Abstract
The role of autologous hematopoietic stem cell transplantation (ASCT) in the management of non-Hodgkin's lymphoma (NHL) is evolving, in the era of novel agents. Multiple histologies and remission stages have been impacted with changing outcomes. In the 1990s, ASCT could cure 50% of relapsed chemosensitive aggressive NHL; now the percentage maybe as low as 20% for patients relapsing within 1 year of completing rituximab-containing induction. Yet recent trials have clarified the value of first remission ASCT for high-grade NHL, the utility of augmented preparative regimens, the efficacy of ASCT in primary CNS lymphoma and in the elderly and analyses have defined strategies to reduce transplant related myeloid malignancies. In addition, optimizing nontransplant induction therapy for mantle cell and double-hit NHL is leading to improved outcomes and a re-examination of the use of ASCT in first complete remission. Caution is needed, however, as delaying transplants may mean that patients will need more morbid allogeneic transplants to achieve long-term control of refractory disease. As an alternative, maintenance therapy trials to improve ASCT outcome in high-risk patients are starting, based on the efficacy of lenolidomide and brentuximab in myeloma and Hodgkin's lymphoma, respectively. In addition, efforts to define early high-risk patients by minimal residual disease (MRD) assessments and genetic profiling, are beginning even for those with “indolent” phenotypes not currently autotransplanted. These efforts should not only refine but also enhance the value of early potentially curative ASCT, especially if novel agents only delay but do not prevent relapse for patients with NHL.
Learning Objectives
To understand the evolution of ASCT for lymphoma over the past 5 years considering the incorporation of novel agents into induction and salvage therapy
To discuss approaches to the use of ASCT in both first and second remission NHL
To update recently completed and planned trials for this form of therapy in NHL
At the Karnofsky lecture in 1983,1 E. Donnall Thomas stated that “there are major problems to be considered before autologous marrow transplantation will have any real role in the treatment of malignant disease.” In fact it was not until 1995 that the Phase III PARMA trial2 finally for the first time validated this form of therapy by demonstrating a survival benefit for patients with relapsed chemosensitive diffuse aggressive non-Hodgkin's lymphoma (NHL). Despite an explosion of subsequent autologous stem cell transplantation (ASCT) trials for all types of NHL, this survival benefit has not been consistently documented in any additional lymphoma subgroup. In fact, data exist that demonstrate that many relapsing patients in the rituximab era with diffuse aggressive NHL treated in a similar fashion to the PARMA trial may no longer benefit as significantly from this therapy.3 Yet given the chemosensitivity of this group of diseases and the high mortality rate of many of the NHL histologies, ASCT procedures continue to be performed buoyed by a decrease in their morbidity and mortality. In addition, data from Phase II studies or analyses of Phase III trials indicate that for certain subgroups there “may” be a survival benefit to ASCT. This however comes at a time when novel therapies for lymphoid malignancies are changing the natural history of these diseases, prompting a re-assessment of the “real role” of these intensive treatments.
