NK4, a 4-kringle antagonist of hepatocyte growth factor (HGF), is a potent inhibitor of tumor angiogenesis and functions independently of its HGF-antagonistic activity. We have shown previously that in vivo genetic modification of tumors with an adenovirus vector that expresses NK4 (AdNK4) restrains tumor angiogenesis and slows the rate of tumor growth in vivo. In the present study, we investigated the hypothesis that this can be made more efficient by also administering bone marrow–generated dendritic cells (DCs) to the tumor. The data show that the growth of mouse subcutaneous tumors is significantly suppressed by direct administration of DCs into established tumors that had been pretreated with AdNK4 3 days previously. The synergistic antitumor effect produced by the combination therapy of AdNK4 with DCs correlated with the in vivo priming of tumor-specific cytotoxic T lymphocytes. Analysis of mice treated with fluorescence-labeled DCs suggested that DCs injected into the flank tumor could migrate to lymphoid organs in vivo for activation of immune-relevant processes. Knockout mice experiments demonstrated that the tumor regression produced by this combination therapy depends on both major histocompatibility complex (MHC) class I antigen presentation of DCs injected into the tumors and CD8+ T cells of the treated host. Finally, a mechanism for this synergism was suggested by the histological observation that tumor necrosis and apoptosis were induced by genetic engineering of the tumors to express NK4. These findings should be useful in designing novel strategies that use a combination of 2 monotherapies directed against the vascular and immune systems for cancer therapy.
Introduction
The fact that many clinical tumors arise under a functional immune system suggests that insufficient antitumor immunity allows even antigenic tumors to escape the immune mechanism.1,2 As a vector to bolster antitumor immunity, dendritic cells (DCs) are drawing interest because of their pivotal role in the immune response.3,4 DCs are professional antigen-presenting cells located at potential pathogen entry sites to sample antigens.3-6 After taking up antigens, DCs migrate to secondary lymphoid tissues and activate CD8+ cytotoxic T cells and CD4+ helper T cells by presenting antigens as peptides bound to MHC class I and class II products, respectively.3-6 Conversely, DCs also can down-regulate immune responses, mainly by generating regulatory T cells that suppress activated T cells.5,7 The mechanism by which DCs mediate such contradictory functions (ie, immunity or tolerance) is outlined as follows: in the steady state, DCs take up apoptotic cells arising from normal cell turnover and induce tolerance to self-antigens in phagocytosed cells. Once infection and inflammation take place, DCs are stimulated to mature into strong activators of T cell–mediated immunity against endocytosed apoptotic cells and pathogens. Recently, exposure to necrotic tumor cells or their supernatant has been identified as a powerful stimulus for DC maturation.8 9Knowledge of DC biology suggests that apoptotic tumor cells together with necrotic tumor cells may be an optimal source of antigens to be captured and presented by DCs for generating tumor-specific adaptive responses in DC-based cancer immunotherapy.
NK4, an antagonist for hepatocyte growth factor (HGF), is composed of the NH2-terminal hairpin domain and 4 subsequent kringle domains of the α-subunit of HGF and is identified as a new angiogenesis inhibitor that has a potential for suppressing tumor growth independently of its activity as an HGF antagonist.10-13 We also have shown that a similar antitumor effect was achieved by genetic modification of tumors to secrete NK4.14 Although the mechanism by which NK4 inhibits angiogenesis is not fully elucidated, NK4 is structurally similar to angiostatin, an angiogenesis inhibitor containing kringle domains from an internal fragment of plasminogen.15Generally, angiogenesis, that is, neovascularization from pre-existing blood vessels, is a critical process in tumorigenesis, and numerous antiangiogenic molecules including angiostatin, endostatin, thrombospondin, matrix metalloprotease inhibitors, quinoline-3-carboxamide linomide, and others have been shown to inhibit tumor growth by driving tumor cells into apoptosis and/or necrosis.15-22
On the basis of our integrated understanding of adaptive immunity and antiangiogenic tumor therapy, we hypothesized that genetic modification of tumors to express NK4 would bring about apoptosis and necrosis of tumor cells by its antiangiogenic function and that, subsequently, administered DCs would take up the apoptotic tumor cells with the necrosis-derived stimuli and then induce tumor-specific T cell–mediated immunity in vivo. In this study, we evaluate this concept in mouse tumor models using an E1− adenovirus (Ad) vector (AdNK4) to transfer the NK4 cDNA to tumor cells and syngeneic bone marrow–derived DCs. The data demonstrate that intratumoral administration of AdNK4 elicits apoptosis and necrosis of tumor cells and that DCs administered to the AdNK4-treated tumor generate tumor-specific CD8+ T-cell immune responses that inhibit the growth of pre-existing tumors.
Materials and methods
Mice
C57Bl/6 (H-2b) and BALB/c (H-2d) female mice, 6 to 8 weeks old, were obtained from Japan Charles River (Atsugi, Japan). Some experiments used 6- to 8-week-old female MHC class I–deficient (B6.129-B2mtm1) and MHC class II–deficient (B6.129-Abbtm1) mice that had been backcrossed to the C57Bl/6 background (Taconic, Germantown, NY).
Cell culture
The Colon-26 colon adenocarcinoma cell line (H-2d), the BALB/3T3 fibroblast cell line (H-2d), and the B16-F10 melanoma cell line (H-2b) were obtained from the Cell Resource Center for Biomedical Research (Tohoku University, Sendai, Japan). E.G7-OVA is a cell line originally derived by transfecting the mouse lymphoma cell line EL4 (H-2b) with a plasmid expressing chicken ovalbumin (OVA) mRNA (obtained from the American Type Culture Collection, Manassas, VA). The 3LL-SA Lewis lung carcinoma cell line was provided by Dr K. Imaizumi (Nagoya University, Nagoya, Japan).23 The Colon-26 and 3LL-SA cell lines were maintained in complete RPMI 1640 media (10% fetal bovine serum, 100 μg/mL streptomycin, and 100 U/mL penicillin). E.G7-OVA was grown in complete RPMI 1640 media containing 400 μg/mL G418 (Invitrogen, Carlsbad, CA). DCs were generated from mouse bone marrow precursors in complete RPMI 1640 media with 10 ng/mL recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN) and 2 ng/mL recombinant mouse interleukin-4 (IL-4; R&D Systems), as described previously.24 25 All other cell lines were maintained in complete Dulbecco modified Eagle medium (DMEM).
