Interleukin 21 (IL-21) has recently been identified as a multifunctional cytokine that induces the proliferation of T cells and B cells and differentiation of natural killer cells. To determine whether IL-21 regulates IL-4–mediated immune responses, we examined the effect of IL-21 on antigen-specific IgE production in mice. We also examined the effect of IL-21 on IL-4–induced IgE production from B cells and antigen-induced T-helper 2 (Th2) cell differentiation. The in vivo injection of IL-21 prevented antigen-specific IgE but not IgG2a production on immunization. IL-21 did not affect Th2 cell differentiation or IL-4 production from CD4+ T cells but directly inhibited IL-4–induced IgE production from B cells at single-cell levels. Moreover, IL-21 inhibited IL-4–induced germ line Cε transcription in B cells without the inhibition of signal transducer and activator of transcription 6 (Stat6) activation. Taken together, these results indicate that IL-21 down-regulates IgE production from IL-4–stimulated B cells through the inhibition of germ line Cε transcription and thus suggest that IL-21 may be useful for the treatment of IgE-dependent allergic diseases.
Introduction
Immunoglobulin E (IgE) plays a crucial role in the pathogenesis of allergic diseases, in which there is a polarization of T-lymphocyte responses toward T-helper 2 (Th2) cells that produces cytokines such as interleukin 4 (IL-4) and IL-5.1-4 During the exposure to antigen stimulation, B cells are activated and switched from expressing IgM to other immunoglobulin isotypes through the mechanism of switch recombination.5,6 Gene-targeting experiments indicate that switch recombination requires the synthesis of specific classes of germ line transcripts,7,8 which are regulated by promoters located upstream of each switch region.6 In conjunction with signals that activate B cells, such as CD40 ligand (CD40L), cross-linking of B-cell receptors, or lipopolysaccharide (LPS) stimulation, specific cytokines regulate the activities of germ line promoters; that is, IL-4, interferon γ (IFN-γ), and transforming growth factor β (TGF-β) selectively direct isotype switching in mouse B cells to IgG1 and IgE, to IgG2a, and to IgA and IgG2b, respectively.5 6
It has recently been shown that IL-21 is a multifunctional cytokine that induces the proliferation of T cells and B cells and differentiation of natural killer (NK) cells.9 IL-21 is a 4-helix bundle type I cytokine with a significant homology to IL-2, IL-15, and IL-4.9 Production of IL-21 is restricted to activated CD4+ T cells9 and the best stimulation for CD4+ T cells to produce IL-21 is the combination of anti-CD3 and anti-CD28.9 The secreted IL-21 enhances IL-2– and IL-15–induced proliferation of T cells.9 Thus, IL-21 may function as an auto-growth factor for T cells like IL-2. On the other hand, IL-21 regulates B-cell proliferation either positively or negatively, depending on the costimuli encountered by the B cells.9 This observation suggests that signaling through IL-21 receptor may function as a fine regulator of B-cell function during immune responses.
IL-21 receptor belongs to the class I cytokine receptor family and exhibits amino acid sequence similarity to IL-2 receptor β chain and IL-4 receptor α chain.9,10 More recently, it has been shown that IL-21 uses a common cytokine receptor γ chain (γc) as a receptor component11 and thus the functional IL-21 receptor is a heterodimer of unique IL-21 receptor and γc. However, the role of IL-21 in the regulation of IL-4–mediated immune responses is as yet unknown.
To determine whether IL-21 regulates IL-4–mediated immune responses, we examined the effect of IL-21 on antigen-specific IgE production in vivo. We also examined the effect of IL-21 on IL-4–induced IgE production from B cells and antigen-induced Th2 cell differentiation in vitro. We found that IL-21 down-regulated IL-4–dependent IgE production from B cells but did not affect Th2 cell differentiation. We also found that IL-21 inhibited IL-4–induced germ line Cε transcription in B cells without the inhibition of signal transducer and activator of transcription 6 (Stat6) activation. The implications of these findings are discussed.
Materials and methods
Mice and cytokines
BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Stat6-deficient (Stat6−/−) mice12 and ovalbumin (OVA)–specific DO11.10 (DO10+) T-cell receptor (TCR) transgenic mice13 were backcrossed for more than 8 generations onto BALB/c mice. All mice were housed in microisolator cages under pathogen-free conditions and all experiments were performed according to the guidelines of Chiba University. Recombinant murine IL-21 and soluble IL-21 receptor (sIL-21R) were purified as described previously.9 Murine IL-2, IL-4, and IL-12 were purchased from R & D Systems (Minneapolis, MN).
Effect of IL-21 on antigen-specific IgE, IgG1, and IgG2a production
BALB/c mice (7-8 weeks old) were immunized intraperitoneally twice with 4 μg OVA (Sigma, St Louis, MO) in 4 mg aluminum hydroxide at a 2-week interval. To examine the effect of IL-21 on antigen-specific immunoglobulin production, these mice were injected intraperitoneally with IL-21 (0.5 μg/mouse) or phosphate-buffered saline (PBS), as a control, twice a week for 4 weeks, with the first injection being performed 4 hours before the first immunization. The in vivo injection of IL-21 did not affect the general condition of the mice. Fourteen days after the second immunization, the titer of anti-OVA IgE antibody in mouse serum was assessed by a 24-hour passive cutaneous anaphylaxis (PCA) reaction as described previously.14 The amounts of anti-OVA IgG1 and IgG2a in sera were measured by enzyme-linked immunosorbent assay (ELISA) as described previously.14
Antigen-induced airway inflammation
BALB/c mice were immunized with OVA as described above. Fourteen days after the second immunization, the sensitized mice were challenged with inhaled OVA (50 mg/mL in 0.9% saline) for 20 minutes as described previously.15 As a control, 0.9% saline alone was administered. To examine the effect of IL-21 on antigen-induced eosinophil and lymphocyte recruitment into the airways, the mice were injected with IL-21 (0.5 μg/mouse) twice a week during the immunization period as well as 2 hours before the inhaled OVA challenge and 22 hours after the challenge. At 36 hours after the inhaled OVA challenge, bronchoalveolar lavage was performed with 3 mL PBS and differential cell counts were examined on cytospin cell preparations stained with Wright-Giemsa solution.
Antigen-induced cytokine production in splenocyte culture
Splenocytes (1 × 106/mL) from DO10+mice were stimulated with OVA323-339 peptide (50 μM) in the presence or absence of IL-21 (10 ng/mL) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 μM β-mercaptoethanol, 2 mM l-glutamine, and antibiotics (complete RPMI 1640 medium) in triplicate in a 96-well microtiter plate at 37°C for 72 hours. As a control, cells were cultured with nonstimulating OVA323-334 peptide (50 μM). The amount of IL-4, IL-5, and interferon γ (IFN-γ) in the culture supernatant was measured by the enzyme immunoassay using murine IL-4, IL-5, and IFN-γ ELISA kits (Pharmingen, San Diego, CA). The assays were performed in duplicate according to the manufacturer's instruction. The detection limits of these assays were 10 pg/mL IL-4 and IL-5, and 50 pg/mL IFN-γ.
Antigen-induced T-cell differentiation
Splenocytes from DO10+ mice were stimulated with OVA323-339 peptide (50 μM) in the presence of IL-21 (10 ng/mL) or sIL-21R (20 μg/mL) in a 24-well microtiter plate at 37°C for 48 hours. Where indicated, IL-12 (7.5 ng/mL) was added to polarize toward Th1 cells (Th1 condition) and IL-4 (7.5 ng/mL) was added to polarize toward Th2 cells (Th2 condition). Cells were washed with PBS and cultured for another 3 days in Th0 (nonpolarizing), Th1, or Th2 condition in the presence of IL-2 (5 ng/mL). Intracellular cytokine analyses for IL-4 versus IFN-γ were performed as described previously.16
B-cell purification from splenocytes
Splenocytes from BALB/c mice or from Stat6−/− mice or the littermate wild-type (WT) mice were incubated with a mixture of fluorescein isothiocyanate (FITC)–labeled antibodies to CD4 (RM4-5; Pharmingen), CD8 (53.6.7; Pharmingen), pan NK (DX5; Pharmingen), and CD11b (M1/70; Pharmingen) for 20 minutes at 4°C, washed 3 times with PBS, and incubated with anti-FITC magnetic microbeads (Miltenyi Biotec, Sunnyvale, CA) for 20 minutes at 4°C. After washing with PBS, cells were passed through the magnetic-activated cell sorting (MACS) separation CS column (Miltenyi Biotec) according to the manufacturer's instructions and cells in the flow-through were collected by centrifugation. These cells were more than 95% pure B220+cells by fluorescence-activated cell sorting (FACS) analysis.
