Abstract
With the recent success of the Bruton's tyrosine kinase (BTK) inhibitor, ibrutinib, and the phosphoinositide-3-kinase (PI3K) inhibitor, idelalisib, in the treatment of patients with relapsed or refractory non-Hodgkin's lymphoma (NHL), a number of new agents targeting the B-cell receptor (BCR) pathway are in clinical development. In addition, multiple trials combining these agents with conventional cytotoxic chemotherapy, immunomodulatory agents, monoclonal antibodies, or other kinase inhibitors are underway. This review will summarize the current data with the use of single agent and combination therapy with BCR inhibitors in NHL. In addition, commonly encountered as well as serious toxicities and hypothesized resistance mechanisms will be discussed. Lastly, this review will examine the future of these agents and opportunities to maneuver them into the front-line setting in selected NHL subtypes.
Learning Objectives
To gain an understanding of possible targets in the B-cell receptor signaling pathway including SYK, PKC-β, PI3K, and BTK and to identify drugs in clinical development inhibiting these kinases with preliminary efficacy in patients with recurrent NHL
To understand the Food and Drug Administration (FDA) approved indications for ibrutinib and idelalisib in the treatment of patients with mantle cell lymphoma, Waldenstrom's macroglobulinemia, and follicular lymphoma
To recognize common and potentially serious toxicities with ibrutinib and idelalisib in the treatment of patients with NHL
To gain an understanding of the results of recently published combination trials with ibrutinib and idelalisib in NHL
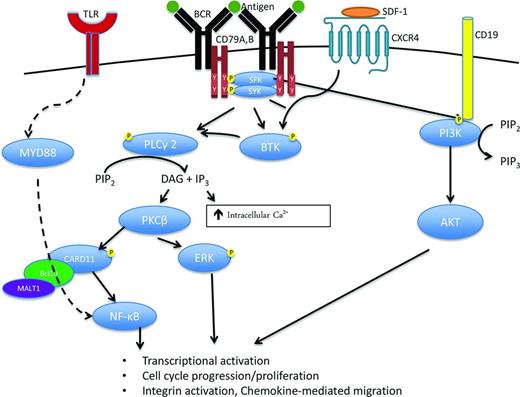
Figure 1 simplifies the complexity of the B-cell receptor (BCR) signaling pathway, depicting the interactions between the BCR, consisting of immunoglobulin heavy (IgH) and light chains (IgL), and CD79A/B with downstream signaling via spleen tyrosine kinase (SYK), Bruton's tyrosine kinase (BTK), phosphoinositide-3-kinase (PI3K), and protein kinase C-β (PKCβ). Specifically, antigen binding to BCR IgH and IgL triggers activation of immunoreceptor tyrosine-based activation motifs (ITAM) in CD79A and CD79B with subsequent SYK phosphorylation.1 SYK recruits B-cell linker protein which in turn phosphorylates BTK and phospholipase C-γ2 (PLCγ2). In addition, SRC-family kinases (SFKs) activated through IgH and IgL binding also phosphorylate CD19, which recruits PI3K to the BCR. These signals ultimately translate into nuclear factor-κB (NF-κB) and AKT activation, which promote proliferation and survival of normal and malignant B cells. Young and Staudt provide a detailed review of this pathway and its specific components in each subtype of B-cell non-Hodgkin's lymphoma (NHL).1 To date, 2 drugs inhibiting BTK and PI3K within the BCR signaling pathway have been FDA approved for the treatment of NHL. Ibrutinib is an oral, irreversible BTK inhibitor that binds cysteine 481 in the active site of BTK and is approved for the treatment of relapsed mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), and Waldenstrom's macroglobulinemia (WM). Idelalisib is an oral PI3-kinase inhibitor with specific activity against the PI3K-δ subunit and is approved for the treatment of relapsed or refractory follicular lymphoma (FL) and CLL.
B-Cell Receptor (BCR) signaling is a critical component of B cell activation, proliferation, survival, and migration in normal as well as malignant B cells. BCR activation, for example after antigen binding, induces CD79A and B immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation and subsequent recruitment of multiple kinases including spleen tyrosine kinase (SYK) and the SRC family kinases (SFK), LYN, SRC, and BLK. Activation of these kinases initiates the signaling cascade leads to phosphorylation, recruitment, and activation of other important kinases and signaling molecules including Bruton's tyrosine kinase (BTK), phospholipase C-gamma 2 (PLCγ2), protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K). The BCR signaling cascade ultimately leads to activation of multiple pro-survival pathways including ERK, NF-κB, and AKT signaling leading to increased transcriptional activation, proliferation, and migration. SDF-1 indicates stromal cell-derived factor 1; CXCR4, chemokine receptor 4; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol triphosphate; DAG, diacyl-glycerol; IP3, inositol triphosphate; and Ca2+, calcium.
B-Cell Receptor (BCR) signaling is a critical component of B cell activation, proliferation, survival, and migration in normal as well as malignant B cells. BCR activation, for example after antigen binding, induces CD79A and B immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation and subsequent recruitment of multiple kinases including spleen tyrosine kinase (SYK) and the SRC family kinases (SFK), LYN, SRC, and BLK. Activation of these kinases initiates the signaling cascade leads to phosphorylation, recruitment, and activation of other important kinases and signaling molecules including Bruton's tyrosine kinase (BTK), phospholipase C-gamma 2 (PLCγ2), protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K). The BCR signaling cascade ultimately leads to activation of multiple pro-survival pathways including ERK, NF-κB, and AKT signaling leading to increased transcriptional activation, proliferation, and migration. SDF-1 indicates stromal cell-derived factor 1; CXCR4, chemokine receptor 4; PIP2, phosphatidylinositol bisphosphate; PIP3, phosphatidylinositol triphosphate; DAG, diacyl-glycerol; IP3, inositol triphosphate; and Ca2+, calcium.