Adenovirus vectors
AdNK4 and AdNull are structurally similar replication-deficient recombinant adenovirus type 5 (Ad5)–based vectors with E1 and E3 deletions in which the NK4 gene and no transgene, respectively, are under transcriptional control of the cytomegalovirus (CMV) immediate-early enhancer and promoter.14,26 The recombinant virus vectors were propagated, purified by CsCl gradient centrifugation, and titered by serial-dilution end-point assay.14,26,27 All vectors were free of replication competent adenovirus.14,26 27
Reverse transcriptase–polymerase chain reaction
To demonstrate the expression of NK4 mediated by the AdNK4 vector, total cellular RNA was extracted from the infected B16-F10 cells or tumors using ISOGEN (Nippon Gene, Tokyo, Japan), and 1 μg of RNA was subjected to reverse transcription using the RNA PCR kit (Takara Shuzo, Kyoto, Japan) at 42°C in a total volume of 20 μL. One tenth of the cDNA was amplified with the following primers specific for either vector-derived NK4 or the control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts: for NK4, 5′-GTGAGGCACTGGGCAGGTGTC-3′ and 5′-CTCGAGGATTTCGACAGTAGT-3′; for GAPDH, 5′-ATGGTGAAGGTCGGTGTGAACGGA-3′ and 5′-TTACTCCTTGGAGGCCATGTAGGC-3′. The amplification profile was 94°C for 2 minutes, 30 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 90 seconds. Each PCR was carried in a volume of 50 μL, and 5 μL of PCR products were resolved on a 1% agarose gel and stained with 0.5 μg/mL ethidium bromide.
Histologic analyses
To examine the in vivo activity of AdNK4, 3 days after the intratumoral injection of Ad vectors into 8-day established Colon-26 tumors, the tumors were removed, and frozen sections (5 μm thick) were prepared. Standard hematoxylin and eosin (H&E) staining, immunohistochemical staining for mouse CD31 (platelet endothelial cell adhesion molecule-1 [PECAM-1]), and the TUNEL (TdT-mediated dUTP nick-end labeling) assay were performed using the tumor sections. For immunohistochemistry, sections fixed in acetone were blocked with phosphate-buffered saline (PBS), pH 7 containing 5% normal goat serum, and incubated with either 12.5 μg/mL anti–mouse CD31 monoclonal antibody or isotype-matched rat IgG (Pharmingen, San Diego, CA) for 30 minutes. After washing, the samples were then incubated with 10 μg/mL goat anti–rat IgG labeled with fluorescein isothiocyanate (FITC; Jackson ImmunoResearch Laboratorie, West Grove, PA) for 30 minutes, followed by nuclear staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Dojindo Molecular Technologies, Gaithersburg, MD). For the TUNEL assay, apoptotic cell death was assessed in the tumor sections using the Apoptosis Detection System, Fluorescein kit (Promega, Madison, WI).
Tumor therapy model
Tumor cells (2 × 105 Colon-26 cells, 3 × 105 B16-F10 cells, 2 × 105 E.G7-OVA cells, 5 × 105 3LL-SA cells) were subcutaneously injected in the right flank of mice on day 0. On day 8, AdNK4 or AdNull (109 plaque-forming units [pfu]) was injected intratumorally, followed by the intratumoral injection of 2 × 105 DCs or vehicle alone (ie, PBS) on day 11. All injections were in 50 μL volume of PBS. The size of each tumor was assessed 3 times weekly by using calipers and was recorded by measuring the largest perpendicular diameters. Mice were killed when they appeared moribund or when their tumors reached 20 mm in diameter. MHC class I–deficient and MHC class II–deficient mice, DCs prepared from these knockout mice, or DCs labeled with PKH26 (Zynaxis, Malvern, PA) were used in the studies of Figure 6B, 6A, or 10, respectively.
Cytotoxic T lymphocytes
To assess the ability of the AdNK4/DC treatment to induce tumor-specific cytotoxic T lymphocytes (CTLs), tumor-bearing mice were treated with AdNK4 and DCs as described in “Tumor therapy model,” and splenocytes were isolated 10 days after the last treatment (day 21). Effector cells were obtained from coculturing splenocytes (3 × 106 cells) with mitomycin C–treated tumor cells (106 cells) corresponding to the treated tumor in 24-well culture plates. The mitomycin-C treatment (100 μg/mL) was performed for blocking cell division of tumor cells before coculture with splenocytes. After restimulation, viable effector cells were collected and tested for their ability to lyse target cells using the lactate dehydrogenase (LDH) cytotoxicity detection kit (Takara Shuzo). The percentage of cytotoxicity was calculated as 100 × ([experimental release] − [spontaneous release])/([maximal release] − [spontaneous release]). Spontaneous or maximal release was determined in the presence of either medium alone or 1% Triton X-100, respectively. In the experiment of Figure 8B, the levels of mouse interferon-γ (IFN-γ) and IL-4 released to the culture medium during the restimulation were assessed by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Proliferation and cytokine assays
E.G7-OVA tumor-bearing mice were treated with AdNK4 and DCs as described in “Tumor therapy model.” Nine days after the last treatment (day 20), splenocytes were prepared by mechanical dissociation, and splenic CD8+ T cells were collected using anti-CD8 magnetic beads (Miltenyi Biotec, Auburn, CA). In 96-well plates, 5 × 105 CD8+ T cells from the treated mice were cocultured with 5 × 104 irradiated DCs (30 Gy) in complete RPMI 1640 media supplemented with or without 50 μg/mL OVA (Sigma Chemical, St Louis, MO). After 4 days of culture, the supernatant was analyzed for the mouse IFN-γ content by ELISA (R&D Systems), and cell proliferation was measured by an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] Colorimetric Assay kit (Promega). The percentage of proliferation was calculated as 100 × ([experimental absorbance] − [background absorbance])/([absorbance at start of the coculture] − [background absorbance]).
Statistical analysis
All data are reported as mean ± standard error. Statistical comparison was made using the 2-tailed Student ttest, and a value of P < .05 was accepted as indicating significance.
Results
Adenovirus-mediated in vitro and in vivo expression of NK4
The expression of NK4 mediated by the AdNK4 vector was confirmed in vitro and in vivo by reverse transcriptase–polymerase chain reaction (RT-PCR) (Figure 1). RT-PCR analysis showed that 0.7-kb fragments corresponding to the NK4 mRNA were amplified with the total cellular RNA from AdNK4-transduced B16-F10 cells, but were not amplified with that from AdNull-transduced or nontransduced B16-F10 cells (Figure 1A). Comparable levels of expression of the housekeeping GAPDH gene in all B16-F10 cells demonstrated the intactness of the RNA. The NK4 mRNA expression was also confirmed in AdNK4-transduced Colon-26, 3LL-SA, and E.G7-OVA cells (not shown). Similar results were achieved in the RT-PCR analysis using B16-F10 tumors in vivo (Figure 1B). As expected, NK4 mRNA expression was detected only in AdNK4-transduced B16-F10 tumors. The intactness of the RNA samples was confirmed by amplification of ubiquitously expressed GAPDH mRNA in the AdNK4 and control AdNull-transduced B16-F10 tumors as well as in the nontransduced B16-F10 tumors.
In vitro and in vivo expression of AdNK4.
(A) Detection of AdNK4-mediated NK4 expression by RT-PCR in B16-F10 cells transduced with AdNK4. The generated cDNA from B16-F10 cells transduced with AdNK4, AdNull, or PBS alone at a multiplicity of infection of 100 for 3 days was amplified with primers for NK4 or GAPDH mRNA. (B) Detection of AdNK4-mediated NK4 expression by RT-PCR in B16-F10 tumors transduced with AdNK4. To demonstrate the expression of AdNK4 in vivo, C57Bl/6 mice were injected subcutaneously in the right flank with 3 × 105 B16-F10 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4, AdNull, or PBS alone. On day 11, the generated cDNA from the transduced B16-F10 tumors was amplified with primers for NK4 or GAPDH mRNA. For both panels, PCR products were resolved in a 1% agarose gel and stained with ethidium bromide.
