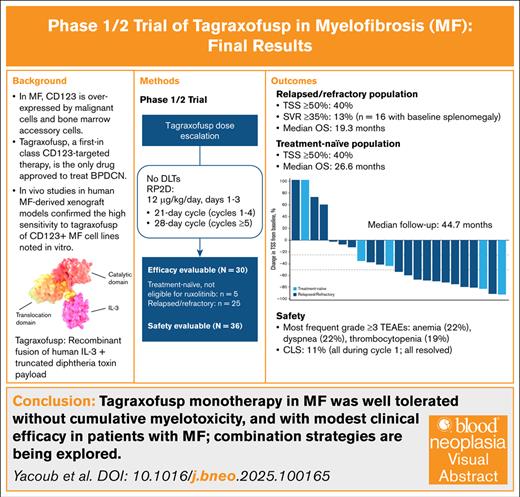
Key Points
In patients with MF, tagraxofusp monotherapy was well tolerated with a manageable and predictable safety profile.
With modest clinical activity in patients with MF, tagraxofusp warrants further investigation, including in combinations.
Visual Abstract
Patients with myelofibrosis (MF) who are resistant to or relapse after Janus kinase inhibitor (JAKi) therapy have limited treatment options and typically poor prognoses. CD123 is overexpressed in various myeloid malignancies, including MF. Tagraxofusp is a first-in-class CD123-targeted therapy, and the only drug approved globally for the rare myeloid malignancy blastic plasmacytoid dendritic cell neoplasm. We conducted a phase 1/2 trial to determine optimal dosing, and evaluate safety and efficacy of tagraxofusp monotherapy in treatment-naïve (n = 5) MF and patients with MF resistant/refractory to JAKi (n = 25) and not eligible for stem cell transplant. There were no dose-limiting toxicities. The recommended phase 2 dose of tagraxofusp was 12 μg/kg per day for 3 consecutive days per cycle. In the safety population (n = 36) treated at 12 μg/kg per day, the most frequent grade ≥3 treatment-emergent adverse events were thrombocytopenia (19%), anemia (22%), and dyspnea (11%). Capillary leak syndrome occurred in 11% of patients, all during cycle 1 with resolution in all patients. Thirty patients treated at 12 μg/kg per day were efficacy evaluable. Of 18 (n = 2 treatment-naïve, n = 16 relapsed/refractory) patients with baseline splenomegaly, 2 relapsed/refractory patients had spleen volume reduction ≥35%. In relapsed/refractory patients, 40% had total symptom score (TSS) ≥50%, and median overall survival (OS) was 19.3 months. In patients who were treatment naïve, 40% had TSS ≥50%, and median OS was 26.6 months. In this trial, tagraxofusp monotherapy in MF was well tolerated, without cumulative myelotoxicity, and with symptom score improvements, warranting further investigation in combination therapy. This trial was registered at www.clincaltrials.gov as #NCT02268253.
Introduction
Myelofibrosis (MF) is a BCR/ABL1-negative myeloproliferative neoplasm (MPN) characterized by stem cell–derived clonal myeloproliferation, which occurs de novo (primary MF) or develops as a sequela to polycythemia vera or postessential thrombocythemia.1 Patients with MF are burdened by constitutional symptoms and serious clinical manifestations (eg, severe anemia, marked hepatosplenomegaly, cachexia, bone pain, splenic infarct, thrombotic and hemorrhagic complications).1,2 The main causes of primary MF-related mortality include leukemic progression, cardiovascular events and other comorbidities, as well as sequelae of cytopenias (eg, infections, bleeding).2 With a marked reduction in life expectancy, patients with MF have a median survival of 5 to 7 years.3
Allogeneic stem cell transplant (SCT) is the only potentially curative treatment for patients with MF,2 but is not feasible in many because of age-related frailty and/or comorbidities, which increase the risk of transplant-related morbidity and mortality. Since 2011, targeted therapies with Janus kinase inhibitors (JAKi) have formed the mainstay of treatment for MF, with the aim of returning the bone marrow (BM) microenvironment to a predisease state.4 Targeted therapies with United States Food and Drug Administration indications to treat patients who were JAKi naïve with MF gained their approvals (ruxolitinib in 2011; fedratinib in 2019; pacritinib in 2022; momelotinib in 2023)5 by demonstrating improvements in some patient-reported symptoms, splenomegaly, and/or anemia.2 Many patients stop taking these medications because of loss of benefit or toxicities. Approximately 50% of patients with MF discontinue ruxolitinib by 3 years due to adverse events (AEs), lack or loss of splenic response, and progression to blast phase.6 Patients who discontinue ruxolitinib have poor outcomes, with reported median survival ranging from 4 to 16 months.6-9 Furthermore, JAKi do not appear to reverse BM fibrosis, or induce durable complete or partial responses.2,10 New therapies with novel mechanisms of action are needed to improve quality of life and survival for patients with MF.