This article will primarily review published single agent data with ibrutinib and idelalisib in selected B-cell NHL subtypes including MCL, diffuse large B-cell lymphoma (DLBCL), FL, and WM. In addition, the activity of other BCR inhibitors including the PKC-β inhibitor, enzasturin, and the SYK inhibitor, fostamatinib, in these diseases will be discussed. Last, ongoing and recently completed combination trials with ibrutinib and idelalisib, the use of these agents in the front-line setting, and ongoing trials with novel BTK, PI3K, PKC- β, and SYK inhibitors will be reviewed with a focus on the future development of these agents and their incorporation into the treatment paradigms for MCL, DLBCL, FL, and WM.
Mantle cell lymphoma
The remarkable success of ibrutinib in patients with relapsed MCL led to the first FDA approval for a drug targeting the BCR pathway in NHL. In the initial phase 1 study,2 9 patients with MCL were enrolled and responses were observed in 7 including 3 complete responses (CRs; Table 1). Ninety-five percent BTK occupancy was observed at doses of at least 2.5 mg/kg/day and with fixed continuous dosing of 560 mg/day. These results prompted a multicenter phase 2 trial utilizing 560 mg/day of ibrutinib in patients with relapsed or refractory MCL (Table 1).3 Patients were classified as bortezomib-naïve or bortezomib-exposed (defined as 2 or more prior cycles of bortezomib). The overall response (OR) was 68% (21% CR), with no differences based on previous bortezomib exposure. These patients were heavily pretreated, having received a median of 3 prior therapies, including hyper-CVAD (30%) and autologous stem cell transplantation (ASCT; 11%). Forty-five percent were refractory to their last regimen and 49% were high risk by the simplified MCL International Prognostic Index (MIPI). Median time to response was 1.9 months and time to CR was 5.5 months. After a median follow-up of 27 months, the median response duration and progression free survival (PFS) were 17.5 and 13 months, respectively.4 At least 29 patients remained on therapy for >2 years. Grade 3-4 toxicities were relatively uncommon even with prolonged, continuous dosing and consisted of neutropenia (16%), thrombocytopenia (11%), diarrhea (6%), dyspnea (4%), and rash (2%). Notably, 4 patients developed subdural hematomas, all in association with aspirin or warfarin use, and it is routinely recommended that patients receiving ibrutinib avoid concomitant anticoagulants, particularly warfarin.
Completed single agent BCR trials in NHL
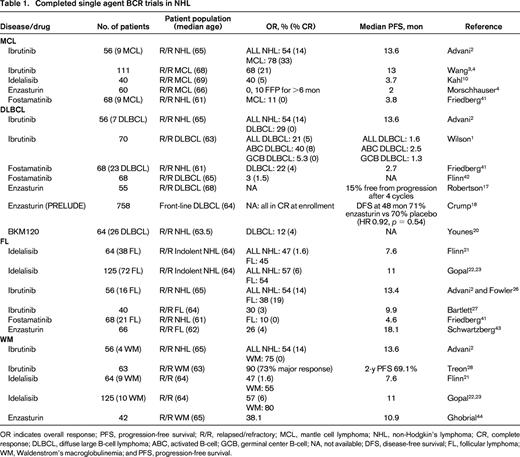
OR indicates overall response; PFS, progression-free survival; R/R, relapsed/refractory; MCL, mantle cell lymphoma; NHL, non-Hodgkin's lymphoma; CR, complete response; DLBCL, diffuse large B-cell lymphoma; ABC, activated B-cell; GCB, germinal center B-cell; NA, not available; DFS, disease-free survival; FL, follicular lymphoma; WM, Waldenstrom's macroglobulinemia; and PFS, progression-free survival.
Recently, a cysteine-to-serine mutation (C481S) in the BTK binding site and PLCγ2 gain of function mutations have been described in patients with CLL that progress while receiving ibrutinib.5 Although the data is limited, initial studies suggest that MCL patients who progress or relapse while receiving ibrutinib have poor outcomes and very few have these same mutations in BTK or PLCγ2. Martin et al described 106 patients with MCL either refractory or relapsed after ibrutinib.6 These 106 patients received ibrutinib for a median of 4 months (range, 0-42) and had a median overall survival of only 2.9 months (95% CI, 1.5-4.9 months) after cessation of ibrutinib. Pretreatment Ki-67, number of prior therapies, response to ibrutinib, or choice of therapy (bendamustine, alkylator, anthracycline, purine analog, cytarabine, lenalidomide, or bortezomib) after ibrutinib failed to correlate with OS in multivariate analysis. Only duration of ibrutinib and pretreatment MIPI were associated with OS. Similarly, in a separate series, Cheah and colleagues determined the median OS was only 8.4 months in 42 patients with MCL who discontinued ibrutinib.7 Using whole exome sequencing, Chiron et al identified 2 patients with partial responses (PRs) to ibrutinib of 14-30 months duration with C481S mutations in the BTK binding site in bone marrow and malignant pleural effusion samples obtained at relapse.8 However, these mutations were not present in serial blood and lymph node samples in 6 patients who failed to respond to ibrutinib or who had responses of <5 months duration. In 25 refractory patients enrolled on the multicenter phase 2 ibrutinib trial with disease progression at the first response assessment (2 months),3 1/25 patients had an identifiable PLCγ2 mutation and 0/25 had a C481S mutation in pretreatment samples.9 Therefore, collectively, these studies demonstrate that patients with MCL who fail to respond or progress on ibrutinib have very poor outcomes, with limited responses to subsequent salvage therapy, and very short OS. The mechanisms of this ibrutinib resistance in MCL are poorly understood, most patients do not have identifiable mutations in BTK or PLCγ2, and development of novel therapeutic agents for use post-ibrutinib is necessary. Unfortunately, based on the presently available data, screening for BTK or PLCγ2 mutations or identification of high-risk clinical factors (ie, Ki-67, pretreatment complex cytogenetics, MIPI, blastoid morphology, etc) cannot be used to reliably identify patients on ibrutinib who will fail to respond or progress.