IgE, IgG1, and IgG2a production from cultured splenic B cells
Splenic B cells (1 × 106/mL) from BALB/c mice were stimulated with lipopolysaccharide (LPS, 10 μg/mL; Sigma) in the presence of IL-4 (50 ng/mL) or IL-21 (0-20 ng/mL) or both in the complete RPMI 1640 medium in a 48-well microtiter plate at 37°C for 7 days. The amount of IgE, IgG1, and IgG2a in the supernatant was measured by ELISA according to the manufacturer's instruction (Pharmingen).
Membrane IgE+ B cells with cell division
Membrane IgE+ cells were detected with cell division as described previously.17,18 Briefly, purified splenic B cells from Stat6−/− mice or littermate WT mice were incubated with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE, 10 μM; Molecular Probes, Eugene, OR) in PBS at 37°C for 10 minutes and then washed with RPMI 1640 medium. CFSE-labeled B cells were cultured at 37°C for 6 days with LPS (10 μg/mL) in the presence of IL-4 (50 ng/mL) and indicated amounts of IL-21 (0-20 ng/mL). Where indicated, sIL-21R (20 μg/mL) was added to the culture. Cells were harvested and Fc receptors were blocked with anti-CD16/32 antibody (2.4G2; Pharmingen) prior to staining. Cells were incubated with biotin-conjugated anti-IgE antibody (R35-72; Pharmingen) at 4°C for 30 minutes, washed with PBS, and then visualized with streptavidin allophycocyanin (APC; Pharmingen) on FACS. In agreement with the previous report,19 acid treatment (pH 4.0) did not affect the percentage of IgE+ cells (data not shown), suggesting that the IgE+ cells were not due to the binding of produced IgE to Fc receptors. Analogously, membrane IgG1+ cells and membrane IgG2a+ cells were detected by FACS using anti-IgG1 antibody (A85-1; Pharmingen) and anti-IgG2a antibody (R19-15; Pharmingen), respectively.
RT-PCR for germ line Cε, Cγ1, and Cγ2a transcripts
Purified splenic B cells from BALB/c mice were stimulated with LPS (10 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both at 37°C for 16 hours. Total cellular RNA was prepared using Isogen solution (Nippon Gene, Tokyo, Japan) and reverse transcription–polymerase chain reaction (RT-PCR) for germ line Cε, Cγ1, and Cγ2a transcripts was performed as described elsewhere.20 21 RT-PCR for β-actin mRNA was also performed to control the sample-to-sample variation in RNA isolation and integrity, RNA input, and reverse transcription. All PCR amplifications were performed at least 3 times with multiple sets of experimental RNAs.
Immunoblotting
Whole cell extracts were prepared and immunoblottings were performed as described previously.16 The following antisera were used: antiphospho-Stat1 (Tyr701; New England Biolabs, Beverly, MA), antiphospho-Stat3 (Tyr705; New England Biolabs), antiphospho-Stat5 (Tyr694; New England Biolabs), antiphospho-Stat6 (Tyr641; New England Biolabs), antimouse Stat6 (M20; Santa Cruz Biotechnology, Santa Cruz, CA), and anti–Bcl-6 (N3; Santa Cruz Biotechnology).
Electrophoretic mobility shift assays
After splenic B cells were stimulated with LPS (10 μg/mL) or IL-21 (10 ng/mL) or both at 37°C for 30 minutes, nuclear extracts were prepared as described elsewhere.22 A nuclear factor-κB (NF-κB) consensus double-stranded oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Promega Biotech, Madison, WI) was labeled with [γ-32P] adenosine triphosphate (ATP). DNA-binding reaction was performed at room temperature for 20 minutes using 5 μg nuclear extracts according to the manufacturer's instruction (Promega Biotech). For DNA competition experiments, a 50-fold molar excess of unlabeled competitor oligonucleotide relative to the labeled probe was incubated in the binding mixture for 20 minutes before addition of the32P-labeled probe. The binding reaction mixtures were electrophoresed on 6% retardation gels (Invitrogen, Carlsbad, CA) and followed by autoradiography.
Luciferase assay
Stat6-dependent reporter plasmid, TPU474,23 was a kind gift from Dr U. Schindler (Tularik, CA). The germ line Cε promoter plasmid, ε-162Luc,24 which contains the segment extending from −163 to +53 relative to the first RNA initiation site of germ line Cε, was a kind gift from Dr J. Stavnezer (University of Massachusetts Medical School, Boston). Another germ line Cε promoter plasmid, ε-623Luc, which contains the segment extending from −623 to +53 of germ line Cε, was constructed as described previously.24 M12.4.5 cells (a kind gift from Dr T. Tokuhisa, Chiba University, Japan) were mixed with 20 μg of either TPU474, ε-162Luc, or ε-623Luc in the presence of 200 ng pRL-TK (Promega Biotech) and 5 μg Stat6-expression plasmid (pcDNA3 Stat6) in 800 μL serum-free RPMI 1640 medium and electroporated at 960 μF/300V. After cells were cultured at 37°C for 12 hours in the complete RPMI 1640 medium, aliquoted cells were left untreated or treated for another 12 hours with IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both. The luciferase activity was measured by dual luciferase assay system (Promega Biotech) according to the manufacturer's instruction. Firefly luciferase activity of TPU474, ε-162Luc, or ε-623Luc was normalized by Renilla luciferase activity of pRL-TK. All values were obtained from experiments carried out in triplicate and repeated at least 4 times.
Decay of germ line Cε transcripts
After splenic B cells were cultured with LPS (10 μg/mL) and IL-4 (20 ng/mL) at 37°C for 15 hours, a transcription inhibitor actinomycin D (10 μg/mL; Calbiochem, La Jolla, CA) was added to the cultures. Cells were then incubated with or without IL-21 (10 ng/mL) at 37°C for 0, 1, 3, and 5 hours. Total RNA was isolated from 5 × 106 cells and the amount of germ line Cε transcripts and β-actin was determined by RT-PCR as described above. Consistent with a previous report,25 no significant loss of cell viability was observed by the trypan blue exclusion method after the incubation.
Data analysis
Data are summarized as mean ± SD. The statistical analysis of the results was performed by the unpaired t test.P < .05 was considered significant.
Results
IL-21 inhibits antigen-specific IgE production and antigen-induced eosinophil recruitment into the airways
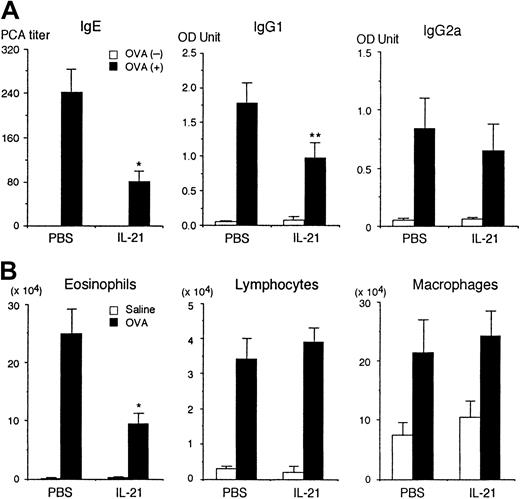
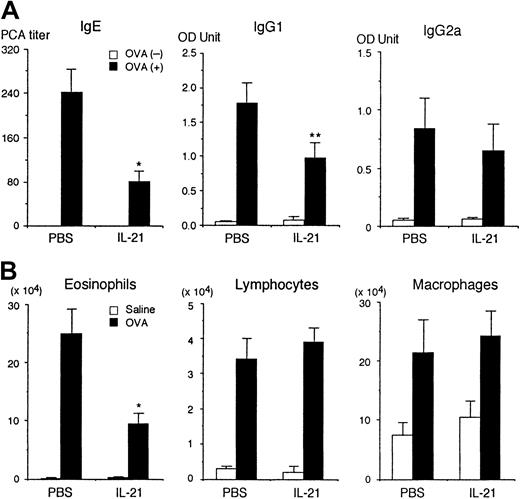
It has recently been shown that IL-21 induces the proliferation of T cells and B cells and differentiation of NK cells.9However, the physiologic role of IL-21 remains elusive. Therefore, we first determined the effect of IL-21 on antigen-specific immunoglobulin production on antigen immunization in mice. BALB/c mice were immunized intraperitoneally twice with OVA and given recombinant IL-21 (0.5 μg/mouse) intraperitoneally twice a week during the immunization. Two weeks after the second immunization, sera were collected and the amounts of anti-OVA IgE, IgG1, and IgG2a antibodies were evaluated. As shown in Figure 1A, IL-21 significantly decreased anti-OVA IgE production (n = 7 mice in each group,P < .005). IL-21 also decreased anti-OVA IgG1 production (P < .01). In contrast, the amount of antigen-specific IgG2a was not significantly affected by IL-21 (Figure 1A). The number of leukocytes and erythrocytes in peripheral blood as well as the number of B cells and T cells in spleen were not significantly affected by the injection of IL-21, either (data not shown).