CD123 is overexpressed on malignant hematopoietic stem cells compared with normal hematopoietic stem cells.11-13 Tagraxofusp, a first-in-class CD123-targeted therapy, is a recombinant fusion protein consisting of human interleukin-3 (IL-3) conjugated to a truncated diphtheria toxin payload approved in the United States and Europe as the only drug to treat blastic plasmacytoid dendritic cell neoplasam based on its clinical efficacy and safety in a registrational trial.14,15 In MF, CD123 is expressed on malignant cells and BM accessory cells that support the proliferation of neoplastic cells.12,16 CD123+ MF cell lines were highly sensitive to tagraxofusp monotherapy in vitro, and this sensitivity was enhanced in the presence of BM accessory cells. This activity was confirmed in in vivo studies utilizing human MF–derived xenograft models.17,18 Importantly, the differential expression of CD123 between malignant and normal cells provides a potential therapeutic window to target MF cells with tagraxofusp while sparing normal BM, thereby reducing myelotoxicity that often leads to JAKi treatment discontinuation.
We report the results for patients with MF within a phase 1/2 trial aimed to determine the optimal dose, and evaluate the safety and efficacy of tagraxofusp monotherapy.
Methods
Study design
This was a nonrandomized, open-label, multicenter, 2-stage phase 1/2 study of tagraxofusp monotherapy in patients with MF or chronic myelomonocytic leukemia (CMML). Stage 1 had a standard 3-plus-3 dose-escalation design to identify the recommended phase 2 dose (RP2D). Stage 2 was a single-arm, dose-expansion phase to characterize the safety and efficacy of tagraxofusp at the RP2D. Results from the CMML population are reported separately.19
Participants
Stage 1 (escalation phase) allowed enrollment of patients who were treatment naïve or relapsed/refractory with a diagnosis of MF, CMML, advanced symptomatic systemic mastocytosis, or advanced symptomatic primary eosinophilic disorder.
In addition to meeting the criteria for stage 1, participants in stage 2 had to meet the 2016 World Health Organization diagnostic criteria for MF, have International Prognostic Scoring System (IPSS)/Dynamic IPSS (DIPSS)/DIPSS-plus intermediate-2 or high-risk disease, and not be eligible for an immediate allogeneic SCT. Patients who relapsed after allogeneic SCT were allowed. Patients with IPSS/DIPSS/DIPSS-plus low or intermediate-1 risk disease were eligible if they had ≥1 of the following symptoms: MF-related anemia (hemoglobin <10 g/dL), splenomegaly (palpable size >10 cm), leukocytosis (white blood cells >25 × 109/L), marked thrombocytosis (platelet count >1000 × 109/L), or constitutional symptoms (weight loss >10% during previous 10 months or fever [>37.5°C or drenching night sweats for >6 weeks]) as recommended by the European LeukemiaNet (ELN)/International Working Group (IWG) criteria.20 Full eligibility is included in the supplemental Methods.
Treatment
During stage 1, patients received doses of 7, 9, or 12 μg/kg tagraxofusp via IV infusion during days 1 to 3 of a 21-day cycle for cycles 1 to 4, a 28-day cycle for cycles 5 to 7, and a 42-day cycle thereafter. During stage 2, patients received 12 μg/kg via IV infusion on days 1 to 3 of a 21-day cycle for cycles 1 to 4, and a 28-day cycle for cycle ≥5. Dosing periods could be extended for up to 10 days for dose delays during each cycle. For both stages, hospitalization was required for the first cycle of tagraxofusp starting on the day of infusion (or the previous day), and ending ∼24 hours after the last infusion. Subsequent cycles could be administered in outpatient settings. Guidelines for managing capillary leak syndrome (CLS) followed tagraxofusp prescribing information,21 and are summarized in the supplemental Methods.