Several smaller studies report the efficacy of other BCR inhibitors like idelalisib, duvelisib, enzasturin, or fostamatinib in MCL (Table 1). Forty patients with relapsed or refractory MCL were treated on a phase 1 study examining idelalisib at doses of 50-350 mg daily or twice daily.10 Similar to the previously described single agent ibrutinib phase 2 study, patients were heavily pretreated with a median of 4 prior therapies, 43% were refractory to their last therapy, 22% had a ASCT, and 35% had a high-risk MIPI. The maximum tolerated dose (MTD) was not reached, but based on pharmacokinetic data, a dose of 150 mg twice daily led to maximal steady state exposure. OR was 40% (CR 5%), median duration of response was 2.7 months, and median PFS of 3.7 months. In patients receiving at least 150 bid of idelalisib, the OR was 69%, although the PFS was no different at 3.7 months. Grade 3-4 toxicities included transaminitis (20%), diarrhea (17%), anorexia (15%), pneumonia (12.5%), neutropenia (10%), nausea (5%), weight loss (5%), and rash (2.5%). Therefore, although the OR with the PI3K inhibitor idelalisib is similar to that observed with ibrutinib in MCL, the median PFS is only 3.7 months, significantly shorter than the 13 months observed with ibrutinib. One potential explanation for the shortened response duration is that idelalisib is a selective PI3K-δ inhibitor and although PI3K-δ is highly expressed in MCL, PI3Kα is also up-regulated in relapsed MCL.11 In vitro, treatment with a dual PI3Kα/δ inhibitor was significantly more active than therapy with idelalisib alone.11
Diffuse large B-cell lymphoma
Gene expression profiling distinguishes two distinct types of DLBCL, germinal center B-cell (GCB) and the activated B-cell (ABC) subtype, with about 15% of cases not classifiable into these 2 categories.12 The ABC subtype of DLBCL is particularly dependent on BCR signaling.1 In ABC DLBCL, 10% of cases demonstrate mutations in CARD1113 (Figure 1) resulting in constitutive downstream activation of NF-kB. In addition, 20% of ABC DLBCL harbor mutations in the ITAMs of CD79A and CD79B13 resulting in downstream kinase activation of SYK, BTK, PI3K, and PKCβ. Last, ∼30% of ABC DLBCL have MYD88 mutations that directly activate the NF-κB pathway. Unlike ABC DLBCL, GCB DLBCL relies primarily upon PI3K/AKT activation rather than NF-κB activation, reviewed in detail by Davis and Staudt,1 and Sehn and Gascoyne.12
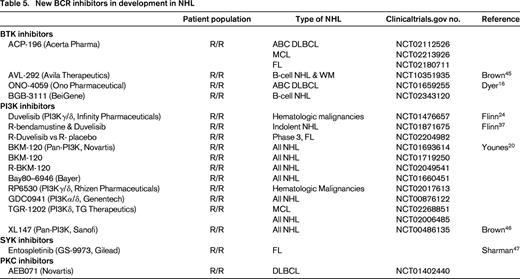
Based on the differences in these subtypes, one would postulate that ABC DLBCL would be particularly sensitive to inhibition of BTK, PKC-β, or SYK. Similarly, inhibition of PI3K may have greater relevance in GCB DLBCL. In a multicenter phase 2 trial of ibrutinib in patients with relapsed DLBCL, Wilson et al demonstrated that BTK inhibition is selectively effective in ABC DLBCL (Table 1).14 Responses were observed in 10 of 29 patients with ABC type DLBCL (40%), compared to 1 of 20 patients (5.3%) with GCB DLBCL. In ABC DLBCL, responses were seen in 3/5 patients with CD79B mutations, 4/4 patients with CD79B and MYD88 mutations, 0/4 patients with isolated MYD88 mutations, and 0/3 patients with CARD11 mutations. The median PFS in responding patients was 5.5 months. As a result of the promising single-agent activity of ibrutinib in ABC DLBCL and recent combination data confirming the safety of ibrutinib combined with RCHOP,15 a randomized phase 3 trial is evaluating the addition of ibrutinib to RCHOP as front-line therapy for patients with ABC DLBCL. In addition, several new BTK inhibitors including ACP-196, AVL-292, and ONO-4059 (see Table 5) are under evaluation in DLBCL. With ONO-4059, 13 patients with relapsed non-GCB DLBCL have been treated with doses ranging from 160 to 480 mg daily, and 6 of 8 evaluable patients have achieved a PR.16