IL-21 decreases antigen-specific IgE production in immunized mice.
(A) BALB/c mice were immunized intraperitoneally twice with (▪) or without (■) OVA at a 2-week interval. The mice were injected intraperitoneally with IL-21 (0.5 μg/mouse) twice a week for 4 weeks, with the first injection of IL-21 being done at 4 hours before the first immunization with OVA. As a control, the mice were injected intraperitoneally with PBS. Two weeks after the second immunization, the sera were collected and antigen-specific IgE, IgG1, and IgG2a were evaluated. Data are means ± SD for 7 mice in each group. Single and double asterisks indicate significantly different from the mean value of the corresponding response in the control mice,P < .005 and P < .01, respectively. (B) IL-21 decreases antigen-induced eosinophil recruitment into the airways. BALB/c mice were immunized with OVA and injected with IL-21 or PBS (as a control) as described above. Two weeks after the second immunization, mice were challenged with the inhaled OVA (▪) or saline (■). In IL-21–injected mice, IL-21 (0.5 μg/mouse) was also administered at 2 hours before and 22 hours after the inhaled OVA challenge. The number of eosinophils, lymphocytes, and macrophages in the bronchoalveolar lavage fluid was evaluated at 36 hours after the inhaled OVA challenge. Data are means ± SD for 5 mice in each group. Asterisk indicates significantly different from the mean value of the corresponding response in the control mice,P < .01.
IL-21 decreases antigen-specific IgE production in immunized mice.
(A) BALB/c mice were immunized intraperitoneally twice with (▪) or without (■) OVA at a 2-week interval. The mice were injected intraperitoneally with IL-21 (0.5 μg/mouse) twice a week for 4 weeks, with the first injection of IL-21 being done at 4 hours before the first immunization with OVA. As a control, the mice were injected intraperitoneally with PBS. Two weeks after the second immunization, the sera were collected and antigen-specific IgE, IgG1, and IgG2a were evaluated. Data are means ± SD for 7 mice in each group. Single and double asterisks indicate significantly different from the mean value of the corresponding response in the control mice,P < .005 and P < .01, respectively. (B) IL-21 decreases antigen-induced eosinophil recruitment into the airways. BALB/c mice were immunized with OVA and injected with IL-21 or PBS (as a control) as described above. Two weeks after the second immunization, mice were challenged with the inhaled OVA (▪) or saline (■). In IL-21–injected mice, IL-21 (0.5 μg/mouse) was also administered at 2 hours before and 22 hours after the inhaled OVA challenge. The number of eosinophils, lymphocytes, and macrophages in the bronchoalveolar lavage fluid was evaluated at 36 hours after the inhaled OVA challenge. Data are means ± SD for 5 mice in each group. Asterisk indicates significantly different from the mean value of the corresponding response in the control mice,P < .01.
We also examined the effect of IL-21 on antigen-induced eosinophil recruitment into the airways of sensitized mice. Interestingly, IL-21 significantly decreased antigen-induced eosinophil recruitment into the airways of sensitized mice (PBS 24.9 ± 4.2 versus IL-21 9.3 ± 2.0 × 104 eosinophils/mouse, n = 5 mice in each group, P < .01; Figure 1B). In contrast, IL-21 did not affect antigen-induced lymphocyte recruitment into the airways (Figure 1B).
IL-21 does not affect IL-4–induced Th2 cell differentiation
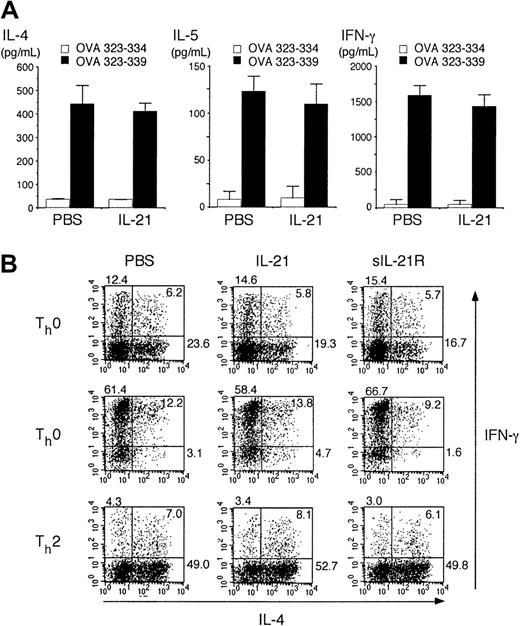
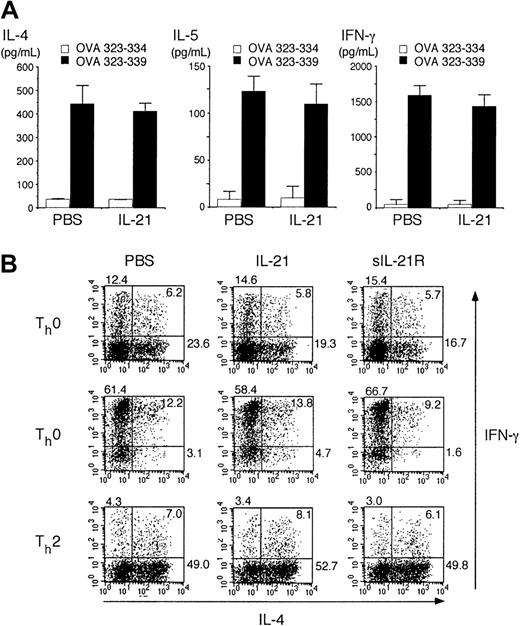
It is well known that antigen-specific IgE production from B cells depends on IL-4 from Th2 cells.4,5,26 27 We therefore attempted to determine whether IL-21 inhibits Th2 cell differentiation. Splenocytes from OVA-specific TCR transgenic mice (DO10+ mice) were stimulated with antigenic OVA peptide (OVA323-339) for 3 days in the presence or absence of IL-21 (10 ng/mL) and cytokine levels in the supernatants were measured by ELISA. The production of Th2 cytokines (IL-4 and IL-5) as well as Th1 cytokine (IFN-γ) was not significantly altered by IL-21 (Figure2A), although IL-21 receptor was expressed in both Th1 cells and Th2 cells at mRNA levels (data not shown).
IL-21 does not inhibit IL-4–induced Th2 cell differentiation.
(A) Splenocytes from DO10+ mice were stimulated with OVA323-339 peptide (50 μM; ▪) or OVA323-334 (50 μM; ■; as a control) for 72 hours in the presence or absence of IL-21 (10 ng/mL). The amounts of IL-4, IL-5, and IFN-γ in the supernatant were determined by ELISA. Data are mean ± SD from 5 independent experiments. (B) Splenocytes from DO10+ mice were stimulated with OVA323-339 peptide (50 μM) for 48 hours in the presence or absence of IL-21 (10 ng/mL) or sIL-21R (20 μg/mL). Where indicated, IL-12 (7.5 ng/mL) or IL-4 (7.5 ng/mL) was added to polarize toward Th1 cells (Th1 condition) or Th2 cells (Th2 condition), respectively. Cells were then cultured in the presence of IL-2 (5 ng/mL) for another 3 days. Intracellular staining for IL-4 and IFN-γ of CD4+ T cells was analyzed by fluorescence-activated cell-sorting (FACS). Shown are representative FACS profiles from 5 mice in each group.