Outcomes
Stage 1 primary objectives were to determine the maximum tolerated dose or the maximum tested dose at which multiple dose-limiting toxicities (DLTs) were not observed for tagraxofusp monotherapy, and to characterize its safety profile. A DLT was defined as any of the following occurring during cycle 1: any treatment-emergent grade 4 transaminase or creatine phosphokinase elevation (confirmed within 24 hours of initial identification) regardless of duration or relationship to tagraxofusp, any grade ≥3 nonhematologic toxicity except for grade 3 laboratory toxicities that resolved to grade ≤1 within 28 days of the last infusion or protocol-specified grade 3 toxicities that resolved to grade ≤1 within 21 days of the last infusion, and grade 4 neutropenia or grade 4 thrombocytopenia with ≥28 days duration. The maximum tolerated dose was defined as the dose preceding the dose level at which ≥2 patients experienced a DLT during cycle 1.
Stage 2 objectives were to characterize the tagraxofusp safety profile, and to evaluate tagraxofusp efficacy based on IWG-MPN research and treatment (IWG-MRT) and ELN consensus response criteria.22 The primary efficacy end point for stage 2 was investigator-assessed objective response rate (ORR), defined as the number and percentage of patients with complete response or partial response (PR). The ORR had to be confirmed ≥12 weeks after the criteria meeting response were initially identified. Because tagraxofusp is not a JAKi and response dynamics were not characterized yet, we included best response at any time to capture the overall dynamics of responses including late responses. Secondary efficacy end points included duration of response, progression-free survival (PFS), and overall survival (OS). Time to objective response and bridge to SCT were also assessed. See the supplemental Methods for details on additional outcome assessments.
Safety assessments included monitoring of treatment-emergent adverse events (TEAEs) through 30 days after the last tagraxofusp infusion, and were summarized by the Medical Dictionary for Regulatory Activities version 19.0. AEs were summarized by National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Translational analyses
Details of the methods for translational analyses, including antidrug antibody (ADA), neutralizing antibody (NAb), and monocytosis are described in the supplemental Methods.
Statistical analysis
Safety analyses included patients in either stage who received ≥1 dose of tagraxofusp at 12 μg/kg per day. Efficacy analyses were conducted using a modified intent-to-treat (henceforth referred to as ITT) population that comprised patients in either stage who received ≥1 dose of tagraxofusp 12 μg/kg per day and were evaluable for efficacy (ie, had an efficacy assessment after first treatment or discontinued due to death or progressive disease). Median follow-up was defined23 as the time from first tagraxofusp infusion date to the study cut-off date. Descriptive statistics were used to summarize baseline characteristics, safety, and efficacy data. PFS and OS were estimated using Kaplan-Meier analysis. Statistical analyses were performed using SAS statistical software version 9.4.
This study was conducted in compliance with the United States Food and Drug Administration Code of Federal Regulations, the principles of the International Conference on Harmonisation Good Clinical Practice Guideline, the ethical principles of the Declaration of Helsinki, and applicable laws from regulatory authorities. The study received institutional review board approval. Before enrollment, all patients provided written informed consent. This trial was registered at www.clinicaltrials.gov as #NCT02268253.
Results
Patient disposition
At the final safety analysis, 2 patients were treated with 7 μg/kg per day tagraxofusp, 1 with 9 μg/kg per day, and 36 with 12 μg/kg per day (safety population). No DLTs were observed among patients treated with tagraxofusp during the dose-escalation phase, which included 4 patients with MF (2 treated at 7 μg/kg per day, 1 at 9 μg/kg per day and 12 μg/kg per day each) and 5 patients with CMML.19 The RP2D, identified as the maximum tested dose, was 12 μg/kg per day. Patients treated at 12 μg/kg per day had a median duration of tagraxofusp therapy of 96.5 days (range, 1-1115) and received a median of 5 cycles (range, 1-30). Reasons for treatment discontinuation are reported in supplemental Table 1.