In vitro and in vivo expression of AdNK4.
(A) Detection of AdNK4-mediated NK4 expression by RT-PCR in B16-F10 cells transduced with AdNK4. The generated cDNA from B16-F10 cells transduced with AdNK4, AdNull, or PBS alone at a multiplicity of infection of 100 for 3 days was amplified with primers for NK4 or GAPDH mRNA. (B) Detection of AdNK4-mediated NK4 expression by RT-PCR in B16-F10 tumors transduced with AdNK4. To demonstrate the expression of AdNK4 in vivo, C57Bl/6 mice were injected subcutaneously in the right flank with 3 × 105 B16-F10 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4, AdNull, or PBS alone. On day 11, the generated cDNA from the transduced B16-F10 tumors was amplified with primers for NK4 or GAPDH mRNA. For both panels, PCR products were resolved in a 1% agarose gel and stained with ethidium bromide.
In vivo effect of AdNK4 on angiogenesis
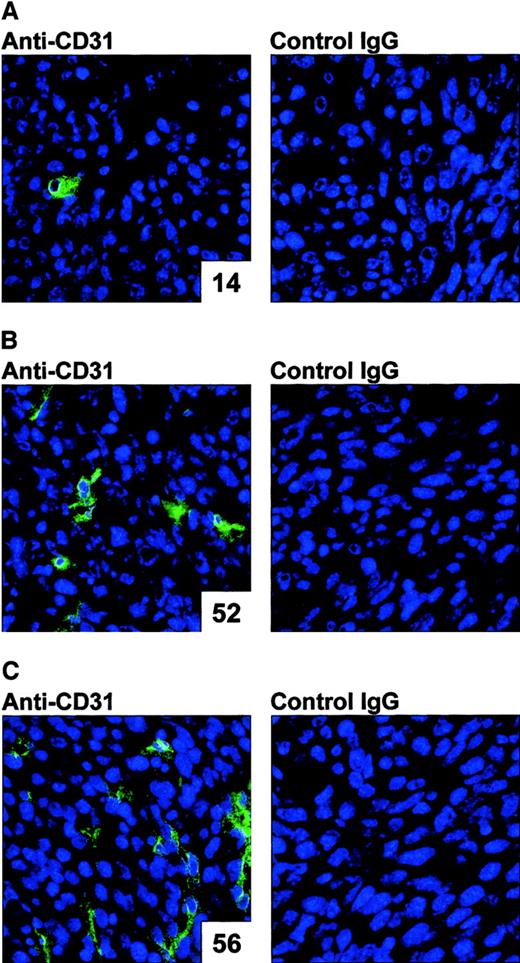
The AdNK4 effect on angiogenesis was confirmed in vivo (Figure2). To assess the antiangiogenic properties of AdNK4 in vivo, Colon-26 tumors injected with AdNK4 were assessed by immunohistochemistry for CD31, a surface marker of endothelial cells. The immunohistochemical assessment demonstrated a marked reduction of intratumoral vascularization within AdNK4-transduced tumors as compared with the AdNull-transduced or nontransduced tumors (number of CD31+ vessels per 10 random high-power fields: AdNK4, 14; AdNull, 52; no transduction, 56; left panels in Figure 2). With isotype-matched control IgG, no significant staining was observed in these tumors with or without Ad transduction (right panels in Figure 2). Similar results were achieved in AdNK4-, AdNull-, and nontransduced E.G7-OVA tumors (not shown).
Demonstration of the ability of AdNK4 to inhibit tumor vascularization.
For immunohistochemical evaluations for CD31, BALB/c mice were injected subcutaneously in the right flank with 2 × 105 Colon-26 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4 (A), AdNull (B), or PBS alone (no transduction, C). On day 11, tumors were dissected, and frozen tumor sections were stained using 12.5 μg/mL rat anti–mouse CD31 antibody, or isotype-matched control rat IgG followed by visualization with FITC-conjugated anti–rat IgG antibody. Nuclei were stained with DAPI (original magnification × 400). The number of CD31+ vessels per 10 random high-power fields is indicated in the lower right corner of each left panel.
Demonstration of the ability of AdNK4 to inhibit tumor vascularization.
For immunohistochemical evaluations for CD31, BALB/c mice were injected subcutaneously in the right flank with 2 × 105 Colon-26 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4 (A), AdNull (B), or PBS alone (no transduction, C). On day 11, tumors were dissected, and frozen tumor sections were stained using 12.5 μg/mL rat anti–mouse CD31 antibody, or isotype-matched control rat IgG followed by visualization with FITC-conjugated anti–rat IgG antibody. Nuclei were stained with DAPI (original magnification × 400). The number of CD31+ vessels per 10 random high-power fields is indicated in the lower right corner of each left panel.
Tumor necrosis and apoptosis induced by AdNK4
The AdNK4-induced inhibition of tumor vascularization resulted in tumor necrosis and apoptosis (Figures 3 and 4, respectively). Histological analysis (H&E staining) revealed extensive areas of necrosis within Colon-26 subcutaneous tumors after the direct administration of 109 pfu AdNK4 (Figure3A). In contrast, AdNull-transduced Colon-26 tumors had relatively small areas of necrosis (Figure 3B), and control Colon-26 tumors without Ad transduction had only some necrotic foci (Figure 3C). Similar results were observed in AdNK4-, AdNull-, and nontransduced E.G7-OVA tumors (not shown). Tumor cell apoptosis was then assessed with the transduced Colon-26 tumors by the TUNEL assay (Figure 4). The analysis demonstrated a marked increase of apoptotic cells in the AdNK4-transduced Colon-26 tumors as compared with AdNull-transduced and nontransduced Colon-26 tumors (number of apoptotic cells per 10 random high-power fields: AdNK4, 149; AdNull, 13; no transduction, 2; Figure 4). Similar results were achieved in AdNK4-, AdNull-, and nontransduced E.G7-OVA tumors (not shown).
Induction of tumor necrosis following intratumoral injection with AdNK4.
BALB/c mice with 8-day established subcutaneous Colon-26 tumors were treated with intratumoral injections of 109 pfu of AdNK4 (A), AdNull (B), or PBS alone (no transduction, C). Tumors were resected 3 days after injection, and frozen tumor sections were stained with H&E. Original magnification × 40.
Induction of tumor necrosis following intratumoral injection with AdNK4.
BALB/c mice with 8-day established subcutaneous Colon-26 tumors were treated with intratumoral injections of 109 pfu of AdNK4 (A), AdNull (B), or PBS alone (no transduction, C). Tumors were resected 3 days after injection, and frozen tumor sections were stained with H&E. Original magnification × 40.
In vivo analysis of apoptosis in Colon-26 tumors treated with AdNK4 using the TUNEL assay.
