Vector-containing medium harvested from murine packaging cell lines has been shown to contain factors that can negatively influence the transduction and maintenance of hematopoietic stem cells. Thus, we generated a human packaging cell line with a gibbon ape leukemia virus pseudotype (Phoenix-GALV), and we evaluated vectors produced by Phoenix-GALV for their ability to transduce hematopoietic progenitor/stem cells. In 3 baboons, we used a competitive repopulation assay to directly compare GALV-pseudotype retrovirus vectors produced by either Phoenix-GALV or by the NIH 3T3–derived packaging cell line, PG13. In 3 additional baboons we compared Phoenix-GALV–derived vectors to more recently developed lentiviral vectors. Gene transfer efficiency into hematopoietic repopulating cells was assessed by evaluating the number of genetically modified peripheral blood and marrow cells using flow cytometry and real-time polymerase chain reaction. Transduction efficiency of hematopoietic repopulating cells was significantly higher using the Phoenix-GALV–derived vector as compared with the PG13-derived vectors or lentiviral vectors, with stable transduction levels up to 25%. We followed 2 animals for more than one year. Flow cytometric analysis of hematopoietic subpopulations in these animals revealed transgene expression in CD13+ granulocytes, CD20+ B lymphocytes, CD3+ T lymphocytes, CD61+ platelets, as well as red blood cells, indicating multilineage engraftment of cells transduced by Phoenix-GALV–pseudotype vectors. In addition, transduction of human CD34+ cells was significantly more efficient than transduction of baboon CD34+ cells, suggesting that Phoenix-GALV–derived oncoretroviral vectors may be even more efficient in human stem cell gene therapy applications.
Introduction
The potential of hematopoietic stem cell gene therapy has recently been demonstrated by the successful treatment of patients with severe combined immunodeficiency syndrome.1In this study, genetic correction of lymphocytes provided a survival advantage over uncorrected cells, and transduced cells were selected in vivo over time. However, in the majority of diseases that may be treatable by a stem cell–directed gene therapy approach, such a selective advantage for genetically corrected cells is not expected. Thus, although gene transfer efficiencies have improved over the past few years, for most clinical applications gene transfer levels achieved with current protocols are still below therapeutically relevant levels. A number of different factors may contribute to the low efficiency of gene transfer into hematopoietic stem cells (HSCs) using retroviral vectors including their predominantly quiescent cell cycle state,2 low viral receptor density,3 and the requirement for relatively long ex vivo culture.4
So far, most studies in humans and large animals have been performed using oncoretroviral vectors produced by mouse-derived packaging cells. Conditioned medium harvested from murine packaging cell lines has been shown to negatively affect the transduction efficiency of the target cells. Xu et al showed that cytokines secreted by the producer cells play a role in the suppression of retrovirus transduction of human CD34+ cells.5Proteoglycans in the conditioned medium of mouse-derived NIH 3T3 packaging cells have also been shown to inhibit retroviral infection.6 7
To avoid these inhibitory factors, we constructed an oncoretroviral producer cell line based on human cells. We used 293 cells because these cells have been used for the production of high-titer vectors by many investigators and vectors produced by 293-based packaging cells have been shown to efficiently transduce hematopoietic cells.8 We chose the gibbon ape leukemia virus (GALV) as an envelope gene, which we previously reported to yield improved gene transfer into nonhuman primate HSCs.9
A limiting factor to efficient stem cell transduction is the quiescent nature of stem cells. Oncoretroviral vectors require cell division to integrate into the host genome, and primitive stem cells are mostly quiescent. Lentiviral vectors based on the human immunodeficiency virus (HIV) genome have been shown to transduce nondividing cells10,11 and, thus, may be a superior vector system than oncoretroviral vectors for transduction of HSCs. Efficient transduction of human nonobese diabetic/severe combined immune-deficient (NOD/SCID) mouse repopulating cells with lentiviral vectors has been reported with levels of up to 50%.12 13
In the current study, we examined gene transfer into baboon hematopoietic repopulating cells using Phoenix-GALV–pseudotyped vectors. We used a competitive repopulation assay in the baboon to directly compare Phoenix-GALV–pseudotyped vectors to PG13-pseudotyped vectors and to lentiviral vectors.
Materials and methods
Construction of retrovirus packaging cell line Phoenix-GALV
The plasmid pMOV-GaLV Seato env (kindly provided by A. D. Miller, Fred Hutchinson Cancer Research Center, Seattle, WA), which contains the GALV envelope gene and was also used for the generation of PG13 packaging cells, was modified by the addition of the human truncated nerve growth factor receptor (NGFR) cDNA under the control of the cytomegalovirus (CMV) immediate early promoter. Helper-virus–free Phoenix-GP packaging cells (kindly provided by G. Nolan, Stanford, CA) were transfected by Ca++ precipitation, and 9 days after transfection, single NGFR-expressing cells were sorted directly into wells of a 96-well, flat-bottomed microtiter plate. Packaging cell clones were rescreened for NGFR expression, and clones with high NGFR expression were transfected with the retroviral vector pMNDEGFPSN (kindly provided by D. Kohn, Los Angeles, CA) to produce transient supernatant. Human CEMSS cells were transduced with retroviral supernatant and the transduction efficiency evaluated by flow cytometry 3 days after infection. The 8 best clones were used to package pLZRSBMNEGFP, and supernatants were screened on human HT1080 cells and human CD34+ peripheral blood cells. The packaging cell clone 100 was identified to produce the highest rate of gene transfer into all cell types and was Phoenix-GALV.