Patient characteristics
In total, 30 patients treated with tagraxofusp 12 μg/kg per day were assessable for efficacy and included in the ITT population. Twenty-five (83%) had relapsed/refractory disease. Five (17%) patients were treatment naïve, not eligible for ruxolitinib (the only approved therapy at the time of study), and had predefined adverse features (Table 1).24
Baseline characteristics of patients treated at 12 μg/kg per day (ITT population)
| Characteristic . | Relapsed/refractory (N = 25) . | Treatment-naïve (N = 5) . |
|---|---|---|
| Age, median (range), y | 70.0 (55-83) | 74.0 (67-81) |
| Sex, n (%) | ||
| Female | 10 (40) | 3 (60) |
| Male | 15 (60) | 2 (40) |
| ECOG performance status, n (%) | ||
| 0 | 2 (8) | 1 (20) |
| 1 | 18 (72) | 2 (40) |
| 2 | 5 (20) | 2 (40) |
| Platelet count (×109/L), n (%) | ||
| Median (range) | 75.0 (16-651) | 36.0 (16-330) |
| >100 | 11 (44) | 2 (40) |
| >50-100 | 5 (20) | 0 |
| ≥20-50 | 8 (32) | 2 (40) |
| <20 | 1 (4) | 1 (20) |
| Hemoglobin, median (range), g/L | 85.0 (63-141) | 75.0 (57-113) |
| Dependent on RBC transfusion, n (%) | 2 (8) | 1 (20) |
| Time since diagnosis, median (range), mo | 43.5 (0-162) | 22.6 (10-152) |
| MF subtype, n (%) | ||
| Primary MF | 16 (64) | 4 (80) |
| Postpolycythemia MF | 5 (20) | 1 (20) |
| Postessential thrombocythemia MF | 4 (16) | 0 |
| DIPSS-plus risk score, n (%) | ||
| Intermediate 1 | 4 (16) | 0 |
| Intermediate 2 | 13 (52) | 4 (80) |
| High risk | 8 (32) | 1 (20) |
| Splenomegaly, n (%) | 16 (64) | 2 (40) |
| Spleen size by MRI/CT, cm3 | (n = 23) | (n = 5) |
| Median (range) | 2091.0 (197-5321) | 1616.0 (724-3183) |
| MPN-SAF tumor symptom score | (n = 20) | (n = 5) |
| Median (range) | 71.0 (6-209) | 48.0 (23-87) |
| Mean (SD) | 78.9 (51.39) | 51.0 (22.99) |
| Prior therapies for MPN | ||
| Median (range) | 2.0 (1.0-8.0) | 0 |
| Mean (SD) | 2.4 (1.58) | 0 |
| Karyotype, n (%) | ||
| Normal | 19 (76) | 4 (80) |
| Abnormal | 6 (24) | 1 (20) |
| Very high-risk for GIPSS/MIPSSv2 | 3 (12) | 1 (20) |
| Unfavorable for GIPSS/MIPSSv2 | 2 (8) | 0 |
| 20q-only | 1 (4) | 0 |
| Unfavorable for DIPSS-plus | 4 (16) | 1 (20) |
| Not reported | 1 (4) | 0 |
| Driver mutations, n (%) | (n = 17) | (n = 5) |
| JAK2 V617F | 16 (64) | 3 (60) |
| CALR exon 9 | 0 | 2 (40) |
| MPL exon 10 | 1 (4) | 0 |
| High-risk molecular mutations, n (%) | 10 (40) | 2 (40) |
| ASXL1 | 5 (20) | 2 (40) |
| SRSF2 | 2 (8) | 0 |
| U2AF1 Q157 | 4 (16) | 1 (20) |
| IDH1 | 1 (4) | 0 |
| IDH2 | 1 (4) | 0 |
| Characteristic . | Relapsed/refractory (N = 25) . | Treatment-naïve (N = 5) . |
|---|---|---|
| Age, median (range), y | 70.0 (55-83) | 74.0 (67-81) |
| Sex, n (%) | ||
| Female | 10 (40) | 3 (60) |
| Male | 15 (60) | 2 (40) |
| ECOG performance status, n (%) | ||
| 0 | 2 (8) | 1 (20) |
| 1 | 18 (72) | 2 (40) |
| 2 | 5 (20) | 2 (40) |
| Platelet count (×109/L), n (%) | ||