To demonstrate the apoptosis induced by AdNK4 in vivo, BALB/c mice were injected subcutaneously in the flank with 2 × 105Conlon-26 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4 (A), AdNull (B), or PBS alone (no transduction, C). On day 11, tumors were dissected, and in frozen tumor sections, the fragmented DNA of apoptotic cells were labeled by fluorescein-12–uridine triphosphate (UTP) incorporation at 3′-OH DNA ends. Following terminal transfer of fluorescein-12–UTP, sections were counterstained with propidium iodide (PI). Each fluorescent image was viewed for fluorescein (green fluorescence shown in the left panel) and PI (red fluorescence shown in the right panel). AdNK4-transduced tumors had some apoptotic cells, whereas control tumors (ie, AdNull-transduced and nontransduced tumors) had minimal apoptotic cells. Original magnification × 400. The number of apoptotic cells per 10 random high-power fields is indicated in the lower right corner of each left panel.
In vivo analysis of apoptosis in Colon-26 tumors treated with AdNK4 using the TUNEL assay.
To demonstrate the apoptosis induced by AdNK4 in vivo, BALB/c mice were injected subcutaneously in the flank with 2 × 105Conlon-26 cells (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4 (A), AdNull (B), or PBS alone (no transduction, C). On day 11, tumors were dissected, and in frozen tumor sections, the fragmented DNA of apoptotic cells were labeled by fluorescein-12–uridine triphosphate (UTP) incorporation at 3′-OH DNA ends. Following terminal transfer of fluorescein-12–UTP, sections were counterstained with propidium iodide (PI). Each fluorescent image was viewed for fluorescein (green fluorescence shown in the left panel) and PI (red fluorescence shown in the right panel). AdNK4-transduced tumors had some apoptotic cells, whereas control tumors (ie, AdNull-transduced and nontransduced tumors) had minimal apoptotic cells. Original magnification × 400. The number of apoptotic cells per 10 random high-power fields is indicated in the lower right corner of each left panel.
Synergistic antitumor effects by AdNK4 and DCs
The combination of AdNK4 with DCs elicited marked synergistic antitumor effects, resulting in suppression of the growth of all 4 established tumors (Figure 5). Intratumoral injection of 109 pfu of AdNK4 on day 8 into established Colon-26 tumors followed by direct injection of 2 × 105 DCs on day 11 significantly inhibited tumor growth in BALB/c mice at the time points from 13 to 17 days (P < .001 compared with all other groups, Figure 5A). Administration of AdNK4 + PBS or AdNull + DCs also had some therapeutic effect on the Colon-26 tumor growth compared with no treatment (day 11, P < .05 for AdNK4 + PBS; days 15-19, P < .05 for AdNull + DCs). Similar results were observed with B16-F10 and 3LL-SA tumors in C57Bl/6 mice (Figure 5B-C). Significant suppression of tumor growth was promoted by the combination of AdNK4 with DCs (B16-F10, days 13-16,P < .0005 compared with all other groups, Figure 5B; 3LL-SA, days 13-20, P < .005 compared with all other groups, Figure 5C). To a lesser extent, the size of tumors treated with AdNK4 + PBS or AdNull + DCs was suppressed significantly compared with the tumors without any treatment (B16-F10,P < .05 over days 11-16 for AdNK4 + PBS,P < .005 over days 13-16 for AdNull + DCs, Figure5B; 3LL-SA, P < .005 over days 11-15 for AdNK4 + PBS, P < .05 over days 13-15 for AdNull + DCs, Figure 5C). In the E.G7-OVA tumor model in C57Bl/6 mice, the tumor size of animals treated with the combination of AdNK4 with DCs also regressed significantly as compared with those of all other groups (days 13-18, P < .05, Figure 5D), whereas neither treatment with AdNK4 + PBS nor AdNull + DCs affected the growth of subcutaneous E.G7-OVA tumors as compared with no treatment (P > .05, Figure 5D). Although the growth of subcutaneous E.G7-OVA tumors after one-time injection of AdNK4 alone (109 pfu) on day 8 was not suppressed as the AdNK4 + PBS treatment in Figure 5D (P > .3 compared with no treatment), 2-time injections of AdNK4 (109 pfu) on days 8 and 10 significantly inhibited the tumor growth (P < .01 compared with no treatment), and the inhibitory effect was significantly augmented by 3-time injections on days 8, 10, and 12 (P < .001 compared with 2-time injection, not shown).
Suppression of growth of pre-existing tumors by intratumoral administration of AdNK4 and DCs.
(A) Colon-26 tumors, BALB/c mice. Colon-26 cells (2 × 105) were implanted subcutaneously in the right flank (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4, and 3 days later (day 11), tumors were inoculated with 2 × 105 DCs (▪). Alternatively, control mice were injected with AdNK4 followed by the day 11 administration of PBS instead of DCs (○) or injected with 109 pfu of AdNull followed by the day 11 inoculation of DCs (▵). (B) B16-F10 tumors, C57Bl/6 mice. This study was similar to that in panel A, but B16-F10 cells (3 × 105) were used. (C) 3LL-SA tumors, C57Bl/6 mice. This study was similar to that in panel A, but 3LL-SA cells (5 × 105) were used. (D) E.G7-OVA tumors, C57Bl/6 mice. This study was similar to that in panel A, but E.G7-OVA cells (2 × 105) were used. For all panels, the size of each tumor was assessed 3 times per week and is reported as the average tumor area (mm2) ± the standard error of n = 5 mice per group. Each experiment included tumor-bearing mice without any treatment as a control (■).
Suppression of growth of pre-existing tumors by intratumoral administration of AdNK4 and DCs.
(A) Colon-26 tumors, BALB/c mice. Colon-26 cells (2 × 105) were implanted subcutaneously in the right flank (day 0). On day 8, tumor-bearing mice were treated by intratumoral injection of 109 pfu of AdNK4, and 3 days later (day 11), tumors were inoculated with 2 × 105 DCs (▪). Alternatively, control mice were injected with AdNK4 followed by the day 11 administration of PBS instead of DCs (○) or injected with 109 pfu of AdNull followed by the day 11 inoculation of DCs (▵). (B) B16-F10 tumors, C57Bl/6 mice. This study was similar to that in panel A, but B16-F10 cells (3 × 105) were used. (C) 3LL-SA tumors, C57Bl/6 mice. This study was similar to that in panel A, but 3LL-SA cells (5 × 105) were used. (D) E.G7-OVA tumors, C57Bl/6 mice. This study was similar to that in panel A, but E.G7-OVA cells (2 × 105) were used. For all panels, the size of each tumor was assessed 3 times per week and is reported as the average tumor area (mm2) ± the standard error of n = 5 mice per group. Each experiment included tumor-bearing mice without any treatment as a control (■).