Oncoretrovirus vectors
The MNDEGFPSN and MNDEYFPSN vectors encode the enhanced green fluorescent protein (EGFP) and its yellow variant EYFP under the control of the 5′ modified viral long terminal repeat (LTR) and the SV40 promoter driving the bacterial neomycin phosphotransferase (neo) gene conveying G418 resistance. Both vectors are identical, with the exception of the 9 nucleotides distinguishing EGFP from EYFP. Expression of EGFP or EYFP protein can be distinguished by flow cytometry. Individual producer cell clones with similar titers were selected for the MNDEGFPSN/MNDEYFPSN comparisons. All vector titers were assessed by infection of HT1080 cells as described earlier.14
Lentivirus vectors
The lentiviral transfer vector RRLsin.cPPT.hPGK.GFP.Wpre (kindly provided by L. Naldini, Torino, Italy) is a self-inactivating HIV-derived vector expressing EGFP from the internal human phosphoglycerate kinase promoter (hPGK), containing a woodchuck hepatitis pre-element and a central polypurine tract.15Vector stocks of VSV-G–pseudotype lentiviral vectors were prepared by calcium phosphate–mediated 3-plasmid transfection of 293T cells. Briefly, 27 μg of the transfer vector construct, 17.5 μg second generation gag-pol packaging construct pCMVΔR8.74, and 9.5 μg VSV-G expression construct pMD.G were used for transfection of 12 × 106 293T cells overnight in 25 mL Dulbecco modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS). The cells were treated with 10 mM sodium butyrate during the first of three 12-hour vector supernatant collections. The supernatant was filtered through a 0.22-μm-pore-size filter and concentrated 100-fold by ultracentrifugation. All vector stocks were titered by transducing HT1080 cells using limiting dilutions of the stock with analysis for EGFP or EYFP expression by flow cytometry. The titers of the concentrated VSV-G–pseudotype vector preparations were between 7.5 × 108 and 8.4 × 108 infectious units/mL.
Gene transfer into human and baboon CD34-enriched cells
Baboon marrow buffy coat cells were labeled with IgM monoclonal antibody (MoAb) 12-8 (CD34) at 4°C for 30 minutes, washed, and incubated with rat monoclonal anti–mouse IgM microbeads (Miltenyi Biotec, Auburn, CA) for 30 minutes at 4°C, washed, and then separated using an immunomagnetic column technique (Miltenyi Biotec) according to manufacturer's instructions. Equal numbers of CD34-enriched cells were prestimulated for 48 hours in tissue culture–treated 75-cm2 canted-neck flasks (Corning, Corning, NY) in Iscove medium containing 10% FBS (Hyclone, Logan, UT) in the presence of either stem cell factor (SCF), granulocyte colony-stimulating factor (G-CSF), and megakaryocyte growth and development factor (MGDF) (animals 00021, F99070, A00066) or interleukin 3 (IL-3), IL-6, SCF, G-CSF, fms-like tyrosine kinase 3 ligand (Flt3-L), and MGDF (animals F99074, F99310, M99267) at 100 ng/mL each and then transferred into 75-cm2 canted-neck flasks (Falcon, Franklin Lakes, NJ) which had been coated with CH-296 (RetroNectin; kindly provided by Takara Shuzo, Japan) at 2 μg/cm2 as described14 and preloaded twice with virally conditioned media (VCM). Cells were exposed to vector (neat VCM for oncoretroviral transductions or concentrated vector diluted in fresh medium for lentiviral transductions) for 4 hours in the presence of the same cytokines, then collected and resuspended in fresh medium with cytokines; the following day, another 4-hour exposure to vector was performed before the cells were harvested and the pooled cell populations reinfused into the irradiated animal. Human CD34-enriched cells from G-CSF–mobilized peripheral blood were transduced using the same transduction protocol as described for the animals 00021, F99070, and A00066.
Animals
Healthy juvenile baboons (Papio cynocephalus cynocephalus or Papio cynocephalus anubis) were housed at the University of Washington Regional Primate Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Studies were conducted under protocols approved by the institutional review board and animal care and use committees. The autologous baboon transplantations including all procedures were performed as previously described.9 14 Prior to bone marrow harvest, animals were treated with recombinant human (rh)–SCF (50 μg/kg) and rh-G-CSF (100 μg/kg) (kindly provided by G. Molineux, Amgen, Thousand Oaks, CA) as single daily subcutaneous injections. After 5 days of growth factor administration, marrow (25 mL to 60 mL) was aspirated from the humeri and/or femora and collected in preservative-free heparin. In preparation for transplantation, all animals received myeloablative irradiation (TBI), 1020 cGy, at 7 cGy/min as 2 equally divided doses 24 hours apart. Animals in the present study were given posttransplant G-CSF, 100 μg/kg, intravenously, once daily starting at day 0 until their peripheral blood neutrophil counts were more than 1000/μL.