| Median (range) | 75.0 (16-651) | 36.0 (16-330) |
| >100 | 11 (44) | 2 (40) |
| >50-100 | 5 (20) | 0 |
| ≥20-50 | 8 (32) | 2 (40) |
| <20 | 1 (4) | 1 (20) |
| Hemoglobin, median (range), g/L | 85.0 (63-141) | 75.0 (57-113) |
| Dependent on RBC transfusion, n (%) | 2 (8) | 1 (20) |
| Time since diagnosis, median (range), mo | 43.5 (0-162) | 22.6 (10-152) |
| MF subtype, n (%) | ||
| Primary MF | 16 (64) | 4 (80) |
| Postpolycythemia MF | 5 (20) | 1 (20) |
| Postessential thrombocythemia MF | 4 (16) | 0 |
| DIPSS-plus risk score, n (%) | ||
| Intermediate 1 | 4 (16) | 0 |
| Intermediate 2 | 13 (52) | 4 (80) |
| High risk | 8 (32) | 1 (20) |
| Splenomegaly, n (%) | 16 (64) | 2 (40) |
| Spleen size by MRI/CT, cm3 | (n = 23) | (n = 5) |
| Median (range) | 2091.0 (197-5321) | 1616.0 (724-3183) |
| MPN-SAF tumor symptom score | (n = 20) | (n = 5) |
| Median (range) | 71.0 (6-209) | 48.0 (23-87) |
| Mean (SD) | 78.9 (51.39) | 51.0 (22.99) |
| Prior therapies for MPN | ||
| Median (range) | 2.0 (1.0-8.0) | 0 |
| Mean (SD) | 2.4 (1.58) | 0 |
| Karyotype, n (%) | ||
| Normal | 19 (76) | 4 (80) |
| Abnormal | 6 (24) | 1 (20) |
| Very high-risk for GIPSS/MIPSSv2 | 3 (12) | 1 (20) |
| Unfavorable for GIPSS/MIPSSv2 | 2 (8) | 0 |
| 20q-only | 1 (4) | 0 |
| Unfavorable for DIPSS-plus | 4 (16) | 1 (20) |
| Not reported | 1 (4) | 0 |
| Driver mutations, n (%) | (n = 17) | (n = 5) |
| JAK2 V617F | 16 (64) | 3 (60) |
| CALR exon 9 | 0 | 2 (40) |
| MPL exon 10 | 1 (4) | 0 |
| High-risk molecular mutations, n (%) | 10 (40) | 2 (40) |
| ASXL1 | 5 (20) | 2 (40) |
| SRSF2 | 2 (8) | 0 |
| U2AF1 Q157 | 4 (16) | 1 (20) |
| IDH1 | 1 (4) | 0 |
| IDH2 | 1 (4) | 0 |
CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; GIPSS, Genetically Inspired Prognostic Scoring System; MIPSS, Mutation-Enhanced International Prognostic Scoring System, version 2; MRI, magnetic resonance imaging; SAF, symptom assessment form; SD, standard deviation.
Patients with relapsed/refractory disease had a median age of 70.0 years; 40% were women; 64% had primary MF; 84% had intermediate 2 or high-risk DIPSS plus scores; and 64% had splenomegaly. These patients had received a median of 2.0 (range, 1-8) prior therapies, including ruxolitinib, hypomethylating agents, and allogeneic SCT. Seventy-six percent of patients in the relapsed/refractory population had normal karyotypes at baseline; 64% had JAK2 V617F as the driver mutation; and 40% had high-risk molecular mutations (Table 1).
Patients in the treatment-naïve population had a median age of 74.0 years; 60% were women; 80% had primary MF; 100% had intermediate 2 or high-risk DIPSS plus scores; and 40% had splenomegaly. Eighty percent of patients who were treatment naïve had normal baseline karyotype; 60% had JAK2 V617F; and 40% had high-risk molecular mutations. High-grade cytopenias were prevalent in both populations.