IL-21 does not inhibit IL-4–induced Th2 cell differentiation.
(A) Splenocytes from DO10+ mice were stimulated with OVA323-339 peptide (50 μM; ▪) or OVA323-334 (50 μM; ■; as a control) for 72 hours in the presence or absence of IL-21 (10 ng/mL). The amounts of IL-4, IL-5, and IFN-γ in the supernatant were determined by ELISA. Data are mean ± SD from 5 independent experiments. (B) Splenocytes from DO10+ mice were stimulated with OVA323-339 peptide (50 μM) for 48 hours in the presence or absence of IL-21 (10 ng/mL) or sIL-21R (20 μg/mL). Where indicated, IL-12 (7.5 ng/mL) or IL-4 (7.5 ng/mL) was added to polarize toward Th1 cells (Th1 condition) or Th2 cells (Th2 condition), respectively. Cells were then cultured in the presence of IL-2 (5 ng/mL) for another 3 days. Intracellular staining for IL-4 and IFN-γ of CD4+ T cells was analyzed by fluorescence-activated cell-sorting (FACS). Shown are representative FACS profiles from 5 mice in each group.
We next examined the effect of IL-21 on the differentiation of Th2 cells at single-cell levels. Consistent with the data shown in Figure 2A, IL-21 did not significantly affect the T-helper cell differentiation in nonpolarizing (Th0) condition (Figure 2B). Moreover, IL-21 did not affect IL-4–induced Th2 cell differentiation or IL-12–induced Th1 cell differentiation (Figure 2B).
Given that activated CD4+ T cells produce IL-21,9 endogenously produced IL-21 may be sufficient for optimal T-helper cell differentiation. Thus, we next examined the effect of sIL-21R, which neutralizes IL-21,9 on T-helper cell differentiation. However, the addition of sIL-21R (20 μg/mL) did not affect either Th1 cell or Th2 cell differentiation (Figure 2B), indicating that endogenously produced IL-21 also plays no significant role in the regulation of T-helper cell differentiation.
IL-21 inhibits IL-4–induced IgE but not IgG1 production from splenic B cells
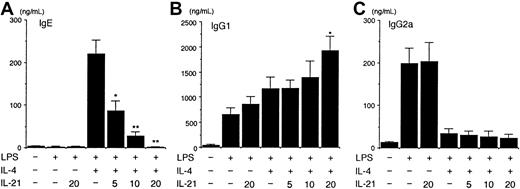
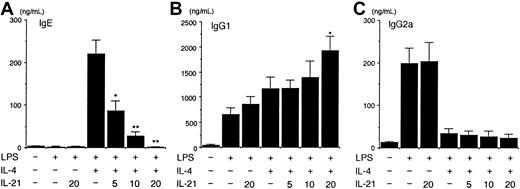
Because IL-21 is not crucial for regulating Th2 cell differentiation (Figure 2), we then examined the effect of IL-21 on IgE production from purified splenic B cells. When splenic B cells from BALB/c mice were stimulated with LPS (10 μg/mL) plus IL-4 (50 ng/mL) for 7 days, IgE was readily detected in the supernatant of the culture (Figure 3A). IL-4 alone was not sufficient for the induction of IgE production from splenic B cells (data not shown). Interestingly, IL-21 (5-20 ng/mL) decreased IgE production from LPS/IL-4–stimulated B cells in a dose-dependent fashion (n = 5 experiments, P < .01-.001; Figure 3A). In contrast, IL-21 (20 ng/mL) enhanced IgG1 production from LPS/IL-4–stimulated B cells (n = 5 experiments,P < .01; Figure 3B), although IL-21 inhibited antigen-specific IgG1 production in vivo (Figure 1A). On the other hand, IL-21 did not affect IgG2a production from LPS-stimulated B cells or did not antagonize the inhibitory effect of IL-4 on IgG2a production from LPS-stimulated B cells (Figure 3C).
IL-21 inhibits IL-4–induced IgE but not IgG1 production from splenic B cells.
Purified splenic B cells from BALB/c mice were stimulated with LPS (10 μg/mL) in the presence of IL-4 (50 ng/mL) or indicated amounts of IL-21 (0-20 ng/mL) or both for 7 days. IgE (A), IgG1 (B), and IgG2a (C) levels in the supernatant of the culture were determined by ELISA. Data are mean ± SD from 5 independent experiments. Single and double asterisks indicate significantly different from the mean value of the corresponding response in the absence of IL-21,P < .01 and P < .001, respectively.
IL-21 inhibits IL-4–induced IgE but not IgG1 production from splenic B cells.
Purified splenic B cells from BALB/c mice were stimulated with LPS (10 μg/mL) in the presence of IL-4 (50 ng/mL) or indicated amounts of IL-21 (0-20 ng/mL) or both for 7 days. IgE (A), IgG1 (B), and IgG2a (C) levels in the supernatant of the culture were determined by ELISA. Data are mean ± SD from 5 independent experiments. Single and double asterisks indicate significantly different from the mean value of the corresponding response in the absence of IL-21,P < .01 and P < .001, respectively.
IL-21 inhibits IL-4–induced IgE but not IgG1 production at single-cell levels
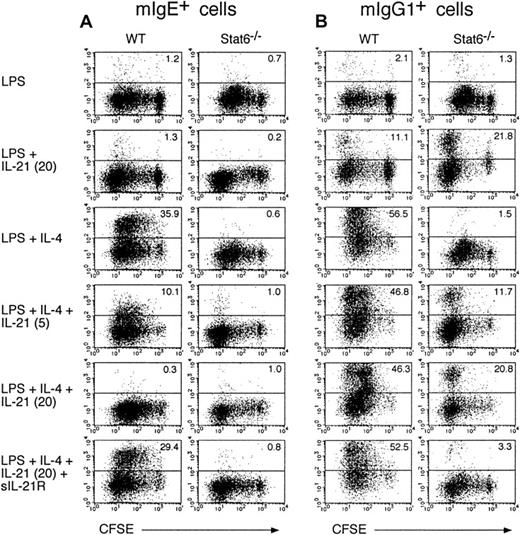
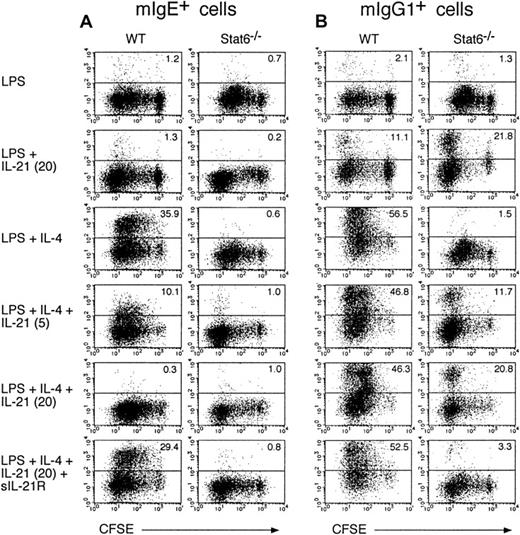
We next examined the effect of IL-21 on IgE production from B cells at single-cell levels. When splenic B cells from WT mice were cultured with LPS plus IL-4 for 6 days, membrane IgE-positive (mIgE+) B cells were significantly increased (Figure4A). In contrast, IL-4 did not increase mIgE+ B cells in Stat6−/− mice (Figure 4A), indicating that Stat6 plays a crucial role in the generation of mIgE+ B cells. Interestingly, IL-21 (5-20 ng/mL) dose dependently decreased mIgE+ cells in LPS/IL-4–stimulated B cells in WT mice (Figure 4A). The addition of sIL-21R, together with IL-21, reversed the inhibitory effect of IL-21 on the generation of mIgE+ cells in LPS/IL-4–stimulated B cells (Figure 4A). In addition, IL-21 negligibly inhibited the cell division of LPS/IL-4–stimulated B cells (Figure 4A), although a high concentration of IL-21 (80 ng/mL) significantly suppressed cell division of LPS/IL-4–stimulated B cells (data not shown). Therefore, these results indicate that IL-21 inhibits IL-4–induced, Stat6-dependent IgE production in B cells.
IL-21 inhibits IL-4–induced IgE production at single cell levels.