Flow cytometric analysis
EGFP and EYFP expression was determined in peripheral blood and marrow cells after red blood cell lysis. Cell subset analysis was performed using flow cytometric quantification of at least 400 000 propidium iodide (2 μg/mL)–excluding forward and right-angle light scatter–gated events on a FACS Vantage (Becton Dickinson, San Jose, CA). Analyses of flow cytometric data was performed using CELLQuest v3.3 software. In all animals only 50% of the transplanted cells were transduced with either vector; thus, the percentage of EGFP+ or EYFP+ cells was multiplied by 2 to account for the 2 experimental arms used in the animals, and the results were then plotted over time in an MS Excel chart. Murine anti–human monoclonal antibodies conjugated to phycoerythrin (PE), which had been shown to bind baboon CD markers, included: anti-CD13 (clone L138), anti-CD20 (clone L27), anti-CD61 (clone S5.2), and matched control (clone X40); all were obtained from Becton Dickinson. Anti-CD3 (clone FN18) was obtained from BioSource International, Carmarillo, CA.
Analysis of EGFP/EYFP expression in a cell colony-forming unit (CFU-C) assay
CD34-enriched cells (1000 per 35-mm plate) were cultured at least in triplicate in a double-layer agar culture system as previously described.14 Briefly, isolated cells were cultured in alpha minimal essential medium supplemented with 25% FBS (Hyclone), 0.1% bovine serum albumin (BSA; fraction V; Sigma, St Louis, MO), 0.3% (wt/vol) agar (BioWhittaker, Rockland, ME) overlaid on medium with 0.5% agar (wt/vol) containing 100 ng/mL of SCF, IL-3, IL-6, granulocyte macrophage–colony-stimulating factor (GM-CSF), G-CSF, and 4 U/mL Epo (provided by G. Molineux, Amgen, Thousand Oaks, CA). Cultures were incubated at 37°C in 5% CO2 in a humidified incubator. Colonies were enumerated and evaluated for EGFP/EYFP expression at day 14 of culture using an inverted fluorescent microscope.
Fluorescent probe PCR assay (TaqMan)
PCR amplification and analysis of the EYFP and EGFP genes were performed by using a quantitative real-time PCR assay (TaqMan). DNA (300 ng) was amplified at least in duplicate with EYFP-specific primers (5′-CTG CAC CAC CGG CAA-3′ and 5′-GTA GCG GGC GAA GCA CT-3′) and a fluorescence-tagged probe (5′-FAM-CCA CCT TCG GCT ACG GCC TG-TAMRA-3′; Synthegen, Houston, TX). For EGFP, the specific primers 5′-CTG CAC CAC CGG CAA -3′ and 5′-GTA GCG GCT GAA GCA CTG-3′ were used with the probe 5′-FAM-CCA CCC TGA CCT ACG GCG TG-TAMRA-3′. These primers and probes were designed using Primer Express software (Perkin-Elmer, Foster City, CA). Standards consisted of dilutions of DNA extracted from cell lines transduced with a single copy of the EGFP or EYFP vector. Negative controls consisted of DNA extracted from peripheral blood mononuclear cells (PBMCs) obtained preinfusion or from control animals or water. A beta-globin–specific primer/probe combination (5′-CCT ATC AGA AAG TGG TGG CTG G-3′, 5′-TTG GAC AGC AAG AAA GTG AGC TT-3′, probe 5′-TGG CTA ATG CCC TGG CCC ACA AGT A-TAMRA-3′) was used to adjust for equal loading of DNA per reaction. Calculated gene marking percentages are adjusted for the fact that 2 experimental arms were compared in each animal and assume that the peripheral blood cells contain only one copy of the corresponding vector per cell. Reactions were run using the ABI master mix (Applied Biosystems, Branchburg, NJ) on the ABI Prism 7700 sequence detection system (Applied Biosystems) using the following thermal cycling conditions: 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
Detection of helper virus
All animals were evaluated for generation of replication competent helper virus at up to 3 time points after transplantation. Peripheral blood mononuclear cell DNA was assayed for recombinant helper virus genomes by PCR using the primers and PCR conditions described previously.9 PCR analysis of peripheral blood samples for the presence of GALV envelope sequences was negative in all animals at a level of detection of less than one in 10 000 cells, indicating the absence of helper-virus infection. Serum of all 3 baboons that received transplants of lentivirally transduced cells (F99074, F99310, and M99267) was tested for the presence of recombinant replication competent lentiviral vectors using the p24 Antigen Assay (Beckman Coulter, Miami, FL) according to the manufacturer's recommendations. p24 antigen was not detectable at any time point (data not shown) indicating the absence of helper-virus production.
Results
Engraftment of transduced cells and follow-up
We used a competitive repopulation assay to study gene transfer into nonhuman primate repopulating cells using GALV-pseudotype oncoretroviral vectors produced by a human producer cell line (Phoenix-GALV). There were 6 baboons that received transplants of transduced CD34-enriched autologous marrow cells to compare oncoretroviral vectors produced by Phoenix-GALV to oncoretroviral vectors produced by PG13 (3 animals) or to lentiviral vectors (3 animals) produced by transient transfection of 293T cells. A stable absolute neutrophil count (ANC) of more than 500/μL was reached at a median of 16.5 days (range, 11-19 days). Average follow-up in the animals is 37 weeks after transplantation.