Safety
In the safety population of 36 patients treated with tagraxofusp 12 μg/kg per day, the most common any-grade nonhematologic TEAEs included hypoalbuminemia (47%), nausea (42%), dyspnea (39%), decreased appetite (36%), and peripheral edema (36%; Table 2). Most nonhematologic TEAEs were grade 1 to 2.
Nonhematologic and hematologic AEs in patients treated at 12 μg/kg per day (safety population; N = 36)
| Event term, n (%) . | Any grade . | Grade 1-2 . | Grade 3-4 . |
|---|---|---|---|
| Nonhematologic TEAEs (any grade occurring in ≥20% of patients) . | |||
| Hypoalbuminemia | 17 (47) | 16 (44) | 1 (3) |
| Nausea | 15 (42) | 15 (42) | 0 |
| Dyspnea | 14 (39) | 10 (28) | 4 (11) |
| Peripheral edema∗ | 13 (36) | 12 (33) | 1 (3) |
| Decreased appetite∗ | 13 (36) | 13 (36) | 0 |
| Dizziness | 12 (33) | 12 (33) | 0 |
| Fatigue∗ | 12 (33) | 10 (28) | 2 (6) |
| Pyrexia | 12 (33) | 9 (25) | 3 (8) |
| Abdominal pain | 10 (28) | 8 (22) | 2 (6) |
| Chills | 10 (28) | 10 (28) | 0 |
| Constipation | 10 (28) | 10 (28) | 0 |
| Headache | 10 (28) | 10 (28) | 0 |
| Hypotension | 10 (28) | 9 (25) | 1 (3) |
| Vomiting | 10 (28) | 10 (28) | 0 |
| Diarrhea | 9 (25) | 7 (19) | 2 (6) |
| ALT increased | 8 (22) | 8 (22) | 0 |
| Confusional state∗ | 8 (22) | 7 (19) | 1 (3) |
| Hypokalemia | 8 (22) | 7 (19) | 1 (3) |
| Tachycardia∗ | 8 (22) | 7 (19) | 1 (3) |
| Weight increased | 8 (22) | 8 (22) | 0 |
| Selected hematologic TEAEs | |||
| Thrombocytopenia∗ | 10 (28) | 3 (8) | 7 (19) |
| Anemia | 8 (22) | 0 | 8 (22) |
| Febrile neutropenia | 2 (6) | 1 (3) | 1 (3) |
| Leukopenia∗ | 2 (6) | 2 (6) | 0 |
| Neutropenia∗ | 1 (3) | 1 (3) | 0 |
| Treatment-related hematologic AEs | |||
| Thrombocytopenia∗ | 5 (14) | 2 (6) | 3 (8) |
| Anemia | 2 (6) | 0 | 2 (6) |
| Febrile neutropenia | 1 (3) | 0 | 1 (3) |
| Leukopenia∗ | 1 (3) | 1 (3) | 0 |
| Neutropenia∗ | 1 (3) | 1 (3) | 0 |
| Event term, n (%) . | Any grade . | Grade 1-2 . | Grade 3-4 . |
|---|---|---|---|
| Nonhematologic TEAEs (any grade occurring in ≥20% of patients) . | |||
| Hypoalbuminemia | 17 (47) | 16 (44) | 1 (3) |
| Nausea | 15 (42) | 15 (42) | 0 |
| Dyspnea | 14 (39) | 10 (28) | 4 (11) |
| Peripheral edema∗ | 13 (36) | 12 (33) | 1 (3) |
| Decreased appetite∗ | 13 (36) | 13 (36) | 0 |
| Dizziness | 12 (33) | 12 (33) | 0 |
| Fatigue∗ | 12 (33) | 10 (28) | 2 (6) |
| Pyrexia | 12 (33) | 9 (25) | 3 (8) |
| Abdominal pain | 10 (28) | 8 (22) | 2 (6) |
| Chills | 10 (28) | 10 (28) | 0 |
| Constipation | 10 (28) | 10 (28) | 0 |
| Headache | 10 (28) | 10 (28) | 0 |