Diffuse aggressive B-cell NHL
Relapsed/refractory disease
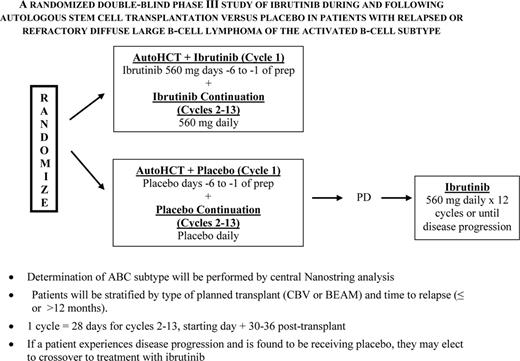
The PARMA trial indicated a 5 year survival advantage of 53% versus 32% for transplant versus ongoing conventional salvage therapy alone with a crossover design for patients relapsing with diffuse aggressive NHL, establishing this as the standard of care for this patient group2 As relapse has remained the primary cause of treatment failure, occurring in ∼40%, efforts to improve on this outcome have come largely from more aggressive salvage therapy prior to transplant, and alterations to transplant regimens. Rituximab added to standard salvage regimens did seem to improve responses for rituximab naïve patients, no difference in response rate was noted when R-DHAP was compared to the newer R-ICE regimen,3 and transplant outcome for relapsing patients was actually similar for these two groups as compared with the PARMA outcome, except perhaps for the germinal center type (GCB) of large-cell NHL, which may respond better to R-DHAP.4 Attempts to improve outcome by augmenting the chemotherapy dose intensity of the preparative regimen have been studied by SWOG and others and little benefit was noted. Based on Phase II trials5 and a prematurely-closed Phase III trial6 with 22 patients per arm data indicating that the combination of radioimmunotherapy combined with a standard preparative regimen was safe and led to a lower relapse rate, the BMT-Clinical Trials Network (BMT-CTN) conducted a Phase large III trial (0401) comparing BCNU, etoposide, cytosine arabinoside, and melphalan with rituximab (R-BEAM) ± 131I-tositumomab in chemosensitive relapsed or persistent diffuse large cell B-cell lymphoma (DLBCL).7 Unfortunately no difference in outcome was seen with a 2 year PFS of 48% for the radioimmunotherapy group versus 47% seen BEAM treated patients alone. Maintenance therapy with rituximab post-transplant has also been investigated and although potentially efficacious in mantle cell NHL (MCL) and indolent NHL, the majority of data in this setting indicate no benefit. However, despite these negative results and based on the efficacy of post-transplant maintenance therapy for multiple myeloma and Hodgkin's lymphoma, and the availability of effective novel targeted agents, this is an area of intense interest. Given the benefit of several novel agents including ibrutinib, lenalidomide, Syc inhibitors and anti-BCL2 agents in the ABC subtype of diffuse aggressive NHL, the use of these agents may have a favorable impact on survival post-transplant.8 One of these, ibrutinib is to be studied in a Phase III Alliance-led intergroup trial that will begin this year (Figure 1). So far no companion study for the GCB subtype is planned although several agents just entering clinical trials including EZH2 inhibitors and anti-BCL-6 compounds bear consideration if found to be efficacious and nontoxic in Phase II trials.
NCTN (alliance-led) and BMT-CTN postautograft phase II trial of ibrutinib for relapsed/refractory ABC NHL.
NCTN (alliance-led) and BMT-CTN postautograft phase II trial of ibrutinib for relapsed/refractory ABC NHL.
One thing has changed for this patient group however. With the improved outcome of patients receiving rituximab-based induction therapy for NHL, those who relapse early have a poor prognosis even with transplant. As indicated by the CORAL study those who relapsed after receiving rituximab based induction >12 months have a long-term survival similar to that seen for the transplant arm of the PARMA trial, whereas those relapsing in the first 12 months have an event free survival of only 20% and if in the high-intermediate or high IPI groups this drops to 18%, a similar survival rate to those entering transplant with disease nonresponsive to salvage chemotherapy (eg, bulk persistent disease >2 cm).3 Although CIBMTR data suggest a better outcome, this new data, as well as data indicating that PET positivity at transplant is as adverse,9 has led to many to no longer consider ASCTt for the former subgroup, rather an allograft with an anticipated long-term survival or 40%. Nevertheless, the treatment related mortality for allografts is such that even these high-risk patients should be considered for novel agent ASCT maintenance therapy trials.