Purified splenic B cells from BALB/c mice or Stat6−/−mice were labeled with CFSE and then stimulated with LPS (10 μg/mL) in the presence of IL-4 (50 ng/mL) or IL-21 (0-20 ng/mL) or both for 6 days. Where indicated, sIL-21R (20 μg/mL) was added to the culture. mIgE+ cells (A) and mIgG1+ cells (B) with the intensity of CFSE were detected by anti-IgE antibody and anti-IgG1 antibody, respectively. Shown are representative FACS profiles from 5 independent experiments and the numbers in the top right-hand corner indicate the percentage of mIgE+ cells and mIgG1+ cells.
IL-21 inhibits IL-4–induced IgE production at single cell levels.
Purified splenic B cells from BALB/c mice or Stat6−/−mice were labeled with CFSE and then stimulated with LPS (10 μg/mL) in the presence of IL-4 (50 ng/mL) or IL-21 (0-20 ng/mL) or both for 6 days. Where indicated, sIL-21R (20 μg/mL) was added to the culture. mIgE+ cells (A) and mIgG1+ cells (B) with the intensity of CFSE were detected by anti-IgE antibody and anti-IgG1 antibody, respectively. Shown are representative FACS profiles from 5 independent experiments and the numbers in the top right-hand corner indicate the percentage of mIgE+ cells and mIgG1+ cells.
We also examined the effect of IL-21 on the generation of membrane IgG1 positive (mIgG1+) and membrane IgG2a positive (mIgG2a+) B cells. IL-4 significantly increased mIgG1+ B cells in WT mice but not in Stat6−/−mice (Figure 4B). Interestingly, in contrast to the inhibitory effect of IL-21 on the generation of mIgE+ B cells by IL-4 (Figure4A), IL-21 did not inhibit the generation of mIgG1+ B cells by IL-4 in WT mice (Figure 4B). Rather, IL-21 increased mIgG1+ cells in LPS-stimulated B cells in WT mice and more strongly in Stat6−/− mice (n = 5, P < .01 and P < .001, respectively; Figure 4B). As anticipated, the effect of IL-21 on the generation of mIgG1+ B cells was canceled when sIL-21R was added to the culture (Figure 4B and data not shown). These results indicate that, although the effect is not so strong as IL-4, IL-21 increases IgG1 production from B cells and this effect is Stat6 independent. On the other hand, IL-21 did not affect the number of mIgG2a+ cells in LPS-stimulated or LPS/IL-4–stimulated B cells from WT mice and Stat6−/−mice (data not shown).
IL-21 inhibits IL-4–induced germ line Cε transcription in B cells
Switch recombination to a particular isotype is preceded by transcription of the corresponding unrearranged germ line transcripts5,6 and IL-4 induces transcripts from the unrearranged Cε genes and directs switching to IgE in LPS-stimulated B cells.26,27 Thus, we next examined whether IL-21 inhibits IL-4–induced germ line Cε transcription. In agreement with a previous report,20 germ line Cε transcripts were detected when splenic B cells were stimulated with LPS plus IL-4 for 16 hours (Figure 5A). Interestingly, IL-21 (10 ng/mL) almost completely suppressed the IL-4–induced germ line Cε transcription in LPS-stimulated B cells (Figure 5A), whereas IL-21 did not significantly affect IL-4–induced germ line Cγ1 transcription in LPS-stimulated B cells or germ line Cγ2a transcription in LPS-stimulated B cells (Figure 5A). In addition, IL-21 did not significantly affect the number of B cells recovered in 16 hours of culture (data not shown). Taken together, these results indicate that IL-21 inhibits IgE production mainly through the inhibitory effect on germ line Cε transcription in B cells. However, because IL-21 slightly (but not statistically significantly) inhibited LPS/IL-4–mediated B-cell proliferation in 48 hours of culture (Figure5B), it is possible that a small antiproliferative effect on B cells may also contribute to the inhibition of IgE production by IL-21.
IL-21 inhibits IL-4–induced germ line Cε transcription in B cells.
(A) Purified splenic B cells were stimulated with LPS (10 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 16 hours. Total cellular RNA was prepared from these cells and RT-PCR for germ line Cε, Cγ1, and Cγ2a transcripts as well as β-actin (as a control) was performed. Shown are representative data from 5 independent experiments. (B) Purified splenic B cells were stimulated with LPS (1 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 48 hours with [3H]-thymidine added for the final 12 hours. Data are mean ± SD from 3 independent experiments.
IL-21 inhibits IL-4–induced germ line Cε transcription in B cells.
(A) Purified splenic B cells were stimulated with LPS (10 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 16 hours. Total cellular RNA was prepared from these cells and RT-PCR for germ line Cε, Cγ1, and Cγ2a transcripts as well as β-actin (as a control) was performed. Shown are representative data from 5 independent experiments. (B) Purified splenic B cells were stimulated with LPS (1 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 48 hours with [3H]-thymidine added for the final 12 hours. Data are mean ± SD from 3 independent experiments.
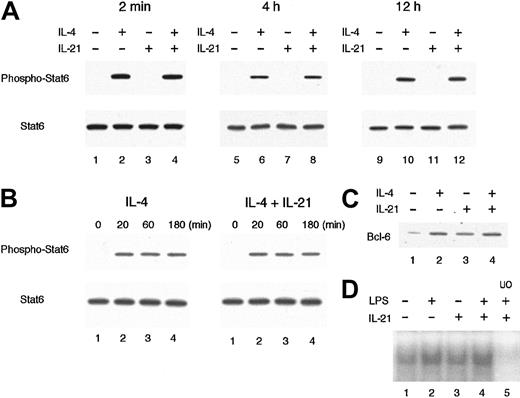
IL-21 does not inhibit IL-4–induced Stat6 phosphorylation or LPS-induced nuclear accumulation of NF-κB
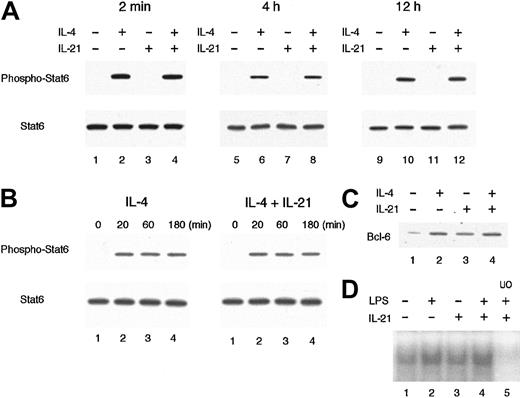
We then examined the effect of IL-21 on IL-4–mediated Stat6 activation, which is known to be essential for germ line Cε transcription and IgE production.12,20,28 29 When splenic B cells were stimulated with IL-4 (20 ng/mL) for 20 minutes, the phosphorylated form of Stat6 was readily detected by antiphospho Stat6 antibody (Figure 6A). IL-21 (10 ng/mL) did not induce the phosphorylation of Stat6 at all (Figure 6A). Moreover, preincubation of B cells with IL-21 for 2 minutes before IL-4 stimulation did not inhibit IL-4–induced phosphorylation of Stat6 (Figure 6A). These results suggest that IL-4–mediated signaling is normally transduced to the activation of Stat6 even in the presence of IL-21.
IL-21 does not inhibit IL-4–induced Stat6 phosphorylation or LPS-induced nuclear accumulation of NF-κB.
(A) Purified splenic B cells from BALB/c mice were incubated with IL-21 (10 ng/mL) for 2 minutes, 4 hours, or 12 hours before IL-4 stimulation and then stimulated with IL-4 (20 ng/mL) for 20 minutes. Whole cell lysates were prepared from the cells and subjected to Western blotting with antiphospho Stat6 antibody (top panels) or anti-Stat6 antibody (bottom panels). Shown are representative blottings from 4 independent experiments. (B) Purified splenic B cells were stimulated with IL-4 (20 ng/mL) in the presence or absence of IL-21 (10 ng/mL). At indicated times after IL-4 stimulation, whole cell lysates were prepared and subjected to Western blotting with antiphospho Stat6 antibody (top panels) or anti-Stat6 antibody (bottom panels). Shown are representative blottings from 3 independent experiments. (C) Purified splenic B cells were stimulated with IL-21 (10 ng/mL) or IL-4 (20 ng/mL) or both for 16 hours. Whole cell lysates were subjected to Western blotting with anti–Bcl-6 antibody. (D) Purified splenic B cells were stimulated with LPS (10 μg/mL) in the presence or absence of IL-21 (10 ng/mL) for 30 minutes. Nuclear lysates were prepared from these cells and the binding to a NF-κB consensus oligonucleotide was determined by electrophoretic mobility shift assays. In lane 5, a 50-fold molar excess of unlabeled competitor oligonucleotide (UO) relative to the labeled probe was added to the lysates.