Comparison between the Phoenix-GALV– and PG13-derived vectors
We first wished to directly compare vectors produced by Phoenix-GALV to vectors produced by PG13. CD34-enriched bone marrow cells were prestimulated and then divided into 2 equal fractions and transduced with either MNDEGFPSN (PG13) or MNDEYFPSN (Phoenix-GALV). The mean expansion of cells during transduction was similar for the PG13-pseudotyped vector and the Phoenix-GALV–pseudotyped vector (2.4-fold versus 2.6-fold).
Gene transfer rates into the bulk population of CD34-enriched cells were determined by flow cytometry on day 3 after the end of transduction (Table 1). Gene transfer efficiency into these cells was significantly higher with the Phoenix-GALV–derived vector (average 20.1%) than with the PG13-derived vector (average 6.9%, P < .02 by paired t test). Gene transfer efficiency into colony-forming cells within the same cell fractions was also determined by quantification of fluorescence-positive CFU-C (Table 1). The overall gene transfer efficiency into CFUs was similar to the overall transduction rate as determined by flow cytometry. The use of the Phoenix-GALV–derived vector led to significantly higher transduction rates of CFUs (P = .04 by paired t test).
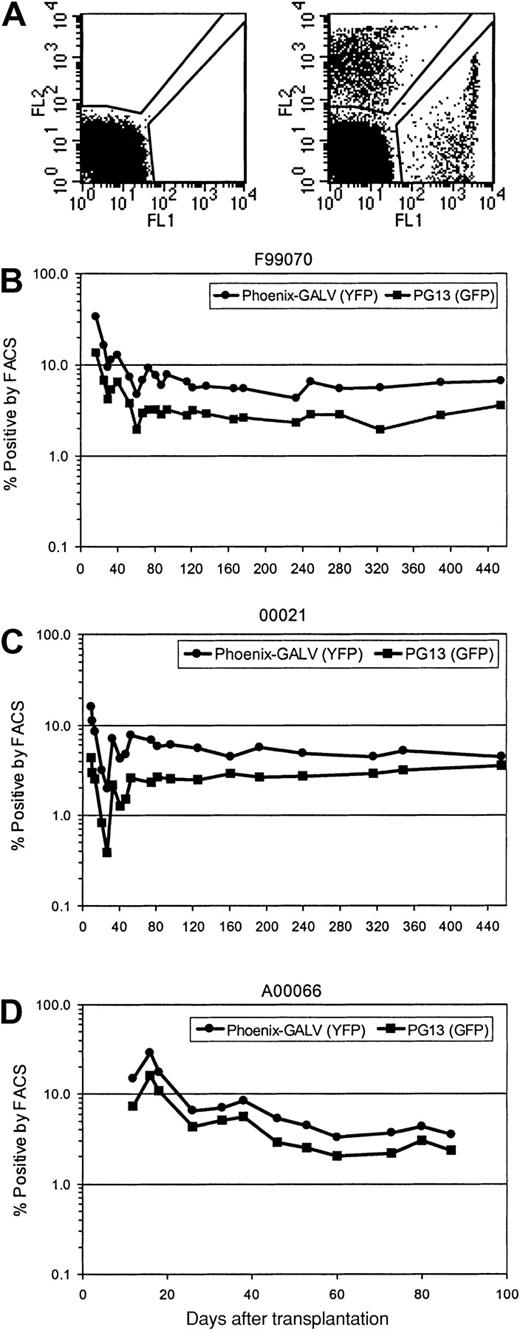
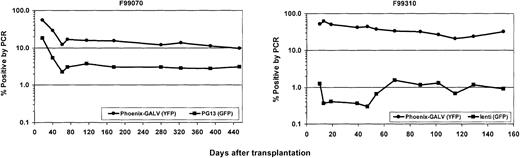
Gene transfer rates after transplantation were measured by simultaneous flow cytometric detection of EGFP and EYFP as well as by real-time PCR of peripheral blood leukocytes. Figure 1illustrates a summary of EGFP and EYFP expression in peripheral blood leukocytes over time in all 3 animals. Initial expression levels between 16% and 34% were achieved for EYFP (Phoenix-GALV) and between 4.3% and 16% for EGFP (PG13). Within the first 10 weeks after transplantation, the percentage of transgene-expressing cells declined to levels between 3.5% and 5.5% for EYFP and 2.3% and 2.8% for EGFP, which was sustained very stably over a period of more than one year in the 2 baboons with the longest follow-up (Figure 1A-B). In all 3 animals, the use of Phoenix-GALV–derived vectors resulted in higher gene transfer levels compared with the PG13-derived vector at every time point examined. The average marking ratios of EYFP+ to EGFP+ in the 3 animals were 1.6 (F99070), 2.8 (00021), and 2.2 (A00066); this difference was statistically significant (P = .02 by paired t test).
Higher in vivo marking levels with Phoenix-GALV–derived vectors than with PG13-derived retroviral vectors in nonhuman primates.