Both SYK and PKC are downstream components of the BCR-pathway and potentially have therapeutic relevance in ABC DLBCL (Figure 1). In a phase 1/2 study of the SYK inhibitor, fostamatinib, in patients with DLBCL, 68 patients including 23 patients with DLBCL were treated at the MTD, with an OR of 22% (n = 5/23) in the DLBCL patients (Table 1).41 The cell of origin (ABC or GCB) of the responders wasn't available. This promising activity in DLBCL patients prompted a multicenter phase 2 study in which 68 patients were enrolled (58% GCB, 30% ABC, 12% unclassifiable).42 These patients had a median of 3 prior therapies and median age of 65 (range, 29-86). OR was only 3%, with responses or stable disease only observed in the patients of GCB or unclassifiable subtype and not ABC type. Mutations in MYD88, CD79A, or CD79B were not detected in the responders. Toxicities were primarily gastrointestinal (nausea or vomiting, 19%-21%), neutropenia (12%), or thrombocytopenia (13%). Therefore, although one would postulate that patients with mutations in BCR signaling, particularly at CD79A/B, would preferentially respond to SYK inhibition, this was not observed and the OR was disappointingly low. In vitro studies have previously demonstrated that fostamatinib can kill both SYK-independent GCB and ABC cell lines,13 suggesting that its cytotoxicity may be due to inhibition of other kinases and not directly due to inhibition of BCR signaling through SYK. A better understanding of fostamatinib's mechanism of action is warranted before this agent is evaluated further in DLBCL.
Enzasturin is a potent inhibitor of PKC-β, and in an initial phase 2 study in relapsed DLBCL, 15% of patients remained free from disease progression after 4 or more cycles.17 Four patients remained free from disease progression after 20-50 months on study drug. In a multicenter, randomized, placebo-controlled phase 3 trial (PRELUDE) reported at the American Society of Hematology (ASH) meeting in 2013,18 patients with DLBCL and International Prognostic Index ≥3 who achieved a CR after 6–8 cycles of RCHOP were randomized to receive 500 mg daily enzasturin or placebo for 3 years. In 758 patients, 504 received enzasturin and 254 received placebo, and disease free survival (DFS) at 24 and 48 months were 79% and 70% in the enzasturin arm and 75% and 71% in the placebo arm, respectively, with a DFS hazard ratio of 0.92 (95% CI 0.69, 1.22, p = 0.54) between the 2 arms. Therefore, there appeared to be no difference in DFS with enzasturin as a maintenance strategy in patients with IPI ≥3. Cell of origin has not yet been reported for the patients enrolled on the PRELUDE trial and hopefully future publications will examine the association of ABC and GCB subtype with PFS in both treatment arms.
Although gene expression profiling data suggests targeting the PI3K pathway may be relevant in GCB DLBCL, limited data exists to date utilizing PI3K inhibitors like idelalisib or duvelisib in aggressive NHL. No data is published regarding the use of idelalisib in DLBCL. In a phase 1 trial of duvelisib, a PI3K-δ/γ inhibitor, 2 patients with DLBCL were treated and none responded.19 However, an update in 2014 on this trial did describe PRs in 2 of 6 patients with aggressive lymphoma (DLBCL or transformed NHL), with both responders having failed prior ibrutinib although cell of origin isn't available. In a phase 2 study with a pan-PI3K inhibitor (inhibits all four isoforms: α, β, δ, and γ), 64 patients with relapsed B-cell NHL were enrolled including 26 patients with DLBCL.20 The OR was 12% in the DLBCL patients and 31% of patients had a decrease in tumor measurements. Two of the 3 responders remained on therapy at 9.2 and 7.4 months and grade 3-4 toxicities included hyperglycemia (23%), nausea (8%), neutropenia (8%), and depression (8%).