Dependence on MHC class I antigen presentation to CD8+ T cells
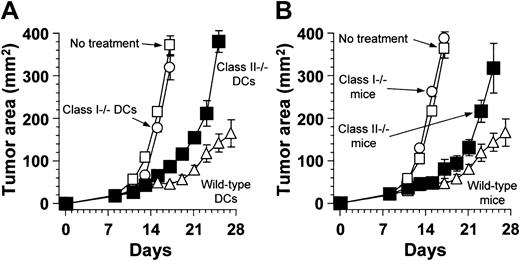
The tumor suppression induced by the combination of intratumor therapy with AdNK4 and DCs required mainly MHC class I antigen presentation of administered DCs and CD8+ T cells of the treated animals (Figure 6). On day 8, B16-F10 tumors established in wild-type C57Bl/6 mice were treated by direct injection of AdNK4, followed 3 days later by intratumoral administration of DCs that had been prepared from MHC class I–deficient (class I−/−), MHC class II–deficient (class II−/−), or wild-type C57Bl/6 mice. The tumors treated with AdNK4 and MHC class I–deficient DCs grew progressively, as did those in untreated animals (P > .1, Figure 6A). When injected into AdNK4-treated tumors, MHC class II–deficient DCs had some beneficial effect compared with MHC class I–deficient DCs as well as no treatment (days 13-17,P < .05, Figure 6A), but the antitumor effect of MHC class II–deficient DCs was partial as compared with that of wild-type DCs (days 17-25, P < .05, Figure 6A). These results on the role of MHC antigen presentation by administered DCs were reflected in the requirement for T-cell subsets of the treated host (Figure 6B). In this context, MHC class I–deficient, MHC class II–deficient, or wild-type mice bearing established B16-F10 tumors were treated with an intratumoral injection of AdNK4 on day 8 and DCs prepared from wild-type mice on day 11, because MHC class I–deficient or MHC class II–deficient mice lack CD8+ or CD4+ T cells, respectively.28 29 MHC class I deficiency completely abrogated the effect on the B16-F10 tumor regression elicited by the AdNK4 and DC combination therapy, and the tumors in MHC class I–deficient mice grew progressively as did those in wild-type mice without any treatment (P > .6, Figure 6B). MHC class II–deficient mice responded to the combination therapy of AdNK4 and DCs, resulting in significant suppression of tumor growth as compared with no treatment and MHC class I–deficient mice (days 13-17,P < .005, Figure 6B). However, the response of MHC class II–deficient mice to the combination therapy was less than that of wild-type mice (days 23-25, P < .05, Figure 6B).
The contribution of MHC class I presentation and CD8+ T cells in suppressing tumor growth by intratumoral administration of AdNK4 and DCs.
(A) Role of MHC class I and class II presentation of DCs coadministered with AdNK4. B16-F10 cells (3 × 105) were implanted subcutaneously in the right flank of wild-type C57Bl/6 mice (day 0). On day 8, established B16-F10 tumors were treated with AdNK4 (109 pfu), and 3 days later (day 11), tumors were inoculated with 2 × 105 DCs prepared from MHC class I–deficient (○), MHC class II–deficient (▪), or wild-type (▵) C57Bl/6 mice. (B) Role of CD4+ and CD8+ T cells in mice treated with AdNK4 and DCs. By subcutaneous injections of B16-F10 cells (3 × 105), tumors were established in the flank of MHC class I–deficient (ie, CD8+ T cell–deficient, ○), MHC class II–deficient (ie, CD4+ T cell–deficient, ▪), or wild-type (▵) C57Bl/6. Eight-day established B16-F10 tumors were treated with direct injection of AdNK4 followed by the injection of DCs prepared from wild-type C57Bl/6 mice 3 days later. For both panels, the size of each tumor was assessed 3 times per week and is reported as the average tumor area (mm2) ± the standard error of n = 5 mice per group. Each experiment included tumor-bearing wild-type mice without any treatment as a control (■).
The contribution of MHC class I presentation and CD8+ T cells in suppressing tumor growth by intratumoral administration of AdNK4 and DCs.
(A) Role of MHC class I and class II presentation of DCs coadministered with AdNK4. B16-F10 cells (3 × 105) were implanted subcutaneously in the right flank of wild-type C57Bl/6 mice (day 0). On day 8, established B16-F10 tumors were treated with AdNK4 (109 pfu), and 3 days later (day 11), tumors were inoculated with 2 × 105 DCs prepared from MHC class I–deficient (○), MHC class II–deficient (▪), or wild-type (▵) C57Bl/6 mice. (B) Role of CD4+ and CD8+ T cells in mice treated with AdNK4 and DCs. By subcutaneous injections of B16-F10 cells (3 × 105), tumors were established in the flank of MHC class I–deficient (ie, CD8+ T cell–deficient, ○), MHC class II–deficient (ie, CD4+ T cell–deficient, ▪), or wild-type (▵) C57Bl/6. Eight-day established B16-F10 tumors were treated with direct injection of AdNK4 followed by the injection of DCs prepared from wild-type C57Bl/6 mice 3 days later. For both panels, the size of each tumor was assessed 3 times per week and is reported as the average tumor area (mm2) ± the standard error of n = 5 mice per group. Each experiment included tumor-bearing wild-type mice without any treatment as a control (■).
Tumor-specific cytotoxic T cells induced by AdNK4 and DCs
The combination of intratumor therapy with AdNK4 and DCs to B16-F10 or E.G7-OVA tumors elicited B16-F10–specific or E.G7-OVA–specific CTL activity, respectively (Figure7). C57Bl/6 mice bearing B16-F10 tumors were treated by intratumoral injection with AdNK4 or AdNull on day 8, followed 3 days later by direct administration of DCs or PBS (Figure 7A-B). Effector cells were generated from splenocytes obtained 10 days after the last treatment by coculture with mitomycin C–treated B16-F10 cells, and then assayed for their ability to lyse B16-F10 cells (Figure7A) or control syngeneic E.G7-OVA cells (Figure 7B). Mice treated with the combination of AdNK4 + DCs exhibited a marked cytotoxic response against the parental B16-F10 tumor cells in their spleens, but no cytotoxic response was achieved using splenocytes from tumor-bearing mice treated with AdNK4 + PBS or AdNull + DCs, or without any treatment (Figure 7A). No splenic cytotoxic response was obtained against E.G7-OVA cells regardless of the treatment (Figure7B). Conversely, in the E.G7-OVA tumor model, only effectors from mice treated with AdNK4 and DCs demonstrated specific lysis of E.G7-OVA target cells (Figure 7C), whereas no apparent lysis was observed against irrelevant but syngeneic B16-F10 cells (Figure 7D).
Induction of tumor-specific CTLs by intratumoral administration of AdNK4 and DCs.
(A-B) C57Bl/6 mice with 8-day established subcutaneous B16-F10 tumors received injection of AdNK4 (▪) or AdNull (▵) followed by the injection of DCs on day 11, or received injection of AdNK4 followed by the injection of PBS on day 11 (○). Spleen cells were isolated 10 days after the last treatment and restimulated in vitro for 5 days with mitomycin C–treated B16-F10 cells. B16-F10 (A) or E.G7-OVA (B) cells were used as targets to assay the cytotoxic function of effector cells. (C-D) This study was identical to that described in panels A and B, except that E.G7-OVA cells, the control targets in panels A and B, were used as the different tumor type. Ten days after the last treatment, the splenocytes were isolated, restimulated with mitomycin C–treated E.G7-OVA cells, and assayed as in panels A and B. Shown are data for E.G7-OVA targets (C) and B16-F10 targets (D). Each experiment included tumor-bearing wild-type mice without any treatment as a control (■). For all panels, results are shown as the means ± the standard error (n = 3 per data point), and E/T denotes effector-target ratio.