(A) Representative example of a flow cytometric analysis of a control animal (no transplant; left panel) and animal F99070 on day 25 after transplantation (right panel). (B-D) Detection of transgene expression over time by flow cytometry in the peripheral blood of 3 baboons transplanted with transduced CD34-enriched marrow. F99070 (B), 00021 (C), A00066 (D).
Higher in vivo marking levels with Phoenix-GALV–derived vectors than with PG13-derived retroviral vectors in nonhuman primates.
(A) Representative example of a flow cytometric analysis of a control animal (no transplant; left panel) and animal F99070 on day 25 after transplantation (right panel). (B-D) Detection of transgene expression over time by flow cytometry in the peripheral blood of 3 baboons transplanted with transduced CD34-enriched marrow. F99070 (B), 00021 (C), A00066 (D).
Comparison between Phoenix-GALV–derived oncoretroviral vectors and lentiviral vectors
In 3 additional animals, we compared Phoenix-GALV–derived vectors to lentiviral vectors. We used a modified growth factor combination for the prestimulation and transduction based on our encouraging results using IL-3, IL-6, SCF, MGDF, Flt3-L, and G-CSF.16 One-half of the cells was transduced with MNDEYFPSN (Phoenix-GALV) while the other half was transduced with the VSV-G–pseudotype lentiviral vector RRLsin.cPPT.hPGK.GFP.Wpre. Mean cell expansion during the transduction time was 4.2-fold, slightly higher than in the first set of animals, but there was no significant difference between the 2 experimental arms in each animal.
Gene transfer rates prior to reinfusion of the cells are shown in Table2. Gene transfer efficiency into these cells as determined by flow cytometry was significantly higher using the Phoenix-GALV–derived vector (average 38.4%) than with the lentiviral vector (average 18.5%, P = .04 by pairedt test). Gene transfer efficiency into CFUs was also higher using the Phoenix-GALV–derived vector as compared with the lentiviral vector (average 29.6% versus 16%).
Pretransplant marking with Phoenix-GALV–derived vectors in the second set of animals was overall somewhat higher compared with the first 3 animals both into the bulk population of cells (38.4% versus 20.1% by flow cytometry on day 3, P = .09) as well as into colony-forming progenitors (29.6% versus 20.4%). This difference is most likely due to the addition of IL-3, IL-6, and Flt3-L to the cytokine cocktail during transduction, although it was not statistically significant.
Figure 2 shows the in vivo gene transfer levels in peripheral blood leukocytes for these 3 animals. Transduction with the Phoenix-GALV–derived vector resulted in a statistically significantly higher gene transfer rate than the lentiviral vector (P = .04 by paired t test). Gene transfer levels with the Phoenix-GALV–derived vector were higher than in the first 3 animals. Transduction efficiency of repopulating cells was up to 60% early after transplantation. In 2 of these animals, levels are still between 20% and 25% at 3 to 6 months after transplantation, and marking/expression levels appear to have reached a plateau. Transduction efficiency with the lentiviral vector was up to 2.4% at an early time point after transplantation and then decreased to levels around 1% after 2 to 3 months.
Higher in vivo marking levels with Phoenix-GALV–derived vectors than with VSV-G–pseudotype lentiviral vectors in nonhuman primates.
(A) Representative example of a flow cytometric analysis of a control animal (no transplant; left panel) and animal M99267 on day 26 after transplantation (right panel). (B-D) Detection of transgene expression over time by flow cytometry in the peripheral blood of 3 baboons that received transplants of transduced CD34-enriched marrow. F99074 (B), F99310 (C), and M99267 (D).
Higher in vivo marking levels with Phoenix-GALV–derived vectors than with VSV-G–pseudotype lentiviral vectors in nonhuman primates.
(A) Representative example of a flow cytometric analysis of a control animal (no transplant; left panel) and animal M99267 on day 26 after transplantation (right panel). (B-D) Detection of transgene expression over time by flow cytometry in the peripheral blood of 3 baboons that received transplants of transduced CD34-enriched marrow. F99074 (B), F99310 (C), and M99267 (D).
Gene expression in multiple hematopoietic lineages
To assess gene expression in different hematopoietic lineages, peripheral blood cells of all 6 baboons were marked with PE-labeled antibodies against granulocytes (CD13), T lymphocytes (CD3), and B lymphocytes (CD20), and analyzed by flow cytometry at different time points. Figure 3 demonstrates a representative example of such a flow cytometric analysis for the animal with the longest follow-up. Both EGFP- and EYFP-positive cells were detected by flow cytometry in all hematopoietic lineages. Gene transfer with the Phoenix-GALV–derived vector was higher than that achieved with PG13-derived vectors or lentiviral vectors in all subsets at every time point examined. Additionally, fluorescence-positive CD61+ platelets and red blood cells were detected (data not shown).
Transduction of repopulating cells with multilineage potential.
EGFP- and EYFP-expressing cells are detected in all hematologic subpopulations in the peripheral blood of F99070 at different time points after transplantation.
Transduction of repopulating cells with multilineage potential.
EGFP- and EYFP-expressing cells are detected in all hematologic subpopulations in the peripheral blood of F99070 at different time points after transplantation.