Follicular NHL
Phosphatidylinositol 3-kinase (PI3K) is a widely-expressed kinase in many tissues with four different isoforms: α, β, γ, and δ. The γ and δ subunits are restricted to hematopoietic cells with the δ subunit particularly relevant in BCR signaling and B-cell malignancy. Idelalisib, a selective PI3Kδ inhibitor represents the first PI3K inhibitor FDA approved (2014) for the treatment of relapsed FL. In a phase 1 trial using idelalisib doses of 50-350 mg daily or twice daily, a MTD was not defined.21 Based on pharmacokinetic data as well as consistently higher OR at doses ≥150 mg bid, this dose was selected at the recommended phase 2 dose. Commonly encountered grade 3-4 toxicities included transaminitis (23.4%), neutropenia (23.4%), thrombocytopenia (10.9%), pneumonia (17.2%), diarrhea (9.4%), anemia (4.7%), rash (3.1%), fever (3.1%), and hyperbilirubinemia (3.1%). In 54 evaluable patients with indolent NHL, lymph-node reductions were observed in 85% and the OR was 47% (CR 1.6%). Responses were observed in 45% patients with FL, 33% patients with MZL, and 55% with WM, with a median time to response of 1.3 months and duration of response of 18.4 months.21
The promising OR of 47% in patients with indolent NHL led to a multicenter phase 2 trial in patients relapsing after at least 2 prior therapies with rituximab and alkylator refractory disease (defined as less than a PR or disease progression within 6 months of rituximab and an alkylating agent).22 One hundred and twenty-five patients, including 72 patients with FL, received 150 mg bid of idelalisib until disease progression or unacceptable toxicity. In all 125 patients, the OR was 57%, with CR in 6%, median duration of response 12.5 months, and median PFS 11.0 months. Fifty-four percent of the 72 patients with FL responded. Grade 3 or higher toxicities included neutropenia (27%), diarrhea (13%), transaminitis (13%), pneumonia (7%), and thrombocytopenia (6%). With additional follow-up, the CR rate increased from 6% to 9.6%, with a median time to CR of 4.5 months and median duration of response of 13.9 months, including a 11.8 month response duration for patients with FL.23 The dual PI3K-δ/γ inhibitor, duvelisib is also under evaluation in indolent NHL.24 In a phase 1 study in 32 patients with FL, MZL, and WM treated with doses of 15-75 mg bid, OR was 65%.24 Five CRs occurred; all in patients with FL. Toxicities are similar to that observed with idelalisib, including transaminitis (41%), diarrhea (22%), and neutropenia (31%). Serious toxicities including colitis, transaminitis, and pneumonitis have been reported with idelalisib, duvelisib, and other PI3K inhibitors. Recently published expert panel recommendations on monitoring, evaluation, and treatment of these potential toxicities25 should be reviewed by anyone prescribing these agents and are summarized in Table 2.
Recommendations for the monitoring and treatment of serious toxicities with idelalisib25
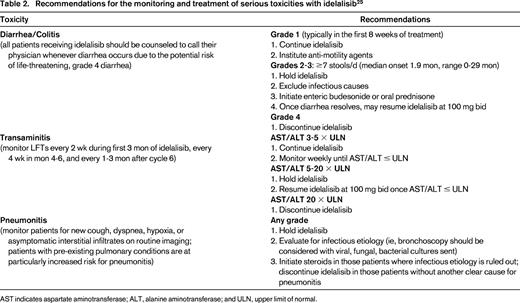
AST indicates aspartate aminotransferase; ALT, alanine aminotransferase; and ULN, upper limit of normal.
BTK inhibitors, including ibrutinib, are currently under evaluation in FL. In the initial phase 1 study of this agent,2 6 of 16 (38%) patients with FL responded, 3 with CRs.26 Bartlett and colleagues presented the results of a multicenter phase 2 study with ibrutinib in patients with relapsed or refractory FL at the ASH 2014 meeting.27 In 40 patients, the median age was 64 (range, 46-82), median prior number of therapies was 3 (range, 1-11), 55% had a FL International Prognostic Index (FLIPI) of 3-5, 45% were rituximab refractory, and 20% had a prior ASCT. The OR in all 40 patients was 30% with only 1 CR at a median follow-up of 6.5 months. Forty-two percent of patients with rituximab-sensitive disease responded compared with 11% of the rituximab refractory patients. Sixty-five percent of patients did demonstrate tumor reduction and some responses were delayed (median time to response 2.4 months, range 1.8-12.9 months), suggesting that additional responses might be observed with further follow-up.
These trials with idelalisib and ibrutinib in FL demonstrate differing efficacy with these agents than that observed in MCL. Whereas in MCL, the OR is higher and response durations appear longer with ibrutinib than idelalisib, the reverse appears to be true in FL. This finding demonstrates that the role of BCR signaling and the necessity of its components in B-cell survival likely differs across B-cell NHL subtypes and specific selection of BCR inhibitors by NHL subtype may be critical to optimizing efficacy and to the maintenance of remissions. Limited data with other inhibitors in the BCR signaling pathway like fostamatinib and enzasturin in FL suggests that the activity of these agents in FL is modest and these studies are summarized in Table 1.
Waldenstrom's
Unlike MCL, FL, and DLBCL, where ibrutinib and idelalisib have variable efficacy depending on NHL subtype, these 2 different agents targeting different components in the BCR signaling pathway both have significant activity in WM (Table 1). The FDA recently approved the use of ibrutinib for patients with relapsed/refractory WM after a multicenter phase 2 trial demonstrated an OR of 90% in this patient population.28 In this trial, ibrutinib was administered at a daily dose of 420 mg (note, this dose is lower than the standard 560 mg dose utilized in other NHL subtypes) to 63 patients with symptomatic WM. Patients were 63 years of age (range, 44-86), with a median of 2 prior therapies (range, 1-9) and adenopathy ≥1.5 cm in 59%. With therapy, the median IGM level decreased from 3520 to 880 mg/dL, median bone marrow involvement decreased from 60% to 25%, and median hemoglobin improved from 10.5 to 13.8 g/dL. Responses included very good PR in 10 patients, PR in 36 patients, and minor response in 11 patients, for an OR of 90% and major response of 73%. Median time to PR was 8 weeks, median duration of treatment was 19.1 months, and estimated 2 year PFS was 69.1%. The response rate was highest in patients with MYD88 mutations. Specifically, the OR was 100%, 85.7%, and 71.4% in patients with MYD88L265PCXCR4WT, MYD88L265PCXCR4WHIM, and MYD88WTCXCR4WT genotypes, respectively. Grade 3-4 toxicities included neutropenia (14%) or thrombocytopenia (13%). Four bleeding events including epistaxis and post-procedural bleeding were observed.