Induction of tumor-specific CTLs by intratumoral administration of AdNK4 and DCs.
(A-B) C57Bl/6 mice with 8-day established subcutaneous B16-F10 tumors received injection of AdNK4 (▪) or AdNull (▵) followed by the injection of DCs on day 11, or received injection of AdNK4 followed by the injection of PBS on day 11 (○). Spleen cells were isolated 10 days after the last treatment and restimulated in vitro for 5 days with mitomycin C–treated B16-F10 cells. B16-F10 (A) or E.G7-OVA (B) cells were used as targets to assay the cytotoxic function of effector cells. (C-D) This study was identical to that described in panels A and B, except that E.G7-OVA cells, the control targets in panels A and B, were used as the different tumor type. Ten days after the last treatment, the splenocytes were isolated, restimulated with mitomycin C–treated E.G7-OVA cells, and assayed as in panels A and B. Shown are data for E.G7-OVA targets (C) and B16-F10 targets (D). Each experiment included tumor-bearing wild-type mice without any treatment as a control (■). For all panels, results are shown as the means ± the standard error (n = 3 per data point), and E/T denotes effector-target ratio.
Splenic responses of mice treated with AdNK4 and DCs
The combination treatment of AdNK4 + DCs elicited tumor-specific cytotoxic T-cell responses and cytokine-secreting reactivity in spleens of BALB/c mice bearing Colon-26 tumors (Figure8). Colon-26 tumors were treated by intratumoral inoculation of AdNK4 or AdNull on day 8, followed 3 days later by direct injection of DCs or PBS, and splenocytes of the treated mice were evaluated for their cytotoxic reactivity against Colon-26 or BALB/3T3 target cells after the in vitro restimulation (Figure 8A). Strong or weak cytotoxic responses against Colon-26 cells were achieved using splenocytes from mice treated with the combination of AdNK4 + DCs or AdNull + DCs, respectively. Other control groups of mice that were either nontreated or treated with AdNK4 + PBS exhibited no cytotoxic activity against Colon-26 cells. The tumor-specific nature of the cytotoxic activity against Colon-26 was demonstrated by the absence of apparent cytotoxicity against irrelevant but syngeneic fibroblast BALB/3T3 cells. Based on these results, splenocytes from Colon-26 tumor-bearing mice treated as described above were assayed for their cytokine profile upon in vitro restimulation with mitomycin C–treated Colon-26 cells (Figure 8B). Spleen cells from AdNK4/DCs-treated mice exhibited significant enhancement of IFN-γ production in the coculture with Colon-26 cells (P < .001 compared with control spleen cells from AdNK4/PBS-treated, AdNull/DC-treated, and nontreated mice). On the other hand, the coculture with the parental Colon-26 cells moderately induced IL-4 release from spleen cells of mice treated with AdNK4 + DCs and AdNull + DCs, and the level of IL-4 in the AdNK4/DC-treated group was 1.5-fold greater than that in the AdNull/DC-treated group (P < .005). No IL-4 release was observed with splenocytes from mice with the AdNK4 + PBS treatment or without any treatment.
Responses of splenocytes from tumor-bearing mice treated with AdNK4 and DCs.
(A) Tumor-specific CTL responses. Eight-day established subcutaneous Colon-26 tumors in BALB/c mice were treated with injection of AdNK4 (AdNK4 + DCs) or AdNull (AdNull + DCs) followed by the injection of DCs 3 days later, or treated with the injection of AdNK4 followed by the injection of PBS (AdNK4 + PBS). Controls included untreated tumor-bearing mice (no treatment). Splenocytes harvested 10 days after the last treatment were assayed for cytotoxic function against Colon-26 or BALB/3T3 target cells at an effector-target ratio of 60:1 after the in vitro restimulation. (B) Cytokine profile. Colon-26 tumor-bearing mice were treated identically to those described in panel A, and 3 × 106 spleen cells taken 10 days after the last treatment were cocultured for 5 days with 106mitomycin C–treated Colon-26 cells in 24-well culture plates. The culture medium was collected, and the levels of mouse IFN-γ and IL-4 were assayed by ELISA. For both panels, results are shown as the means ± the standard error (n = 3 per data point).
Responses of splenocytes from tumor-bearing mice treated with AdNK4 and DCs.
(A) Tumor-specific CTL responses. Eight-day established subcutaneous Colon-26 tumors in BALB/c mice were treated with injection of AdNK4 (AdNK4 + DCs) or AdNull (AdNull + DCs) followed by the injection of DCs 3 days later, or treated with the injection of AdNK4 followed by the injection of PBS (AdNK4 + PBS). Controls included untreated tumor-bearing mice (no treatment). Splenocytes harvested 10 days after the last treatment were assayed for cytotoxic function against Colon-26 or BALB/3T3 target cells at an effector-target ratio of 60:1 after the in vitro restimulation. (B) Cytokine profile. Colon-26 tumor-bearing mice were treated identically to those described in panel A, and 3 × 106 spleen cells taken 10 days after the last treatment were cocultured for 5 days with 106mitomycin C–treated Colon-26 cells in 24-well culture plates. The culture medium was collected, and the levels of mouse IFN-γ and IL-4 were assayed by ELISA. For both panels, results are shown as the means ± the standard error (n = 3 per data point).
Antigen-specific CD8+ T-cell responses induced by AdNK4 and DCs
CD8+ T cells from tumor-bearing mice treated with the combination of AdNK4 + DCs proliferated and secreted IFN-γ in a tumor antigen–specific manner (Figure9). In C57Bl/6 mice, E.G7-OVA tumors expressing ovalbumin as a model tumor antigen were treated with direct injection of AdNK4 or AdNull on day 8, and DCs or PBS on day 11. Splenic CD8+ T cells were isolated from treated animals 9 days after the last treatment and were assayed for their proliferating reactivity to ovalbumin in the presence of naive DCs as antigen-presenting cells (Figure 9A). CD8+ T cells from AdNK4/DC-treated mice markedly responded to ovalbumin (2-fold increase,P < .0005 compared with ovalbumin depletion, Figure 9A). In contrast, no proliferative response to ovalbumin was achieved using splenic CD8+ T cells from control mice nontreated or treated with AdNK4 + PBS or AdNull + DCs (P > .05 compared with ovalbumin depletion in each splenocyte type, Figure 9A). The antigen-specific CD8+T-cell proliferation from AdNK4/DC-treated mice was reflected in the IFN-γ level secreted from CD8+ T cells (Figure 9B). Although the addition of ovalbumin in the culture with splenic CD8+ T cells resulted in an increase of IFN-γ production regardless of the CD8+ T-cell type (P < .05,P < .05, P < .0005, orP < .005; AdNK4 + DCs, AdNK4 + PBS, AdNull + DCs, or no treatment compared with ovalbumin depletion in each splenocyte type, respectively, Figure 9B), the ovalbumin-stimulated IFN-γ level of CD8+ T cells from AdNK4/DC-treated mice was much greater than that of any other CD8+ T-cell types (P < .05, Figure 9B). Both AdNK4/DCs and AdNull/DCs treatments slightly induced nonspecific IFN-γ release from CD8+ T cells cultured without ovalbumin, whereas AdNK4/PBS treatment did not affect the IFN-γ production in ovalbumin-depleted culture, as was the case with no treatment.