IL-21 does not inhibit IL-4–induced Stat6 phosphorylation or LPS-induced nuclear accumulation of NF-κB.
(A) Purified splenic B cells from BALB/c mice were incubated with IL-21 (10 ng/mL) for 2 minutes, 4 hours, or 12 hours before IL-4 stimulation and then stimulated with IL-4 (20 ng/mL) for 20 minutes. Whole cell lysates were prepared from the cells and subjected to Western blotting with antiphospho Stat6 antibody (top panels) or anti-Stat6 antibody (bottom panels). Shown are representative blottings from 4 independent experiments. (B) Purified splenic B cells were stimulated with IL-4 (20 ng/mL) in the presence or absence of IL-21 (10 ng/mL). At indicated times after IL-4 stimulation, whole cell lysates were prepared and subjected to Western blotting with antiphospho Stat6 antibody (top panels) or anti-Stat6 antibody (bottom panels). Shown are representative blottings from 3 independent experiments. (C) Purified splenic B cells were stimulated with IL-21 (10 ng/mL) or IL-4 (20 ng/mL) or both for 16 hours. Whole cell lysates were subjected to Western blotting with anti–Bcl-6 antibody. (D) Purified splenic B cells were stimulated with LPS (10 μg/mL) in the presence or absence of IL-21 (10 ng/mL) for 30 minutes. Nuclear lysates were prepared from these cells and the binding to a NF-κB consensus oligonucleotide was determined by electrophoretic mobility shift assays. In lane 5, a 50-fold molar excess of unlabeled competitor oligonucleotide (UO) relative to the labeled probe was added to the lysates.
The ligation of IL-21 receptor has been shown to result in the activation of Stat1, Stat3, and Stat5.9-11 Because Stat proteins regulate the expression of a variety of genes, including negative regulators of cytokine signaling such as SOCS/CIS/SSI family proteins,30 it was possible that IL-21 induced these genes and thus inhibited the subsequent IL-4–mediated Stat6 activation. Therefore, we examined IL-4–induced Stat6 phosphorylation in splenic B cells that were pretreated with IL-21 for either 4 hours or 12 hours. However, the pretreatment with IL-21 did not affect the subsequent activation of Stat6 in IL-4–stimulated B cells (Figure 6A). In addition, the duration of IL-4–induced phosphorylation of Stat6 was not affected by the presence of IL-21 (Figure 6B). Consistent with these results, the expression levels of IL-4 receptor α chain as well as γc on cultured splenic B cells were not significantly affected by IL-21 (data not shown).
It has been shown that IL-4–induced IgE production is down-regulated at the transcriptional level by Bcl-6,31 a site-specific transcriptional repressor belonging to the POZ/zinc-finger family.32 An in vitro–defined binding site for Bcl-6 demonstrates a marked similarity to the consensus element recognized by Stat633 and, in fact, Bcl-6 binds to Stat6 site of germ line Cε promoter and down-regulates the IL-4–induced transcription.31 To address the possibility that IL-21 might inhibit the IL-4–induced germ line Cε transcription through the induction of Bcl-6, we examined the expression of Bcl-6 in IL-21–stimulated B cells at protein levels. As shown in Figure 6C, Bcl-6 was expressed in unstimulated splenic B cells at low levels. IL-21 modestly increased Bcl-6 expression in B cells (Figure 6C). However, the levels of Bcl-6 were similar in IL-4–stimulated B cells in the absence and presence of IL-21 (Figure 6C). Thus, it is suggested that the induction of Bcl-6 cannot account for IL-21–mediated inhibition of germ line Cε transcription in B cells, although it is still possible that IL-21 may regulate the function of Bcl-6 protein by posttranslational modification.
We also examined the possibility that IL-21 inhibits LPS-mediated NF-κB activation, which is known to be required for the induction of germ line Cε transcription in LPS/IL-4–stimulated B cells.26,27 As reported previously,34 35nuclear accumulation of NF-κB was observed in unstimulated splenic B cells (Figure 6D) and LPS further increased the levels of nuclear accumulation of NF-κB (Figure 6D). IL-21 itself did not increase nuclear accumulation of NF-κB in splenic B cells (Figure 6D). In addition, IL-21 did not suppress LPS-mediated nuclear accumulation of NF-κB (Figure 6D), suggesting that NF-κB is not a target of the IL-21–mediated inhibition of germ line Cε transcription.
IL-21 does not inhibit Stat6-mediated transcription from core germ line Cε promoter
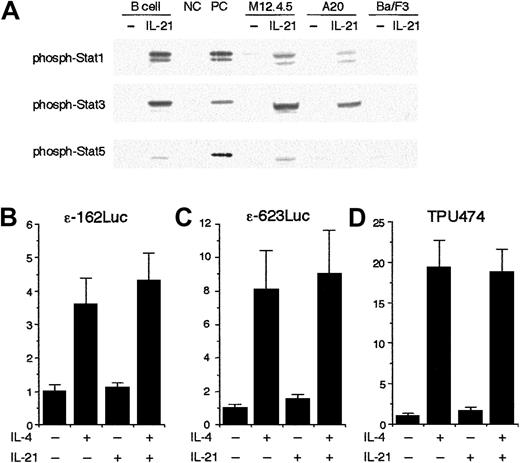
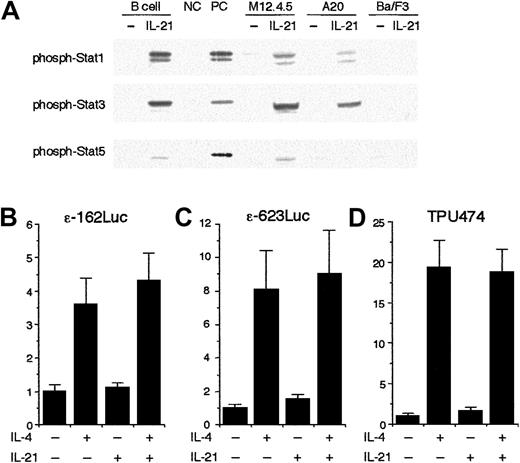
We next examined the effect of IL-21 on the transcription from germ line Cε promoter using a reporter assay. To perform the assay, we first tried to identify a murine cell line that exhibited IL-21 responsiveness similar to splenic B cells. As shown in Figure7A, when splenic B cells were stimulated with IL-21 (10 ng/mL) for 20 minutes, strong phosphorylation of Stat1 and Stat3 and weak phosphorylation of Stat5 were observed (Figure 7A). A similar phosphorylation pattern of Stat proteins was observed when M12.4.5 cells and A20 cells were stimulated with IL-21 (Figure 7A). In contrast, IL-21 did not induce phosphorylation of Stat proteins in Ba/F3 cells, suggesting that Ba/F3 cells may lack the expression of IL-21 receptors (Figure 7A).
IL-21 does not inhibit Stat6-mediated transcription from germ line Cε promoter construct.
(A) IL-21 induces phosphorylation of Stat1, Stat3, and Stat5 in B cells. Purified splenic B cells, M12.4.5 cells, and A20 cells were stimulated with IL-21 (10 ng/mL) for 20 minutes. Ba/F3 cells were starved from IL-3 for 2 hours and then stimulated with IL-21 (10 ng/mL) for 20 minutes. Whole cell lysates were prepared and subjected to Western blotting with antiphospho Stat1 antibody, antiphospho Stat3 antibody, or antiphospho Stat5 antibody. Positive controls (PC) were lysates of IFN-γ–stimulated splenocytes, IL-6–stimulated splenocytes, and IL-7–stimulated thymocytes for antiphospho Stat1 blotting, antiphospho Stat3 blotting, and antiphospho Stat5 blotting, respectively. Negative controls (NC) were unstimulated splenocytes and unstimulated thymocytes for Stat1 and Stat3 and for Stat5, respectively. Shown are representative blottings from 3 independent experiments. (B-D) IL-21 does not inhibit the transcription from Stat6-dependent reporter plasmids. M12.4.5 cells were transfected with either ε-162Luc (B), ε-623Luc (C), or TPU474 (D) in the presence of murine Stat6 expression plasmid (pcDNA3 Stat6) and pRL-TK. Twelve hours after transfection, cells were stimulated with IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for another 12 hours and the luciferase activity was measured by the dual luciferase reporter system. Data are means ± SD from 4 experiments.
IL-21 does not inhibit Stat6-mediated transcription from germ line Cε promoter construct.
(A) IL-21 induces phosphorylation of Stat1, Stat3, and Stat5 in B cells. Purified splenic B cells, M12.4.5 cells, and A20 cells were stimulated with IL-21 (10 ng/mL) for 20 minutes. Ba/F3 cells were starved from IL-3 for 2 hours and then stimulated with IL-21 (10 ng/mL) for 20 minutes. Whole cell lysates were prepared and subjected to Western blotting with antiphospho Stat1 antibody, antiphospho Stat3 antibody, or antiphospho Stat5 antibody. Positive controls (PC) were lysates of IFN-γ–stimulated splenocytes, IL-6–stimulated splenocytes, and IL-7–stimulated thymocytes for antiphospho Stat1 blotting, antiphospho Stat3 blotting, and antiphospho Stat5 blotting, respectively. Negative controls (NC) were unstimulated splenocytes and unstimulated thymocytes for Stat1 and Stat3 and for Stat5, respectively. Shown are representative blottings from 3 independent experiments. (B-D) IL-21 does not inhibit the transcription from Stat6-dependent reporter plasmids. M12.4.5 cells were transfected with either ε-162Luc (B), ε-623Luc (C), or TPU474 (D) in the presence of murine Stat6 expression plasmid (pcDNA3 Stat6) and pRL-TK. Twelve hours after transfection, cells were stimulated with IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for another 12 hours and the luciferase activity was measured by the dual luciferase reporter system. Data are means ± SD from 4 experiments.
To examine the effect of IL-21 on the transcription from the core germ line Cε promoter, ε-162Luc reporter plasmid,24 which contains most of the regulatory elements known to be important for germ line Cε transcription,24,26,27 was transiently transfected to M12.4.5 cells. The luciferase activity obtained on transient transfection of ε-162Luc in M12.4.5 cells was increased an average of 4-fold in cells cultured for 12 hours in the presence of IL-4 (20 ng/mL; Figure 7B). IL-21 (10 ng/mL) itself did not significantly induce the promoter activity of ε-162Luc (Figure 7B). Surprisingly, IL-21 did not inhibit IL-4–induced transcription from ε-162Luc (Figure 7B). Similar results were obtained when another germ line Cε reporter construct that contained an additional 460-bp fragment of murine germ line Cε promoter (ε-623Luc) was used as a reporter plasmid (Figure 7C). IL-21 did not inhibit IL-4–induced transcription from the other Stat6-dependent reporter construct, TPU474, either23 (Figure 7D). Therefore, these results indicate that IL-21 does not inhibit the core promoter activity of germ line Cε.
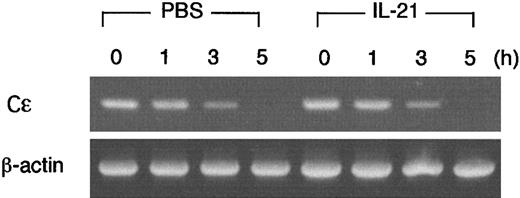
IL-21 does not accelerate the decay of IL-4–induced germ line Cε transcripts
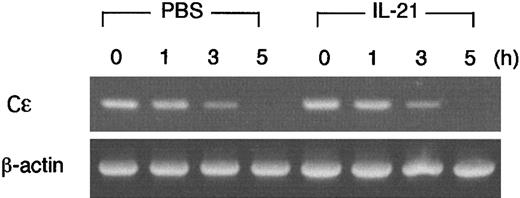
We found that IL-21 decreased IL-4–induced germ line Cε transcripts in B cells (Figure 5), but IL-21 did not inhibit the transcription from the core promoter of germ line Cε (Figure 7). These data raised the possibility that IL-21 decreased the stability of germ line Cε transcripts. To address this possibility, we examined the effect of IL-21 on the decay of germ line Cε transcripts in splenic B cells. After splenic B cells were stimulated with LPS plus IL-4 for 15 hours, de novo transcription was inhibited by the addition of actinomycin D (10 μg/mL). Cells were then incubated with or without IL-21 (10 ng/mL) for 1, 3, or 5 hours and remaining germ line Cε transcripts were measured by RT-PCR (Figure8). Contrary to our expectation, IL-21 did not accelerate the decay of germ line Cε transcripts, indicating that the inhibitory effect of IL-21 on germ line Cε transcription is not due to an accelerated decay of germ line Cε transcripts.
IL-21 does not accelerate the decay of IL-4–induced germ line Cε transcripts in B cells.
Purified splenic B cells were stimulated with LPS (10 μg/mL) and IL-4 (20 ng/mL) for 15 hours and then incubated with or without IL-21 (10 ng/mL) in the presence of actinomycin D (10 μg/mL) for 1, 3, or 5 hours. Cells were harvested and RT-PCR analysis for germ line Cε transcripts and β-actin (as a control) was performed. Shown are representative data from 3 independent experiments.
IL-21 does not accelerate the decay of IL-4–induced germ line Cε transcripts in B cells.
Purified splenic B cells were stimulated with LPS (10 μg/mL) and IL-4 (20 ng/mL) for 15 hours and then incubated with or without IL-21 (10 ng/mL) in the presence of actinomycin D (10 μg/mL) for 1, 3, or 5 hours. Cells were harvested and RT-PCR analysis for germ line Cε transcripts and β-actin (as a control) was performed. Shown are representative data from 3 independent experiments.
Discussion
In this study, we show a novel function of IL-21 in the regulation of IgE production. The in vivo injection of IL-21 prevented antigen-specific IgE production on immunization (Figure 1). IL-21 did not inhibit IL-4 production or Th2 cell differentiation from CD4+ T cells (Figure 2) but directly inhibited IL-4–induced IgE production from B cells at single-cell levels (Figures 3 and 4). Moreover, IL-21 inhibited IL-4–induced germ line Cε transcription in B cells (Figure 5). Therefore, these results indicate that IL-21 down-regulates IgE production from IL-4–stimulated B cells by the inhibition of germ line Cε transcription. Given that IL-21 is a product of activated CD4+ T cells9including Th1 cells (A.S. et al, unpublished observation, March 2002), these results suggest that IL-21 may be involved in Th1 cell–mediated down-regulation of IgE production in immune responses.
We show that IL-21 inhibits IL-4–induced germ line Cε transcription in B cells. Among downstream molecules under IL-4 signaling, Stat6 acts as a direct connection between the cytokine receptor and the nucleus, where dimerized Stat6 binds the Stat6 site in the germ line Cε promoter.20,24 Recent studies with Stat6−/−mice have shown that the Stat6 pathway is the principal signaling pathway for IgE isotype switching in B cells.12,20,28,29In addition to germ line Cε transcription, we also found that IL-21 inhibited IL-4–induced expression of CD23 on B cells (data not shown), whose expression is also known to be Stat6 dependent.12 28These findings raised the possibility that IL-21 inhibited IL-4–induced Stat6 activation. However, we found that IL-21 did not inhibit Stat6 activation, even when B cells were pretreated with IL-21 (Figure 6). These findings suggest that neither the competition of γc between IL-4 and IL-21 at the receptor levels on B cells nor the induction of negative regulators that inhibit IL-4 signaling at the upstream of Stat6 activation is responsible for the inhibitory effect of IL-21 on IL-4–induced germ line Cε transcription.
In addition to Stat6, several other molecules have been demonstrated to be involved in the regulation of germ line Cε transcription.26,27 For instance, LPS as well as CD40L induce nuclear accumulation of NF-κB and enhance germ line Cε transcription through 2 NF-κB sites located downstream of the Stat6 site in the promoter.24,36 37 However, we found that IL-21 did not inhibit either steady-state or LPS-induced nuclear accumulation of NF-κB in splenic B cells (Figure 6), suggesting that NF-κB activation is not a target of IL-21–mediated inhibition of germ line Cε transcription.
Despite the inhibitory effect of IL-21 on germ line Cε transcription in LPS/IL-4–stimulated B cells (Figure 5), IL-21 did not inhibit IL-4–induced transcription from the core germ line Cε promoter (ε-162Luc or ε-623Luc; Figure 7) that contained most of the regulatory elements known to be important in germ line Cε regulation, including Stat6 site and NF-κB sites.24,26 27 In addition, we found that IL-21 did not accelerate the decay of germ line Cε transcripts in LPS/IL-4–stimulated B cells (Figure 8). These results suggest that IL-21 decreases germ line Cε transcripts by modulating the transcriptional activity through an undefined element that located outside the core promoter region of germ line Cε gene or by regulating chromatin remodeling around the germ line Cε gene. Further studies are required to address the molecular basis for the inhibitory effect of IL-21 on germ line Cε transcription.
It is well documented that IL-4 directs isotype switching in mouse B cells not only to IgE but also to IgG1.5,6,26,27 However, it has been shown that switching to IgG1 is at most partially reduced in Stat6−/− mice, whereas switching to IgE is undetectable in Stat6−/− mice.12,28,29,38 It has also been shown that the IL-4–responsive element of the germ line Cγ1 promoter is less inducible by IL-4 than that of the Cε promoter, although each promoter contains a binding site for Stat6.39 Therefore, it is indicated that these isotypes differ in their dependence on Stat6 signaling. In this regard, we found that whereas IL-21 inhibited IgE production from LPS/IL-4–stimulated B cells (Figures 3A and 4A), IL-21 rather increased IgG1 production from LPS-stimulated B cells (Figure 4B). Surprisingly, IL-21–mediated increase of IgG1 production was significantly enhanced in Stat6−/− mice (Figure 4B). Taken together, these results indicate that there is an IL-21–dependent but Stat6-independent pathway for IgG1 production, suggesting that this pathway may be involved in the remaining IgG1 production in Stat6−/−mice.
Although IL-21 increased IgG1 production from LPS-stimulated B cells (Figure 4B), IL-21 did not significantly enhance germ line Cγ1 transcription in LPS-stimulated B cells (Figure 5A). These results suggest that IL-21 increases IgG1 production by the mechanism that is independent from the induction of germ line Cγ1 transcription. Regarding the point, Yoshida et al40 have shown that switch recombination to IgE occurs at least in part in a successive manner: first from IgM to IgG1, and then from IgG1 to IgE. Therefore, it is possible that IL-21 increases IgG1-producing cells through the inhibition of switching from IgG1 to IgE.
In contrast to the in vitro effect of IL-21 on IgG1 production from splenic B cells (Figures 3B and 4B), we found that IL-21 rather inhibited antigen-specific IgG1 production in vivo (Figure 1A). These results suggest that the inhibition of in vivo IgG1 production by IL-21 is mediated by an indirect mechanism. It has recently been shown that IL-21 enhances IFN-γ production by activated NK cells and CD8+ T cells.41 Therefore, it is plausible that the in vivo administration of IL-21 increases IFN-γ production from NK cells or CD8+ T cells and the produced IFN-γ inhibits IgG1 production from B cells. It is also possible that the produced IFN-γ regulates the Th1/Th2 balance toward Th1 type and then inhibits IgG1 production. These possibilities are under investigation in our laboratory.
Interestingly, whereas IL-21 strongly inhibited LPS/IL-4–induced IgE production in vitro (Figures 3 and 4), the effect of IL-21 on antigen-specific IgE production in vivo was modest (Figure 1). This difference may be simply due to a short half-life of recombinant IL-21 in vivo. However, we found that when unfractionated splenocytes instead of purified splenic B cells were used as a source of B cells, much higher concentrations of IL-21 were required to achieve comparable inhibitions of LPS/IL-4–induced IgE production (data not shown). Therefore, non-B cells may provide a signal that antagonizes IL-21–induced inhibition of IgE production to B cells. Because it has been demonstrated that IL-21 enhances the proliferation of B cells that are stimulated with anti-CD40 antibody but not the proliferation of B cells that are stimulated with IL-4 plus anti-IgM,9CD40/CD40L interaction may provide a signal to antagonize the IL-21–mediated inhibition of IgE production.
In summary, we have shown that IL-21 prevents antigen-specific IgE production and antigen-induced eosinophil recruitment into the airways of sensitized mice. Because both IgE production and eosinophil recruitment into the tissue are believed to be involved in the pathogenesis of allergic diseases, it is suggested that IL-21 itself or the mimic of IL-21 signaling may be useful for the treatment of allergic diseases, such as allergic asthma and allergic rhinitis.
We thank Dr K. M. Murphy for DO10+ mice, Drs S. Akira and K. Takeda for Stat6-deficient mice, Dr U. Schindler for TPU474, Dr J. Stavnezer for ε-162Luc, Dr T. Tokuhisa for M12.4.5 cells and valuable discussion, and Dr A. Nelson for critical comments.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-04-1115.
Supported in part by grants from Mitsubishi Pharma Research Foundation, the Ministry of Education, Science and Culture, Japan; and Health Science Research Grants, Japan.
A.S. and H.N. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Nakajima, Department of Internal Medicine II, Chiba University School of Medicine, 1-8-1 Inohana, Chiba City, Chiba 260-8670, Japan; e-mail:nakajimh@intmed02.m.chiba-u.ac.jp.

![Fig. 5. IL-21 inhibits IL-4–induced germ line Cε transcription in B cells. / (A) Purified splenic B cells were stimulated with LPS (10 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 16 hours. Total cellular RNA was prepared from these cells and RT-PCR for germ line Cε, Cγ1, and Cγ2a transcripts as well as β-actin (as a control) was performed. Shown are representative data from 5 independent experiments. (B) Purified splenic B cells were stimulated with LPS (1 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 48 hours with [3H]-thymidine added for the final 12 hours. Data are mean ± SD from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/13/10.1182_blood-2002-04-1115/4/m_h82423553005.jpeg?Expires=1769291151&Signature=Hyn5j-8HzznT-OAXnnsbQbPboRO4SobG218onrKJT4olB2rBkjAJ20IWIBQE712~hw4Vt-y-1p9hCVqRNh0Hi8JcT1ckIeaiObreVM315cg5cNIt-z2C5XqjgbWgvfaojF1VBbMCdxL7ikFzqmm5MDZXIHlIidlfQvdDzwmY8V63TniaNSwnQLomDvrFuEsSy3G8-jxRwdCVs21feUcjjZsghD-MXPwk7zwbw-JkMW0QEYUU0gHqODZCe4GzWMdY9R3JMmGKoWT5bQngi~va5zSOsu8UCPKFqFUWiUXWGxSoiBvaK7L~3XqdPAVodg9YDmKo6yFUKJy7kmbkIhfDfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. IL-21 inhibits IL-4–induced germ line Cε transcription in B cells. / (A) Purified splenic B cells were stimulated with LPS (10 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 16 hours. Total cellular RNA was prepared from these cells and RT-PCR for germ line Cε, Cγ1, and Cγ2a transcripts as well as β-actin (as a control) was performed. Shown are representative data from 5 independent experiments. (B) Purified splenic B cells were stimulated with LPS (1 μg/mL) in the presence of IL-4 (20 ng/mL) or IL-21 (10 ng/mL) or both for 48 hours with [3H]-thymidine added for the final 12 hours. Data are mean ± SD from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/13/10.1182_blood-2002-04-1115/4/m_h82423553005.jpeg?Expires=1769337243&Signature=sMUHq6h8ghyjgFLtt2eil8KkEO5d3tg~rOGUNy-2Mq24ET-h94H7KOZNc~OHnmUSlJLII8NPo~kRd7ANVQt2j6WWt1uu~B2yUmAcLyDXP2LsbajL0PiCbKKq1F5~PRB40ASef9MdaNNziTRbQScls3ieKEkAloWDQujj~U8B99aT6FhelTCTmRIiLkKNklvT6SSdBi-nHaStUic5gLA-BEU5ZH4SULpsYeL6gmgCxo4IUw8vYpTnClfGzEcp6tHfnlW5cCsqx85wNrHqv-g328RzRwYgmOr4i2M78KpHhMdZyNoYVGUlG-GE37UqdrC8mRuK5VVdQimyuXWnu-kn4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)