Similar to ibrutinib, idelalisib also has significant activity in WM, although fewer patients have received this agent. In the phase 1 trial of idelalisib in indolent NHL, responses were observed in 55% of the 9 patients with WM.21 In the subsequent multicenter phase 2 trial in patients with indolent NHL who had at least 2 prior therapies with alkylator and rituximab refractory disease,22 the OR was 80% in 10 patients with WM. The median duration of response in these patients has not yet been reached and median PFS was 22.2 months.23
Combination trials utilizing BCR inhibitors in NHL
Due to the favorable toxicity profiles and efficacy of ibrutinib and idelalisib, a number of studies have been recently completed or are underway examining combinations of these BCR inhibitors with conventional cytotoxic chemotherapy, immunotherapy, and other novel targeted drugs in the relapsed and front-line settings. These combination regimens may overcome potential resistance mechanisms, resulting in higher CR rates and prolonged response durations. Tables 3 and 4 list these combination trials in B-cell NHL.
Combination clinical trials with ibrutinib in NHL
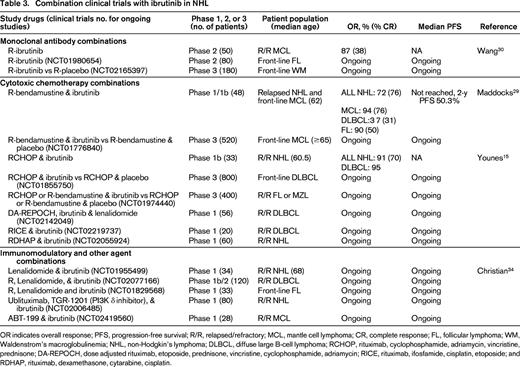
OR indicates overall response; PFS, progression-free survival; R/R, relapsed/refractory; MCL, mantle cell lymphoma; CR, complete response; FL, follicular lymphoma; WM, Waldenstrom's macroglobulinemia; NHL, non-Hodgkin's lymphoma; DLBCL, diffuse large B-cell lymphoma; RCHOP, rituximab, cyclophosphamide, adriamycin, vincristine, prednisone; DA-REPOCH, dose adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, adriamycin; RICE, rituximab, ifosfamide, cisplatin, etoposide; and RDHAP, rituximab, dexamethasone, cytarabine, cisplatin.
Combination clinical trials with idelalisib in patients with NHL
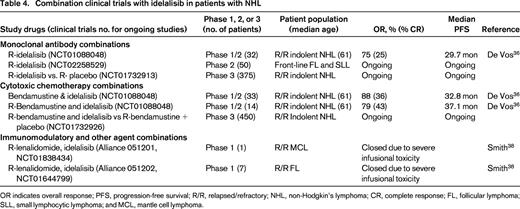
OR indicates overall response; PFS, progression-free survival; R/R, relapsed/refractory; NHL, non-Hodgkin's lymphoma; CR, complete response; FL, follicular lymphoma; SLL, small lymphocytic lymphoma; and MCL, mantle cell lymphoma.
We recently reported the results of a phase 1/1b study of rituximab (R)-bendamustine in combination with ibrutinib in patients with relapsed NHL.29 In this trial, 48 patients were enrolled and received rituximab 375 mg/m2 day 1, bendamustine 90 mg/m2 days 1 and 2, and ibrutinib 280-560 mg days 1-28 for 6 cycles followed by ibrutinib alone until disease progression. No dose limiting toxicity (DLT) was observed. Seventeen patients with newly diagnosed (n = 5) or relapsed MCL (n = 12) were treated and the OR in this group was 94% (76% CR). In addition, 16 patients with DLBCL (11 ABC type) and 12 patients with relapsed FL were enrolled with OR of 37% (31% CR) and 90% (50% CR), respectively. Four of the 6 DLBCL responders were ABC type by immunohistochemistry. Twenty-six of all 48 patients treated were able to complete 6 cycles of R-bendamustine and ibrutinib and continued to receive ibrutinib alone until disease progression. Median PFS has not been reached and the estimated 2 year PFS in all patients was 50.3 months. The only unexpected toxicity was a 25% incidence of grade 3 rash not attributable to bendamustine alone as 11 of the 12 patients with rash were retreated with full-dose R-bendamustine and dose reduced ibrutinib without recurrence of the rash. This combination of R-bendamustine and ibrutinib is now being studied in the front-line setting for patients over 65 with MCL (NCT01776840).
Similarly, a recent phase Ib trial demonstrated the safety of RCHOP in combination with ibrutinib.15 In this trial, 17 patients with DLBCL (n = 8), MCL (n = 5), and FL (n = 4) received 280-560 mg ibrutinib with standard RCHOP and 16 additional patients with DLBCL received the combination at the recommended phase 2 dose of 560 mg ibrutinib with RCHOP. The most common grade 3-4 events were neutropenia (73%), anemia (18%), and febrile neutropenia (18%). Twenty-nine of the 33 patients completed all 6 cycles; and OR was 91% (70% CR). This combination of RCHOP and ibrutinib is under evaluation in the front-line setting for patients with ABC DLBCL (NCT01855750).