Responses of CD8+ T cells from tumor-bearing mice treated with AdNK4 and DCs in an antigen-specific manner.
(A) Antigen-specific proliferation. C57Bl/6 mice with 8-day established subcutaneous E.G7-OVA tumors were inoculated with AdNK4 (AdNK4 + DCs) or AdNull (AdNull + DCs) followed 3 days later by intratumoral injection of DCs or inoculated with AdNK4 followed by the injection of PBS (AdNK4 + PBS). Controls included untreated tumor-bearing mice (no treatment). Nine days after the last treatment, splenic CD8+ T cells were isolated from treated or nontreated mice using the magnetic beads. In 96-well culture plates, 5 × 105 CD8+ T cells were cocultured for 4 days with 5 × 104 irradiated DCs with or without 50 μg/mL ovalbumin. The number of viable cells was determined using the MTS assay. The data are presented as the percentage increase over baseline on the initiation of the coculture. (B) IFN-γ production. In the termination of the coculture described above, the culture medium was collected, and the levels of murine IFN-γ were assayed by ELISA. For both panels, results are shown as the means ± the standard error (n = 3 per data point).
Responses of CD8+ T cells from tumor-bearing mice treated with AdNK4 and DCs in an antigen-specific manner.
(A) Antigen-specific proliferation. C57Bl/6 mice with 8-day established subcutaneous E.G7-OVA tumors were inoculated with AdNK4 (AdNK4 + DCs) or AdNull (AdNull + DCs) followed 3 days later by intratumoral injection of DCs or inoculated with AdNK4 followed by the injection of PBS (AdNK4 + PBS). Controls included untreated tumor-bearing mice (no treatment). Nine days after the last treatment, splenic CD8+ T cells were isolated from treated or nontreated mice using the magnetic beads. In 96-well culture plates, 5 × 105 CD8+ T cells were cocultured for 4 days with 5 × 104 irradiated DCs with or without 50 μg/mL ovalbumin. The number of viable cells was determined using the MTS assay. The data are presented as the percentage increase over baseline on the initiation of the coculture. (B) IFN-γ production. In the termination of the coculture described above, the culture medium was collected, and the levels of murine IFN-γ were assayed by ELISA. For both panels, results are shown as the means ± the standard error (n = 3 per data point).
Migration of administered DCs to spleens
When injected intratumorally to subcutaneous tumors, DCs migrated to lymphoid organs, including the spleens in vivo (Figure10). To track DCs administered into Colon-26 tumors established in the flank of BALB/c mice in vivo, DCs were labeled with red fluorescence PKH26 before administration. The PKH26-labeled DCs were intratumorally injected to AdNK4- or AdNull-treated tumors, and the spleens were harvested 3 days after the DC injection. An assessment of spleens from mice treated with AdNK4 + DCs and AdNull + DCs demonstrated cells labeled with red fluorescence (Figure 10A,C). In contrast, control spleens from mice nontreated or treated with AdNK4 + PBS had no red fluorescence–labeled cells in minimal auto-fluorescence (Figure10B,D). Interestingly, assessment of AdNK4-treated tumors 4 hours after the injection of PKH26-labeled DCs demonstrated some red fluorescence–labeled cells in areas of necrosis (not shown).
Demonstration of the ability of intratumorally inoculated DCs to traffic to the spleen.
BALB/c mice with 8-day established subcutaneous Colon-26 tumors received injections of AdNK4 (A) or AdNull (C) followed by the injection of PKH26 (red fluorescence)–labeled DCs on day 11, or received injections of AdNK4 followed by the injection of PBS (B). Controls included untreated tumor-bearing mice (D). Spleens were dissected 3 days after the last treatment, and the frozen spleen sections were examined with a fluorescence microscope. In all panels, nuclei were stained with DAPI (blue fluorescence). Original magnification × 400.
Demonstration of the ability of intratumorally inoculated DCs to traffic to the spleen.
BALB/c mice with 8-day established subcutaneous Colon-26 tumors received injections of AdNK4 (A) or AdNull (C) followed by the injection of PKH26 (red fluorescence)–labeled DCs on day 11, or received injections of AdNK4 followed by the injection of PBS (B). Controls included untreated tumor-bearing mice (D). Spleens were dissected 3 days after the last treatment, and the frozen spleen sections were examined with a fluorescence microscope. In all panels, nuclei were stained with DAPI (blue fluorescence). Original magnification × 400.
Discussion
In this study, we hypothesized that, when placed in the milieu of combined apoptosis and necrosis of a pre-existing tumor induced by an angiogenesis inhibitor NK4, DCs would acquire tumor-associated antigens from apoptotic tumor cells for the presentation and mature into activators of T-cell immunity by the necrotic stimuli in the tumor-bearing host, resulting in stimulation of tumor-specific immune responses that would suppress the growth of the tumor. Several pieces of evidence support this hypothesis. Local administration of the AdNK4 vector to tumors impaired tumor angiogenesis with a consequent increase of apoptotic and necrotic cell death in the AdNK4-treated tumors. Although the antitumor effects that followed the local delivery of the NK4 gene alone were only partial, subsequent injection of DCs to the AdNK4-pretreated tumors markedly intensified the antitumor effect. The observed tumor suppression was coupled with the induction of tumor-specific CTLs in vivo, and the involvement of CD8+T-cell immune responses specific to a tumor-associated antigen in their generation was suggested by the in vitro CD8+ T-cell proliferation and cytokine analyses. The studies carried out using knockout mice demonstrated that MHC class I antigen presentation by administered DCs to CD8+ T cells of the treated host is central to the therapeutic efficacy of DCs in combination with AdNK4 pretreatment.
Synergistic antitumor activity of AdNK4 and DCs
DCs, professional antigen-presenting cells located in most tissues, sample and process antigens and migrate to lymphoid organs, where they display antigens bound to MHC class I and class II molecules to stimulate CD8+ and CD4+ T cells, respectively.3,5,6 Evidence from the literature suggests that DCs cannot drive CD8+ T cells to expand clonally, produce cytokines, and develop into CTLs unless they first undergo maturation.3,5,6,30 The maturation of DCs is usually accomplished by multiple stimuli, including signaling molecules such as CD40 ligand on CD4+ helper T cells, inflammatory cytokines such as IL-1β and tumor necrosis factor-α (TNF-α), viral and microbial constituents such as double-stranded RNA and lipopolysaccharide, and unmethylated CpG oligonucleotides.3,5,6,30,31 Additionally, exposure to necrotic cells or their soluble derivatives, in contrast to apoptotic cells, was recently reported to be a requisite maturation signal for DCs.8,9 As necrotic cell death is typically associated with nonphysiologic settings such as inflammation and infection, DC maturation induced by necrotic stimuli is likely to be common.32,33 In regard to the relevance of DC biology to the 2 types of cell death (ie, apoptosis and necrosis), it also has been demonstrated that immature DCs efficiently phagocytose both necrotic and apoptotic cells and that only apoptotic cells, but not necrotic cells, can serve as sources of antigen for cross-presentation to CD8+ T cells.34
Based on the above, we envision the following scenario: first, when put in contact with combined apoptotic and necrotic tumor cells, DCs would acquire dying cells via phagocytosis and receive a maturation signal that could be provided by necrotic cells and/or their soluble derivatives; second, the matured DCs would migrate to lymphoid tissues and cross-present tumor-associated antigens from phagocytosed apoptotic cells on MHC class I molecules with the enhanced T-cell stimulatory capacity (eg, costimulatory molecules and cytokines); and third, CD8+ T cells would clonally expand in an antigen-specific manner and develop into tumor-lysing CTLs, resulting in the generation of antitumor immunity in the host.