Gene expression in bone marrow leukocytes
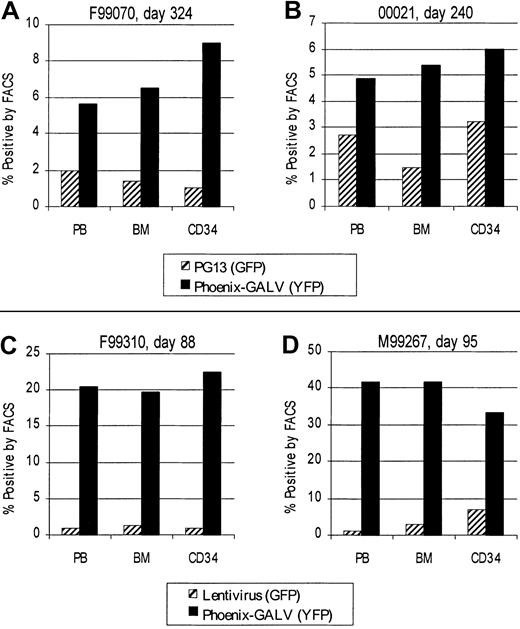
To assess gene transfer levels in bone marrow leukocytes, periodic bone marrow aspirates were obtained after transplantation and the percentage of transgene-expressing cells in peripheral blood, whole marrow, and marrow CD34+ cells was determined by flow cytometry. Representative analyses are shown in Figure4. No obvious difference between the percentage of gene-modified cells in peripheral blood and bone marrow or CD34+ cells was observed.
Similar gene marking/expression levels in bone marrow and peripheral blood.
Displayed are representative examples of transgene expression in leukocytes from peripheral blood (PB), bone marrow (BM), and CD34+ cells from the marrow (CD34) of F99070 at day 324 (A), 00021 at day 240 (B), F99310 at day 88 (C), and M99267 at day 95 (D) after transplantation.
Similar gene marking/expression levels in bone marrow and peripheral blood.
Displayed are representative examples of transgene expression in leukocytes from peripheral blood (PB), bone marrow (BM), and CD34+ cells from the marrow (CD34) of F99070 at day 324 (A), 00021 at day 240 (B), F99310 at day 88 (C), and M99267 at day 95 (D) after transplantation.
Gene marking as determined by quantitative real-time PCR
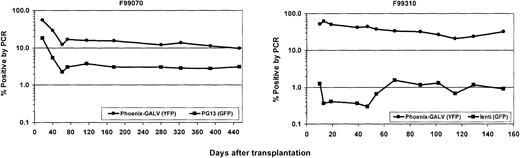
To ensure that the difference in the percentage of fluorescence-positive cells between the 2 experimental arms in the animals that received transplants was not due to differential silencing of the oncoretroviral vector and the lentiviral vector, quantitative real-time PCR was performed on DNA extracted from peripheral blood leukocytes. Both transgenes EGFP and EYFP were detectable at slightly higher levels than by flow cytometry, but there was very good correlation with the flow cytometric data. Figure5 demonstrates the PCR results for animals F99070 and F99310. Similar results were obtained in other animals (data not shown).
No significant silencing of transgene expression over time.
The transgenes EGFP and EYFP were quantified by real-time quantitative PCR and follow the same pattern as for transgene expression, indicating that there is no significant progressive silencing over time. The difference between the 2 vectors is even more pronounced on the DNA level than it is by flow cytometry.
No significant silencing of transgene expression over time.
The transgenes EGFP and EYFP were quantified by real-time quantitative PCR and follow the same pattern as for transgene expression, indicating that there is no significant progressive silencing over time. The difference between the 2 vectors is even more pronounced on the DNA level than it is by flow cytometry.
Transduction of human CD34-enriched cells with Phoenix-GALV–derived vectors
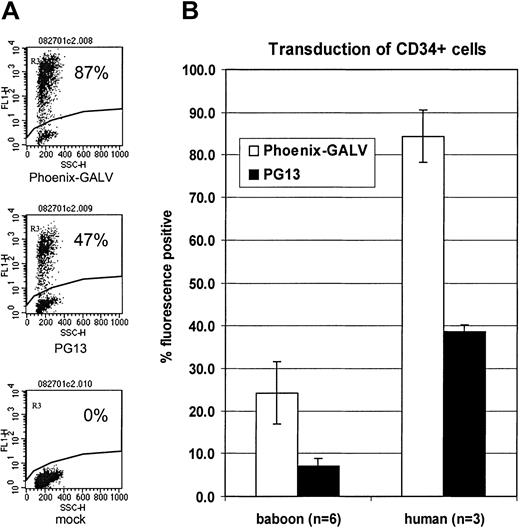
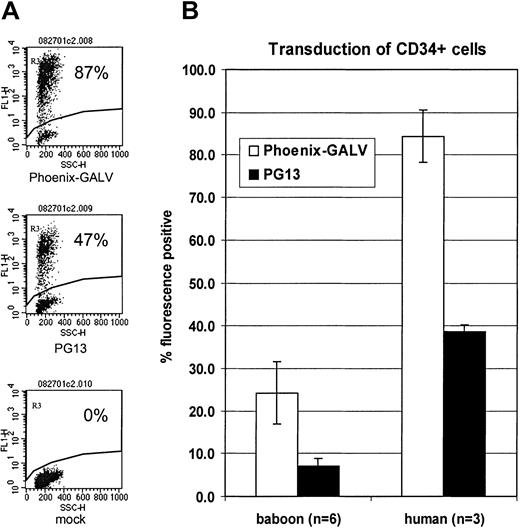
To determine the effect of vectors produced by the different GALV-pseudotype packaging cell lines (Phoenix-GALV versus PG13) on the transduction of human progenitors, human CD34+ cells were enriched from G-CSF–mobilized peripheral blood and transduced with either MNDEGFPSN (PG13) or MNDEYFPSN (Phoenix-GALV). On average, 84.3 ± 6.1% of cells were transduced with the Phoenix-GALV–derived vector while 38.7 ± 1.5% were transduced with the PG13-derived vector (Figure 6A-B),P < .01. Also, the overall transduction rate in human CD34+ cells was higher than in baboon CD34+ cells (Figure 6B), suggesting that gene transfer into human repopulating cells may also be higher compared with baboon repopulating cells.