Consolidation of first remission
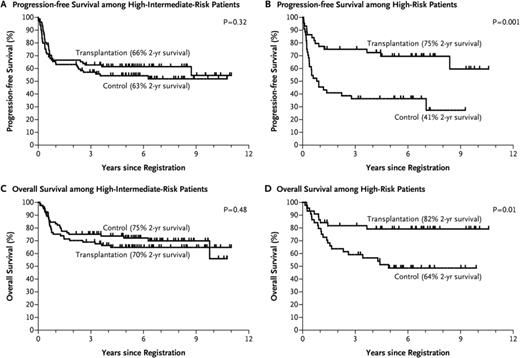
In the pre-rituximab era, although there were several trials indicating both a PFS and a survival benefit for patients undergoing consolidative autotransplant in first remission,11,12 the majority of such studies did not show a survival benefit. However, given the high dropout rate in many of these studies, and the inclusion in most the ∼15% who progress and die quickly, most of the studies were underpowered to answer the question. The US/Canadian Phase III intergroup led by SWOG (S9704) sought to answer the question in the rituximab era (although it began pre-rituximab) in high-risk patients with a design that eliminated most dropouts and early relapsing patients (patients randomized if responding after 5 courses of CHOP/R-CHOP).13 This design was similar to the LNH-87 study which in a retrospective subgroup analysis did show a survival advantage for transplant in the high and high-intermediate IPI subgroups.10 Although the prospective S9704 study showed a PFS advantage for transplant, owing to the success of salvage transplants for the conventional therapy group, there was no survival advantage for up-front transplant. In a retrospective subgroup analysis, those in the high IPI group at diagnosis did appear to have a survival advantage however (2 yr OS of 82 vs 64%; p = .01), leading many in the absence of a formal prospective trial to continue to perform autotransplants on this group, largely given the low PFS (2 yr PFS of 41%) for the conventional arm for this subgroup and the fact that IPI stage was a stratification factor for this trial (Figure 2). Of note, the trial results were similar for those receiving rituximab with induction versus those who did not. Other subgroups may also benefit from early transplant including those with C-Myc positivity as suggested in a subsequent analysis from this study.14 Although persistent PET positivity has also been considered a reason for an early autotransplant data would indicate that this is not always a reliable indicator of active disease after the completion of induction therapy.15
Survival rates among eligible patients who underwent randomization after 5 cycles of CHOP/CHOP-R to either CHOP/CHOP-R ×3 or CHOP/CHOP-R ×1 with an ASCT, according to IPI risk category. Reprinted from Stiff et al13 with permission.
Survival rates among eligible patients who underwent randomization after 5 cycles of CHOP/CHOP-R to either CHOP/CHOP-R ×3 or CHOP/CHOP-R ×1 with an ASCT, according to IPI risk category. Reprinted from Stiff et al13 with permission.
Indolent non-Hodgkin's lymphoma
Relapsed/refractorydisease
Although a pre-rituximab era randomized Phase III trial indicated a survival benefit for autotransplants in patients with relapsed follicular NHL with a 70 versus 42% 5 year OS (p = .026),16 the advent of more effective induction and maintenance, as well as salvage therapy incorporating rituximab, has seemingly eliminated this benefit in subsequent studies. However, a carefully done case-controlled analysis indicated that those in second complete remission (CR) who may indeed have a prolonged PFS beyond 8-12 years with autotransplant.17-19 As allografts frequently can “cure” these patients a randomized trial was begun in the BMT-CTN group, which compared autotransplant to reduced intensity allografts designed to further define the role of autotransplant in this setting. The trial was closed prematurely due to poor accrual likely due to the advent of a number of new effective salvage drugs/regimens, leaving transplant consideration to those with far advanced, bulky disease for which ASCT are ineffective. Of note, the NCTN Lymphoma Working Group is currently attempting to define and treat early, with new novel agents/combinations, those 20% of patients who do not enter a CR with chemoimmunotherapy induction or progress within the first 1-2 years who unlike the remaining 80% ultimately have a poor long-term survival. Although these high-risk patients would traditionally be candidates for an ASCT in first incomplete remission or after their typical early relapse following aggressive second line chemotherapy, it is hoped that if the novel therapy is effective in inducing a CR in this high-risk group, transplant would be delayed to better ascertain the long-term effectiveness of this therapy. If a CR however were not obtained, then an early ASCT would be pursued.
ASCT also continues to have a role in transformed follicular NHLs that are chemosensitive and have nonbulky disease after salvage chemotherapy (<2 cm max bulk), having a survival similar to those with diffuse aggressive NHL who have relapsed and seemingly better than continued conventional salvage therapy alone.20 Per a recent CIBMTR analysis, ASCT provides superior outcomes to allografts especially using myeloablative regimens.21 Later relapses do occur, albeit with the indolent phenotype however. A similar benefit is seen in patients with multiply relapsed Waldenstrom's macroglobulinemia or marginal-zone NHL, although the numbers of such patients treated are small. New effective novel agents are emerging for these patients, which may move ASCT to a later time point or eliminate it altogether. Again however, for drug-resistant disease a RIC allograft may be needed for optimal outcome by the time transplant is finally considered.
Consolidation of first remission
In the pre-rituximab era, multiple randomized trials indicated no benefit to consolidative ASCT, for indolent NHL although they did show an EFS/PFS benefit. This lack of a survival benefit was due to both effective salvage therapies and a higher second-malignancy risk for those undergoing early transplant. A subsequent GITMO trial that incorporated rituximab reported an identical outcome with a 4 year EFS and survival rate of 61% versus 28% and 81% versus 80%, for the transplant and conventional therapy groups, respectively, although follow-up time is short given the natural history of this disease.22 Although this option has largely been abandoned, due to the effectiveness of salvage therapies including autotransplant, a recent retrospective analysis of 203 autotransplants performed by the Spanish GELTAMO group in first CR indicates that in follow-up ≤15 years, there may indeed be a plateau in the PFS/OS curves for first remission autotransplant at >70% that might equate with cure.19 However, landmark analyses of CR1 patients treated with novel conventional regimens seem to yield similar results. Thus, until we can accurately define a truly high-risk group at diagnosis, it is unlikely that this modality will soon be retested in first remission patients.
Mantle-cell NHL
Relapsed/refractory disease
Given the early relapses seen in this type of NHL, most of the transplant clinical trial data have been in the first remission setting. However, with more effective standard dose induction regimens, particularly in the elderly or unfit patients, the PFS for patients with this disease not undergoing autotransplants is improving, making the case to defer transplant therapy for such high-risk patients. Early ASCT trials in patients with relapsed/refractory disease suggested little benefit for patients undergoing ASCT after relapse with the majority relapsing before 2 years, which led to an even bigger push for first remission transplants. Subsequent trials have been few and small in size with most recommending an allograft after first relapse. The largest autotransplant trials to date are from the Seattle group initially incorporating 131I-tositumomab with high-dose etoposide and cyclophosphamide based on earlier data indicating a more favorable outcome for patients undergoing radiation-based preparative regimens from the EBMT for patients with MCL in first PR. In this Seattle study a 3 year PFS and survival of 61% and 91% were seen. A follow-up study of 67 patients indicated that those without a high MIPI score at transplant or B symptoms at diagnosis and a higher remission quotient (months from diagnosis to autotransplant divided by the number of prior therapies) had a 5 year PFS of 58% despite the fact that the favorable risk group accounted for only 1/3 of their patients. Recently the CIBMTR reported a series of MCL transplants from 1996-2007 including 132 patients who underwent ASCT for relapsed/refractory disease of whom 51% were in a CR. Although the 5 year survival was 44% for the entire cohort, seemingly better than earlier analyses, there was again no plateau in the relapse curve. With the percentage and the total number of patients not transplanted in CR1/PR1 likely to grow with the availability of more effective induction therapies and the development of effective novel salvage therapies developed leading to a higher percentage with a second or subsequent CR, the numbers of late autotransplant eligible patients will likely grow. This population then should be prospectively evaluated. For those relapsing without favorable prognostic factors and a minimal disease state, a potentially curative allograft is still currently recommended.
Consolidation of first remission
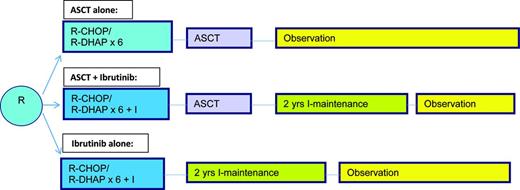
Given the dismal outcome of patients diagnosed with this disease first reported by SWOG in 1995,27 many have considered ASCT to be an important component of induction therapy. Indeed a European study indicated that compared with interferon therapy after an initial remission with non-rituximab based induction, an autotransplant led to a PFS of nearly 4 years and a borderline survival advantage for patients with this disease.28 Longer follow-up in an intent-to-treat subset did show a survival advantage although none was seen for the entire group.29 This led to the wide scale adaptation of this strategy, with subsequent trials building on the success of this first trial; however, no additional randomized trials re-examining transplant have been performed. As the CR subgroup had the best outcome,27 subsequent efforts have been focused on optimizing the pretransplant induction therapy and significant advances have been made incorporating both rituximab and cytosine arabinoside into the induction followed by ASCT,30,31 with time to treatment failure now in excess of 8 years. As there is emerging increasing data in the non-fit patients that nontransplant therapy can lead to prolonged remissions, the emergence of even more effective yet less toxic induction therapy with novel agents there is a shift away form doing first remission ASCT. With the emergence of a molecular marker for this disease that is not only predictive but can be easily followed during the course of therapy,32 and retrospective analyses indicating a similar outcome for aggressive induction regimens to first remission transplants,33 it is appropriate at this time to reconsider the use of autotransplants in the young, fit patient. Such a study is to begin in late 2015 (TRIANGLE) in Europe and Great Britain (Figure 3), which incorporates perhaps the most effective novel agent reported for this disease to date (per a recent meta-analysis) ibrutinib. An alternative approach would be to reserve autotransplants to those clinically responding but with residual molecular positive disease after induction. Until then it would appear that ASCT should continue to be routinely performed for those in CR1, but especially those in CR1 with very poor risk features (high Ki67+, blastic subtype, SOX11-positive), those with a high MCL International Prognostic Index (MIPI) score, those with a PR to induction or those who required multiple regimens to achieve a clinical CR.
EU MCLNet younger TRIANGLE study design for fit patients with newly diagnosed MCL (provided by M. Dreyling, University of Munich, Munich, Germany).
EU MCLNet younger TRIANGLE study design for fit patients with newly diagnosed MCL (provided by M. Dreyling, University of Munich, Munich, Germany).
T-cell NHL
Relapsed/refractory disease
Given both the rarity and heterogeneity of this group of lymphomas, and the poor prognosis of all subtypes other than ALK+ anaplastic large cell lymphoma (ALCL) most transplants for T-cell disease are performed in first remission. ASCTs after initial relapse, leads to a survival similar to B-cell aggressive lymphomas, with the best outcome for patients in a second remission with chemosensitivity, a low secondary IPI classification and most often the anaplastic large cell phenotype.34,35 However the number of such patients is quite small making it difficult to draw firm conclusions due to the high percentage of relapsed patients who are ineligible for ASCT due to either refractory disease or an early progression after a partial response to salvage therapy. Because of this RIC allografts are being increasingly performed for this patient group with either similar or slightly better outcomes compared to ASCT as demonstrated in a recent CIBMTR analysis.36 Effective novel agent therapy may change this in the future, but finding agents, other than brentuximab for CD30-positive disease, that are as effective as some of those recently approved for B-cell NHL has been elusive.