This study extends this concept by using the angiogenesis inhibitor NK4. NK4 is composed of the NH2-terminal hairpin domain and subsequent 4-kringle domains of the α-subunit of HGF, and was originally prepared as a competitive antagonist for HGF.12In addition to its antagonistic function toward HGF, NK4 also has been shown to have an antiangiogenic activity.10,11,13 In this context, Kuba et al demonstrated that repetitive administration of recombinant NK4 slowed the rate of growth of lung and mammary carcinoma tumors subcutaneously implanted in mice, and that this delayed tumor growth was mediated by a remarkable decrease in the microvessel density within the NK4-treated tumors through a mechanism distinct from its activity as an HGF antagonist.11 We also have shown that in vivo genetic modification of human lung tumors with an adenovirus vector engineered to express NK4 (AdNK4) restrains tumor angiogenesis and suppresses tumor growth in vivo.14 Additionally, previous studies of cancer antiangiogenic therapy have suggested that the sustained blockage of the angiogenic process by angiogenesis inhibitors impedes tumor growth by driving tumor cells into apoptosis and/or necrosis.15-23 35 Therefore, impairment of tumor angiogenesis by genetic engineering of tumor cells to secrete NK4 most likely brings about apoptotic and necrotic tumor cells, which are expected to be favorable for eliciting antitumor immunity after subsequent DC injection.
The observations in this study are consistent with the idea that DCs injected into AdNK4-pretreated tumors efficiently initiate therapeutic antitumor immunity. After adenovirus gene transfer of the NK4 cDNA, NK4 expression could be detected only in AdNK4-injected tumors, where both tumor apoptosis and necrosis were revealed in association with reduced tumor vessel density. The suppression of tumor growth by AdNK4 alone (AdNK4 + PBS) in the present study was only partial as compared with that in our previous study because of differences in the experimental design, that is, single administration of AdNK4 in the present study as opposed to repetitive administration of AdNK4 in our previous study.14 However, the subsequent administration of DCs to AdNK4-pretreated tumors induced the development of tumor-specific CTLs in vivo, and the immune response correlated with the tumor regression obtained by the AdNK4/DC combination in the tumor therapy model. The observed antitumor response to the combined treatment with AdNK4 and DCs was completely abrogated when the injected DCs or treated animals were deficient in MHC class I antigen presentation or CD8+ T cells, respectively. Additional evidence for this concept comes from the observation that DCs administered in the flank tumor were detected in the spleen of the treated mice, suggesting that DCs can migrate from the tumor to the spleen.
Cancer immunotherapy and adjuvants
Tumors evade the adaptive immune response in various ways such as by the secretion of immunosuppressive cytokines (eg, transforming growth factor-β and IL-10) or down-regulation of MHC molecules or the tumor antigens themselves.1,2 It also has been proposed that immature DCs constantly sample apoptotic tumor cells in the absence of “danger signals” necessary for DC maturation and present the captured antigens without costimulation, thereby preventing the immune system from responding to the tumor, as in self-tolerance.1,2,31 Several strategies to break the tolerant environment in tumor-bearing hosts have been studied for cancer immunotherapy.1 2 In earlier immunotherapeutic strategies, inflammatory substances (eg, Bacillus Calmette-Guérin [BCG]) were tested as adjuvants for tumor immunity. More recently, immunologically defined adjuvants have been evaluated, including immunostimulatory cytokines, heat shock proteins, and genetic engineering to express costimulatory molecules.
In contrast to the strategy of converting tolerance to immunity by exogenous immunostimulatory molecules, the strategy in the present study of using necrotic cells induced by angiogenesis inhibitors as endogenous stimuli has several theoretical advantages of potential clinical interest. First, any tumor growth is, at least in part, angiogenesis-dependent, and hence, the desired response in tumors could be expected to result from the antiangiogenic treatment itself.35 Second, it is generally assumed that apoptosis as well as necrosis is induced by inhibiting tumor angiogenesis, and apoptotic tumor cells are likely the optimal source of multiple tumor-associated antigens to promote antitumor immunity. Albert et al reported that DCs exclusively cross-presented antigens from phagocytosed apoptotic cells, as opposed to necrotic cells, stimulating MHC class I–restricted CD8+ CTLs.34 Third, necrosis, but not apoptosis, is thought to be the common machinery of cell death associated with inflammation, and thus necrosis-derived stimuli might be the most physiologically relevant form of signals that would allow DCs to mature into potent immunostimulatory cells.32,33 Fourth, it has been reported that some cytokines associated with antitumor immunity (eg, IFN-γ) have a function of impairing tumor angiogenesis.36 The antitumor immunity induced by the angiogenesis inhibitor NK4 may further enhance its antiangiogenic effect. Finally, necrotic tumor cells induced by the angiogenesis inhibitor NK4 in vivo can directly activate the immune response without the possible loss of necrosis-derived stimuli by in vitro manipulation. Although heat shock proteins and cytokines provided by necrotic cells are known to be involved in DC maturation, both the nature of the activating molecules and the mechanisms underlying DC activation remain unclear.31 37 The involvement of the entire repertoire of necrosis-derived stimuli may be favorable for inducing the optimal antitumor response.
It is conceivable that the beneficial effect of the AdNK4 and DCs combination strategy in the present study depends mainly on the specific molecule (ie, NK4) and its specific delivery system (ie, gene transfer). Definitive proof of the general usefulness of angiogenesis inhibitors in stimulating antitumor immunity awaits further studies, using other angiogenesis inhibitors and delivery systems.
We thank Dr Yasuo Saijo for useful discussion and Brent Bell for reading the manuscript.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-04-1096.
Supported in part by Kurokawa Cancer Research Foundation, Sendai, Japan; Takeda Science Foundation, Osaka, Japan; Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (nos. 13218014, 13770293, 14021006, and 14030007).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshiaki Kikuchi, Department of Respiratory Oncology and Molecular Medicine, Institute of Development, Aging and Cancer, Tohoku University, 4-1 Seiryomachi, Aobaku, Sendai 980-8575, Japan; e-mail: kikuchi@idac.tohoku.ac.jp.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal