation to DLI, GVHD prior to relapse, and relapse type should be taken into account in future prospective studies.
The authors are extremely grateful to Anja van Biezen and Nelleke Tazelaar for their assistance in collecting and analyzing data and to the transplant centers (listed in “”) that reported patients.
The lists the responsible individual, center, city, and state by decreasing number of cases (in parentheses reported for this study).
F. Dazzi, Hammersmith Hospital, London, United Kingdom (66); D. Bunjes, University of Ulm, Ulm, Germany (29); L. Verdonck, University Medical Center Utrecht, Utrecht, The Netherlands (25); A. Schattenberg, University Medical Center St Radboud, Nijmegen, The Netherlands (24); H. J. Kolb, Klinikum Grosshadern, Munich, Germany (20); P. Ljungman, Huddinge University Hospital, Huddinge, Sweden (17); A. Devergie, Hospital St Louis, Paris, France (16); A. Urbano-Ispizua, Hospital Clinic, Barcelona, Spain (16); W. Arcese, Università“La Sapienza,” Rome, Italy (13); A. Bacigalupo, Ospedale San Martino, Genova, Italy (12); M. Michallet, Hospital E. Herriot, Lyon, France (12); V. Runde, University of Essen, Essen, Germany (10); A. Zander, University Hospital Eppendorf, Hamburg, Germany (9); D. Niederwieser, University of Leipzig, Leipzig, Germany (9); W. Siegert, Charité-Virchow Klinikum, Berlin, Germany (8); H. Greinix, AKH Vienna, Vienna, Austria (7); S. McCann, St James Hospital Trinity College, Dublin, Ireland (7); R. Willemze, Leiden University Medical Center, Leiden, The Netherlands (7); J. Y. Cahn, Hospital Jean Minjoz, Besancon, France (7); E. Conde, Hospital University “Marques de Valdecilla,” Santander, Spain (6); G. Gastl, University Hospital Innsbruck, Innsbruck, Austria (6); B. Hertenstein, Medical School of Hannover, Hannover, Germany (6); N. Jacobsen, Rigshospitalet, Copenhagen, Denmark (6); A. Ferrant, Cliniques Universitaire St Luc, Brussels, Belgium (5); K. Paloczi, NIH and Immunology, Budapest, Hungary (5); A. Vitek, Institute of Hematology, Prague, Czech Republic (5); A. Gratwohl, Kantonsspital, Basel, Switzerland (4); T. Ruutu, University of Helsinki, Helsinki, Finland (4); A. Fauser, Klinik fur KMT und Hamatologie, Idar-Oberstein, Germany (4); G. Bandini, Hospital San Orsola, Bologna, Italy (3); B. Chapuis, Hopital Cantonal Universitaire, Geneva, Switzerland (3); J. Finke, University of Freiburg, Freiburg, Germany (3); C. Cordonnier, Hopital Henri Mondor, Creteil, France (2); J. Sierra, Hospital Santa Creu I Sant Pau, Barcelona, Spain (2); R. Haas, Heinrich Heine University, Dusseldorf, Germany (2); C. Urban, University Children's Hospital, Graz, Austria (2); P. Bordigoni, CHU, Nancy, France (2); J. Gmur, Klinik im Park, Zurich, Switzerland (1); L. Brinch, Rikshospitalet, Oslo, Norway (2); J. Fernandez-Ranada, Hospital de la Princesa, Madrid, Spain (2); J. Cornelissen, Academic Hospital Rotterdam, Rotterdam, The Netherlands (2); M. Boogaerts, University Hospital Gasthuisberg, Leuven, Belgium (2); I. Franklin, Glasgow Royal Infirmary, Glasgow, Scotland (2); J. Jouet, Hopital Claude Huriez, Lille, France (2); H. Prentice, Royal Free Hospital and School of Med., London, United Kingdom (1); J. Davies, Western General Hospital, Edinburgh, United Kingdom (1); P. Di Bartolomeo, Ospedale Civile, Pescara, Italy (1); N. Schmitz, Christian-Albrechts-University, Kiel, Germany (1); S. Lenhoff, University Hospital, Lund, Sweden (1); P. Alessandrino, IRCCS Policlinico San Matteo, Pavia, Italy (1); B. Labar, University Hospital Centre-Rebro, Zagreb, Croatia (1); A. Hunter, Leicester Royal Infirmary, Leicester, United Kingdom (1); B. Rotoli, “Federico II” Medical School, Napoli, Italy (1); A. Bosi, Ospedale di Careggi, Firenze, Italy (1); A. Fassa, George Papanicolaou General Hospital, Thessaloniki, Greece (1); R. Schots, University Hospital VUB, Brussels, Belgium (1); V. Koza, Charles University Hospital, Pilsen, Czech Republic (1); D. Beelen, University of Saarland, Homburg, Germany (1); A. Goldstone, University College London Hospital, London, United Kingdom (1); C. Uderzo, Clinica Ped. Ospedale San Gerardo, Monza, Italy (1); and R. Scime, Ospedale V. Cervello, Palermo, Italy (1).
Supported in part by Sezione di Roma della Associazione Italiana contro le Leucemie (ROMAIL).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cesare Guglielmi, Cattedra di Ematologia, Università “La Sapienza,” via Benevento 6, 00161 Roma, Italy; e-mail: guglielmi@bce.uniroma1.it.