Consolidation of first remission
The prognosis of aggressive T-cell NHL, treated with standard induction therapy and leaving out ALK+ ALCL is ∼30% at 5 years. Although there are no randomized clinical trials, prospective first remission autotransplant Phase II trials for this patient group indicate a 40%-50% 5 year survival, a seemingly better outcome.37 A recent prospective trial of the Nordic Oncology Group treated 160 patients with T-cell disease, excluding Alk+ ALCL with dose-dense induction followed by an autotransplant demonstrating a 5 year survival of 51%, which included 26% who dropped out early due to progressive disease.38 Retrospective trial reports, ie, those just considering just transplant outcome report a higher survival for patients with chemosensitive disease in most analyses of 60%-70% but these exclude up to 1/3 of patients who dropout due to early progression of disease. The S9704 US/Canadian intergroup trial included T-cell disease in the design of the trial and this subgroup when analyzed had the same outcome as those with B cell disease, ie, an improved PFS but not OS. As they were also included in the retrospective analysis of the high IPI group only, which suggested a survival advantage to ASCT, one could make the case for first remission ASCT for those with high IPI disease. Although together these results seem to suggest a benefit for early ASCT, 50% of all patient with aggressive T-cell NHL die early of their disease suggesting to some that allografts be used in first remission to take advantage of a graft versus lymphoma effect. However, available data does not suggest a significantly higher survival rate as indicated by a prospective study that randomized patients based on the availability of a donor to an auto- or allotransplant.39
Other indications
Double-hit diffuse aggressive NHL
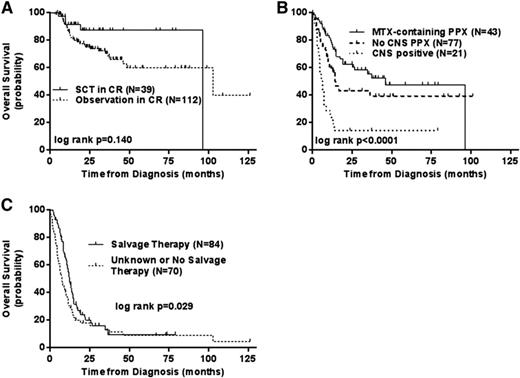
Although only ∼7% of all diffuse aggressive B-cell NHLs, this subgroup has traditionally had a very poor prognosis with survivals usually <1 year. Defined as being double-hit based on cytogenetic or FISH rearrangements of the MYC and either the BCL2 of BCL6 genes the WHO classification for this group of tumors is usually B-cell unclassifiable with features between DLBCL and Burkitt's NHL. Given the high-risk features and the adverse prognosis, these patients have long been considered appropriate candidates for a consolidative auto- or allotransplant if an initial remission was obtained and data from small series has reported a favorable outcome for this strategy. However recent large retrospective analyses have suggested that transplants are either ineffective or unnecessary.40,42 The first is a retrospective analysis of 311 patients from 23 centers in which patients were treated with a variety of rituximab-based induction regimens followed in 39 by a consolidative transplant (28 auto, 11 allo) in CR. The authors found only a trend for a difference in outcome for these CR1 patients for either type of transplant compared with observation alone (p = .14). However, firm conclusions are hard to claim as only 13% of patients underwent transplant (Figure 4).40 What was important in this analysis was the validation that dose adjusted EPOCH-R, or other aggressive induction regimens produce the highest CR rates for these patients as compared to standard R-CHOP This is similar to the MD Anderson single institution experience who also reported that although there was an improvement in 2 year EFS of 67% versus 25% for R-EPOCH over R-CHOP in 129 patients.42 Like the first report, there was again only a trend to a benefit to a consolidative transplant (p = .155). With both series indicating a trend to a transplant benefit but both suffering from patient selection bias for transplant, to fully ascertain the benefit to transplant in CR1 a randomized trial would need to be performed. For those with relapsed/refractory disease unfortunately any salvage transplant therapy is associated with a <20% long-term survival.
Retrospective analysis of the outcome of double-hit-positive NHL based on the use of transplant (A), CNS involvement or prophylaxis (B), or the use of salvage therapy after relapse (C). Reprinted from Petrich et al40 with permission.
Retrospective analysis of the outcome of double-hit-positive NHL based on the use of transplant (A), CNS involvement or prophylaxis (B), or the use of salvage therapy after relapse (C). Reprinted from Petrich et al40 with permission.
Although only ∼7% of diffuse-aggressive NHLs are double-hit by cytogenetic/FISH analysis, another 20%-30% have only MYC/BCL2 protein overexpression. This subgroup may have an improved survival as compared to the true double-hit patients but still inferior to non-overexpressers. Whether this group benefits from an autotransplant is unknown but a retrospective analysis performed on the S9704 trial did suggest a trend to an improved survival for those with MYC protein overproduction who were randomized to transplant, albeit in a very small cohort of patients and in patients treated with what is likely inferior induction with CHOP/R-CHOP. More aggressive induction therapy may negate this effect as well if a CR is obtained.43 For now however, ASCT is an acceptable therapy for the management of these patients in first remission, especially if only a PR.