Additional combinations of ibrutinib with immunotherapy are also being explored in patients with MCL. Wang et al reported the results of a phase 2 study of ibrutinib until progression with rituximab for up to 2 years.30 In 50 patients, the OR was 87% (CR 38%) and median PFS not yet reached. Fewer responses were observed in patients with Ki-67 staining of 50% or greater (OR 50% compared with 100% in patients with Ki-67 <50%). In contrast, in the analysis of ibrutinib non-responders by Martin,6 a number of patients (8 of 19) with Ki-67 <30% were found to have early ibrutinib failure with the single agent and this association of Ki-67 with response to ibrutinib needs to be validated in larger single-agent and combination trials.
Recent data suggests that ibrutinib may not only inhibit BTK, but also interleukin-2-inducible T-cell kinase (ITK) on T cells, altering Th1 and Th2 T-cell subsets.31 An intriguing preclinical study from Stanford suggests that combined therapy with ibrutinib and an anti-programmed death ligand 1 (PD-L1) antibody actually can suppress growth of lymphoma cell lines that are insensitive to ibrutinib in vivo, suggesting that this anti-tumor activity may not be due to direct B-cell cytotoxicity but due to enhanced T-cell immunity.32 Similarly, this same group demonstrated that combination therapy with ibrutinib and intratumoral injection of CpG oligodeoxynucleotides can induce lymphoma cell death of tumors at distant sites through stimulation of antigen-presenting cells via toll-like receptor 9 combined with enhanced T-cell autoimmunity.33 Both of these papers usher in the exciting concept of using ibrutinib not as a direct B-cell killer but rather as a mechanism to enhance T cell-mediated immunity that may have clinical applications in other hematologic malignancies and solid tumors.
In addition to the novel immunologic approaches described above with ibrutinib in lymphomas, ibrutinib is also under evaluation in combinations with lenalidomide and the bcl-2 inhibitor, ABT-199. At the ASH 2014 meeting, Christian et al reported the preliminary results of a phase 1 combination of ibrutinib and lenalidomide in patients with relapsed B-cell NHL.34 In this study, 7 patients received combination therapy with 15 mg lenalidomide and 420 mg ibrutinib with DLT in 2 patients consisting of ischemic stroke and grade 3 rash. Dose de-escalation to 10 mg lenalidomide and 280 ibrutinib in 6 patients was well tolerated and an intermediate dose of 10 mg lenalidomide and 420 mg ibrutinib is currently under evaluation. Responses have been observed in patients with DLBCL, MCL, and transformed NHL. Similar combinations of rituximab, lenalidomide, and ibrutinib are being evaluated in patients with FL front-line through Alliance Co-Operative group (NCT01829568) and in a multicenter study in patients with relapsed DLBCL (NCT02077166). Based on preclinical data in MCL demonstrating synergy of ibrutinib when combined with the Bcl-2 inhibitor, ABT19935 ; a multicenter phase 1 study of this combination is also underway (NCT02419560).
Combinations of idelalisib with cytotoxic chemotherapy like R-bendamustine and with immunomodulatory agents like lenalidomide are also under evaluation (Table 4). Idelalisib can be safely administered at 150 mg bid with rituximab, with bendamustine (90 mg/m2 days 1 and 2), or with R-bendamustine for up to 6 cycles.36 In 79 patients with indolent B-cell NHL treated on a phase 1 study with these combinations, grade 3-4 toxicities included neutropenia (41%), thrombocytopenia (8%), diarrhea (15%), rash (9%), pneumonia (19%), transaminitis (17%), pneumonitis (3%), and febrile neutropenia (3%). Eleven of 14 (79%) patients treated with R-bendamustine and idelalisib responded with 34% CR and a median PFS of 37.1 months. A similar phase 1 combination trial of rituximab, bendamustine, or R-bendamustine with duvelisib is ongoing in patients with relapsed NHL and CLL.37 Grade 3 rash was observed in 25% of patients receiving R-bendamustine and duvelisib, compared with only 14% with R-duvelisib.
The Alliance conducted two phase 1 studies of idelalisib, lenalidomide, and rituximab in patients with relapsed MCL and FL. Unfortunately, both of these studies closed prematurely due to significant, unexplained, and uncontrollable infusional type toxicity.38 Serious life-threatening toxicities were observed in 4 of 8 patients treated at doses of 10-15 mg qd of lenalidomide, 150 mg bid of idelalisib, and 375 mg/m2 rituximab weekly. These events consisted of grade 3-4 transaminitis, fevers, chills, hypotension, and rash on days 11-22 of cycle 1, requiring intensive care unit support in 3 patients. These studies and the prior studies combining R-bendamustine or lenalidomide with ibrutinib29,34 demonstrate that unexpected toxicities including life-threatening cytokine storm reactions, as well as severe rashes, can occur when these BCR-signaling inhibitors are combined with other agents. Careful phase 1 dose escalation and toxicity evaluation should be performed for every new combination regimen incorporating BCR inhibitors.
Future directions
Due to the recent success of the previously described combination trials with ibrutinib and idelalisib, a number of exciting trials are underway in MCL, DLBCL, FL, and WM utilizing these agents in the front-line setting. The results of these ongoing front-line trials will determine the impact these agents have as prolonged maintenance therapy and may ultimately shift the therapeutic paradigms from combination cytotoxic chemotherapy to less intensive, less toxic, and long-term kinase inhibitor therapy particularly for patients with FL, WM, and elderly patients with MCL. For younger MCL and DLBCL patients, these agents may become routinely incorporated into aggressive combination strategies with RCHOP, ASCT, and post-ASCT in an effort to eliminate minimal residual disease (MRD).
In MCL, trials are underway to explore the efficacy of ibrutinib in the front-line setting. As mentioned previously, a randomized phase 3 study is examining the impact of ibrutinib when added to R-bendamustine in previously untreated patients with MCL who are 65 years or older (NCT01776840). Depending on the results of this trial, one can also envision studies comparing R-bendamustine with ibrutinib to ibrutinib alone in elderly patients with MCL. For younger patients, future studies will likely incorporate ibrutinib into standard induction and ASCT regimens, will utilize ibrutinib as post-transplant maintenance therapy, and will explore its ability to eliminate MRD in patients who are still MRD positive post-transplant. One such planned study is the European TRIANGLE Study where patients ≤65 are randomized to one of 3 arms: 1. RCHOP/RDHAP induction followed by ASCT, 2. RCHOP/RDHAP with ibrutinib followed by ASCT and 2 years of ibrutinib maintenance, or 3. RCHOP/RDHAP with ibrutinib and 2 years of ibrutinib maintenance and no ASCT. This trial will be one of the first trials to provide data on the success of ibrutinib in the induction and post-ASCT maintenance settings in inducing MRD negativity in both high- and low-risk MIPI patients. The study will also examine if incorporation of ibrutinib into induction and maintenance therapies approaches PFS currently achieved with ASCT in young patients with MCL. Last, whereas most studies administer ibrutinib until progression, this trial will be the first to examine planned discontinuation of the drug at 2 years. Additional study will be needed to determine if patients who stop ibrutinib due to cost, toxicity, or achievement of CR can be retreated with similar efficacy upon disease progression or upon detection of MRD. Lastly, improving our ability to predict which patients will fail to respond or relapse early will be critical to the therapeutic advancement of ibrutinib in MCL. Additional study in these front-line trials of potential resistance mechanisms and clinical predictors of relapse (MIPI, Ki-67, complex karyotype, etc) will be critical.
In DLBCL, a number of combination studies utilizing ibrutinib with cytotoxic chemotherapy including RCHOP, REPOCH, RICE, and RDHAP are underway (Table 3). In the front-line setting, a randomized phase 3 trial of RCHOP versus RCHOP with ibrutinib in ABC DLBCL will determine whether the addition of ibrutinib can improve PFS in this poor prognostic group. This study could potentially usher in an era of cell of origin directed therapy in DLBCL. An Alliance sponsored trial incorporating ibrutinib into pre-transplant induction and post-transplant maintenance for patients with relapsed ABC DLBCL is also planned (Alliance A051301). In addition, novel combinations with lenalidomide, ABT-199, PD-1/PDL1 inhibitors, and monoclonal antibody conjugates may further improve the efficacy of ibrutinib in patients who relapse after ASCT. Further study of idelalisib alone and in combination with these novel agents may also provide new therapeutic regimens with potential efficacy in GCB DLBCL dependent upon PI3K signaling. Lastly, although multicenter trials of the SYK inhibitor, fostamatinib, and the PKC-β inhibitor, enzasturin, failed to confirm the efficacy observed in earlier phase 1 studies, newer SYK and PKC-β inhibitors are being evaluated in DLBCL that may potentially have greater target selectivity (Table 5). Next generation BTK, PI3K, SYK, and PKC-β inhibitors (Table 5) may also have greater efficacy in DLBCL patients.
In FL, a greater understanding of the differential activity observed with PI3K and BTK inhibitors is necessary, particularly with respect to the selection of the optimal agent to take forward into the front-line setting. In an effort to develop nontoxic oral regimens in this disease, use of BCR inhibitors alone or in combination with monoclonal antibodies or immunomodulatory therapy is increasingly relevant. Results from ongoing studies in the front-line setting including multicenter phase 2 studies of R-ibrutinib (NCT01980654), R-lenalidomide, and ibrutinib (NCT01829568), and R-idelalisib (NCT02258529) will inform the design of future randomized phase 3 studies comparing these regimens to conventional RCHOP or R-bendamustine chemotherapy. The identification of poor prognosis patients (ie, high FLIPI or early progression within 24 months) and analysis of their outcomes with these less-intensive approaches compared with conventional cytotoxic chemotherapy will be important. Additionally, long-term observation with respect to risks of late toxicities or infections, second malignancies, and transformation will be necessary in these trials.
In WM, both ibrutinib and idelalisib have significant activity in the relapsed setting, although fewer patients have been treated with idelalisib. Little is known about the impact of these agents on complications of WM including hyperviscosity, neuropathy, cold agglutinin induced anemia, amyloidosis, or cryoglobulinemia. An ambitious randomized phase 3 study (NCT02165397) will accrue ∼180 patients to determine whether combined R-ibrutinib is superior to rituximab alone in patients with newly diagnosed WM. This study will hopefully answer some of the questions regarding the incidence and resolution of common WM complications with long-term use of BCR-targeted therapy.
Correspondence
Kristie A. Blum, Associate Professor of Medicine, Section Head, Lymphoma Program, Division of Hematology, The Ohio State University, B315 Starling Loving Hall, 320 West 10th Ave, Columbus, OH 43210; Phone: 614-293-3507; Fax: 614-293-7484; e-mail: Kristie.blum@osumc.edu.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Cephalon.
Author notes
Off-label drug use: None disclosed.