High-level transduction of human CD34+progenitor cells.
(A) Flow cytometric detection of EGFP or EYFP in human CD34+ cells transduced with either PG13- or Phoenix-GALV–derived vectors in vitro on day 3 after transduction. (B) Comparison of transduction efficiencies obtained with either PG13 or Phoenix-GALV in human and baboon CD34+ cells in vitro.
High-level transduction of human CD34+progenitor cells.
(A) Flow cytometric detection of EGFP or EYFP in human CD34+ cells transduced with either PG13- or Phoenix-GALV–derived vectors in vitro on day 3 after transduction. (B) Comparison of transduction efficiencies obtained with either PG13 or Phoenix-GALV in human and baboon CD34+ cells in vitro.
Discussion
In the current study, we show in a nonhuman primate model highly efficient gene transfer into hematopoietic repopulating cells using a GALV-pseudotype oncoretroviral vector produced by the newly established human-derived producer cell line Phoenix-GALV. Up to 60% of hematopoietic repopulating cells were transduced early after transplantation and up to 25% after transduction levels stabilized at 3 to 6 months. All animals had persistent marking and expression, suggesting that neither transcriptional transgene silencing nor immune responses to genetically modified cells occurred. In addition, the use of Phoenix-GALV–derived vectors resulted in significantly higher gene transfer into human CD34-enriched cells than PG13-derived vectors, emphasizing its potential for human stem cell gene therapy applications.
Murine-derived producer cell lines have been reported to produce inhibiting factors, which can negatively affect gene transfer and/or stem cell maintenance.6,17 More recently, conditioned medium from human HT1080 cells has also been described to have a negative, differentiating effect on the maintenance of NOD/SCID repopulating cells.18 Such negative effects have not been reported with human packaging cells based on 293 cells.8,19 20 Thus, we generated a GALV-pseudotype producer cell line based on the 293-derived Phoenix cells, and we evaluated the use of oncoretroviral vectors produced by Phoenix-GALV in our competitive repopulation assay in the baboon. The purpose of these studies was not to determine a single factor potentially affecting differences between cell lines but, rather, to compare the different conditioned media from PG13 and Phoenix-GALV for their ability to transduce and maintain hematopoietic repopulating cells. There were 3 animals that received transplants of transduced CD34+ cells to compare vectors derived from Phoenix-GALV with vectors derived from PG13. In all 3 animals, transduction efficiency into hematopoietic repopulating cells was higher with the Phoenix-GALV–derived vector than with the PG13-derived vector. This difference was statistically significant.
In a second series of 3 baboons, we performed a competitive repopulation assay in which we compared the oncoretroviral vector produced by the Phoenix-GALV producer cell line to a more recently described lentiviral vector construct. Lentiviral vectors may be superior to oncoretroviral vectors due to their ability to integrate into nondividing cells.10,11 Based on the rather low transduction efficiency of long-term repopulating cells with shorter transduction protocols and minimal growth factor support during transduction,21-23 we decided to use the 3-day transduction protocol optimized for oncoretroviral vectors. We used IL-3, IL-6, SCF, Flt3-L, G-CSF, and MGDF for prestimulation and transduction based on our recently published and encouraging results with this growth factor combination.16 In all 3 animals, preinfusion transduction efficiency into progenitor cells was higher with the Phoenix-GALV–derived vector than with the lentiviral vector. In vivo transduction efficiency in repopulating cells with the Phoenix-GALV–derived vector was up to 60% early after transplantation and then plateaued after 2 to 3 months at levels of approximately 20% to 30% for 2 of the animals and around 5% for the third animal. Transduction with a lentiviral vector in a 3-day protocol in the presence of multiple recombinant growth factors did not markedly improve the transduction efficiencies obtained with these vectors in shorter transduction protocols or with less cytokine support.21-23 Other research groups have also reported low gene transfer levels using lentiviral vectors in nonhuman primate transplantation models.21,22 These findings are in contrast to the high transduction efficiencies obtained with these vectors in human NOD/SCID repopulating cells.12,13,24 A species-specific difference in susceptibility to lentiviral transduction between humans and nonhuman primates such as baboons and rhesus macaques has been considered but would not explain the high transduction efficiency into baboon CFUs23 and baboon NOD/SCID repopulating cells.25 An alternative explanation would be that this difference is a result of assaying different cell populations in the NOD/SCID xenotransplant system versus the autologous transplant setting. We are currently investigating these different possibilities.
Many different growth factor combinations have been used for oncoretroviral transduction of HSCs, attempting to maintain a balance between sufficient stimulation of HSCs to allow for vector integration and maintenance of HSCs to ensure engraftment of transduced cells.1,4,9,14,21,22,26 27 The addition of IL-3, IL-6, and Flt3-L to the growth factor combination in the second set of animals (comparison between Phoenix-GALV–derived vectors and lentiviral vectors) resulted in a higher expansion rate and slightly higher pretransplantation gene transfer rates using Phoenix-GALV–derived vectors compared with the first set of animals (Phoenix-GALV versus PG13). More importantly, the use of Phoenix-GALV–derived oncoretroviral vectors in combination with IL-3, IL-6, SCF, Flt3-L, G-CSF, and MGDF led to the highest gene transfer rates into hematopoietic repopulating cells in baboons. While we did not intend to specifically investigate the impact of the different growth factor combinations, SCF, G-SCF, and MGDF versus IL-3, IL-6, Flt3-L, SCF, G-SCF, and MGDF, on transduction efficiency, the improved in vivo results with the latter cytokine combination, although statistically not significant due to the small group sizes, seem noteworthy.
Several studies have previously demonstrated progressive silencing of oncoretroviral transgenes in the mouse bone marrow transplantation model.28-30 To address whether transgene silencing played a significant role in our study we compared transgene expression as determined by flow-cytometry with gene marking as assessed by quantitative real-time PCR. The results from the 2 assays correlated very well; with both flow cytometric and real-time PCR analysis we observed an initial decline followed by a stable level of genetically modified cells. These data suggest that the loss of transgene-positive cells rather than progressive transcriptional silencing is responsible for the dynamics of EGFP- and EYFP-expressing cells in the peripheral blood of the animals. The finding that transgene silencing of oncoretroviral vectors is far less pronounced in nonhuman primates than in mice is consistent with previous publications by our group31 and other research groups.32 33
At most time points gene transfer levels determined by real-time PCR were slightly higher than by flow cytometry. This difference may be caused by low or absent expression of the transgene in part of the marked cells due to variegation of expression,34 by multiple transgene copies in at least part of the transduced cells, or by the technical difficulties associated with absolute quantification of copy number by real-time PCR.35
Quantitative real-time PCR also confirmed the differences in gene marking between oncoretroviral vectors and lentiviral vectors. Thus, differential expression from the lentiviral and oncoretroviral vectors does not account for the differences in gene marking between the 2 vector systems.
We followed 2 animals for more than one year, and the level of marking has been very stable during that time. Transgene-expressing cells were found in all hematopoietic subsets, suggesting that Phoenix-GALV–derived vectors are capable of transducing cells with multilineage potential.
We also compared the frequency of transgene-expressing leukocytes in the peripheral blood and in the bone marrow. No significant differences between the percentage of transgene-expressing cells in bone marrow and peripheral blood were detected at any time point (Figure 5). This is consistent with the findings reported by Sellers et al.36Thus, we did not find evidence for a block in differentiation or a specific immune rejection of mature cells in our study as had previously been suggested by other investigators.37 38
To ensure that the improvement in gene transfer with the human-derived producer cell line was not a baboon-specific effect, we compared Phoenix-GALV–derived and PG13-derived vectors for their ability to transduce primary human CD34-enriched cells from G-CSF–mobilized peripheral blood. Phoenix-GALV–derived vectors led to significantly higher transduction rates than PG13-derived vectors. It is noteworthy that both vectors were 3- to 5-fold more efficient in transducing human cells than baboon cells, which suggests that even higher gene transfer into long-term repopulating cells in humans could be achieved with this vector system.
In conclusion, our data show highly efficient transduction of nonhuman primate hematopoietic repopulating cells and sustained multilineage engraftment of transduced cells using GALV-pseudotype retroviral vectors produced by a human packaging cell line. Transduction efficiency of short-term repopulating cells was up to 60%, with levels up to 20% to 30% at 3 months after transplantation and later. Importantly, transduction efficiencies of human CD34-enriched cells with vectors produced by the human producer cell line Phoenix-GALV were significantly higher than with PG13-derived vectors, suggesting that Phoenix-GALV–derived vectors may also result in more efficient gene transfer into human hematopoietic stem cells.
The authors wish to thank Bobbie M. Thomasson, Laura J. Peterson, and Jennifer Potter for their technical assistance; Mike Gough and the staff of the University of Washington Regional Primate Research Center for assistance with the animals; and Bonnie Larson and Helen Crawford for their help in preparing the manuscript.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-05-1359.
Supported in part by National Institutes of Health grants and contracts P50 HL54881, P30 CA15704, NO1 AI35191, NIHRROO166, CA18029, and P30 DK47754. P.A.H. was supported by the German Krebshilfe. M.S.T. received support from the German Forschungsgemeinschaft (T0 208/1-1). H.-P.K. is a Markey Molecular Medicine Investigator.
P.A.H. and M.S.T. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hans-Peter Kiem, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109; e-mail: hkiem@fhcrc.org.