Primary CNS lymphoma
ASCT for primary CNS NHL based on very recent data, is one of the least controversial uses of ASCT to treat patients with NHL. Treated with standard NHL regimens with radiation consolidation or high-dose methotrexate/high-dose cytosine arabinoside regimens alone, a 50% long-term PFS rate is seen. Although the high incidence of cognitive abnormalities has been essentially eliminated by the chemotherapy only approach, the high relapse rate has led to the consideration of autotransplants as part of primary therapy. The Memorial Sloan–Ketteing group recently reported on such a combination of rituximab, procarbazine, vincristine, and high-dose methotrexate followed by an ASCT with thiotepa, cyclophosphamide, and busulfan demonstrating a 2 year PFS of 79% and a 2 year OS of 81%.44 Importantly this non-radiation-based treatment schema was associated with little cognitive changes. These results are similar to a report from Boston where a similar 2 year PFS of 81% using an identical transplant regimen.45 Based on these favorable results an Alliance-led Phase III trial is nearing completion and should validate the role of autotransplant in this condition.
Elderly patients
Until recently autotransplants were reserved for patients under age 70, with data from the EBMT showing in a registry analysis a higher nonrelapse mortality and shorter 3 year PFS (51% vs 62%) and OS (60%-70%) for patients undergoing transplant for diffuse aggressive NHL when patients >60 were compared to younger patients.45 However the elderly had higher risk disease, a higher percentage of having received 2 or more regimens and a higher percentage of prior rituximab, suggesting that although differences do occur as patients age, consideration of transplants in the >60 were appropriate to consider. Recent data suggest that this is indeed the case with age not being an adverse prognostic factor for an ASCT outcome considering patient age to 70 and beyond.45,46 In lieu of age, the newer geriatric assessment tools are being investigated as a means of patient selection and should provide guidance into which elderly patients are at high-risk of a life-threatening ASCT complication.
Summary
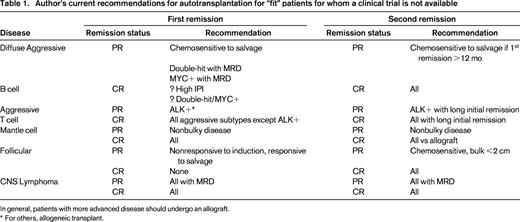
Although ASCT still currently plays a significant role in the management of NHL (Table 1), it is expected that over time, based on carefully controlled clinical trials that incorporate novel agents into induction and salvage regimens, that its use will evolve. A decrease in histology-based transplants will likely give way, at least in part, to targeted transplants based on genomic fingerprint or molecular measurements of minimal residual disease, and will include histologies not now considered appropriate for such therapy. With fewer first remission transplants, and more effective standard dose salvage therapies, the percentage of patients referred for ASCT with drug-resistant disease will likely increase. ASCT for this group will need to improve and consolidation and/or maintenance therapy with novel therapies after transplant is one such strategy that appears to have some promise. Otherwise, an increasing percentage of patients with NHL needing a transplant will be required to undergo potentially more risky allografts to achieve long-term remissions for their drug resistant disease. Finally, although there may be clinical benefit for prolonged nontransplant novel therapy in lieu of ASCT, the relative costs of these two options need to be considered going forward to achieve not only the best clinical outcome, but also the least costly.
Correspondence
Patrick Stiff, Cardinal Bernardin Cancer Center, Loyola University Medical Center, 2160 S First Ave, Maywood, IL 60153; Phone: 708-327-3216; Fax: 708-327-3220; e-mail: pstiff@lumc.edu.
References
Competing Interests
Conflict-of-interest disclosures: The author has received research funding from Seattle Genetics, Incyte, Plasmacyclics, Gilead, Eisai, Amgen, and Fate Therapeutics; has consulted for Seattle Genetics, Incyte, Plasmacyclics, and Gilead; and has received honoraria from Seattle Genetics, Incyte, Plasmacyclics, and Gilead.
Author notes
Off-label drug use: Ibrutinib: improve outcomes in lymphoma as part of transplant therapy.