| Hypotension | 10 (28) | 9 (25) | 1 (3) |
| Vomiting | 10 (28) | 10 (28) | 0 |
| Diarrhea | 9 (25) | 7 (19) | 2 (6) |
| ALT increased | 8 (22) | 8 (22) | 0 |
| Confusional state∗ | 8 (22) | 7 (19) | 1 (3) |
| Hypokalemia | 8 (22) | 7 (19) | 1 (3) |
| Tachycardia∗ | 8 (22) | 7 (19) | 1 (3) |
| Weight increased | 8 (22) | 8 (22) | 0 |
| Selected hematologic TEAEs | |||
| Thrombocytopenia∗ | 10 (28) | 3 (8) | 7 (19) |
| Anemia | 8 (22) | 0 | 8 (22) |
| Febrile neutropenia | 2 (6) | 1 (3) | 1 (3) |
| Leukopenia∗ | 2 (6) | 2 (6) | 0 |
| Neutropenia∗ | 1 (3) | 1 (3) | 0 |
| Treatment-related hematologic AEs | |||
| Thrombocytopenia∗ | 5 (14) | 2 (6) | 3 (8) |
| Anemia | 2 (6) | 0 | 2 (6) |
| Febrile neutropenia | 1 (3) | 0 | 1 (3) |
| Leukopenia∗ | 1 (3) | 1 (3) | 0 |
| Neutropenia∗ | 1 (3) | 1 (3) | 0 |
ALT, alanine aminotransferase.
Aggregated events by United States Food and Drug Administration Medical Dictionary for Regulatory Activities analysis of selected TEAEs.
Four (11%) patients had CLS deemed related to tagraxofusp, 2 (6%) grade 1 to 2, and 1 each (3%) grade 3 and grade 4. All CLS events occurred during cycle 1, with a median time to any grade CLS of 4 days (range, 2-8). All patients recovered from CLS with a median time to resolution of 6 days (range, 4-12). Two patients who had CLS (1 with grade 2 and 1 with grade 4) discontinued treatment, and 2 patients continued treatment at the same dose after recovery.
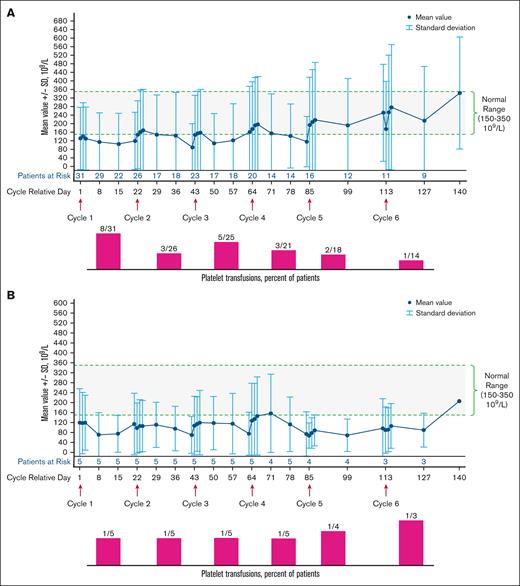
The most common (any grade) hematologic TEAEs (Table 2) were thrombocytopenia (28%) and anemia (22%). Notably, 6 of 10 patients who had an AE of thrombocytopenia reported by the investigator during treatment already had grade ≥2 thrombocytopenia at baseline; similarly, 7 of 8 patients who had anemia reported during the study had grade ≥2 anemia at baseline. Treatment-related grade 3 to 4 hematologic TEAEs were thrombocytopenia (8%), anemia (6%), and febrile neutropenia (3%). In the relapsed/refractory population, platelet counts were stable over time, and the number of patients receiving platelet transfusions did not increase after cycle 3 (Figure 1A). Similar results were observed for the treatment-naïve population (Figure 1B).
Platelet levels (109/L mean ± SD) and platelet transfusions among patients treated with tagraxofusp 12 μg/kg per day over time. (A) Platelet levels and platelet transfusions in the relapsed-refractory population. (B) Platelet levels and platelet transfusions in the treatment-naïve population. SD, standard deviation.
Platelet levels (109/L mean ± SD) and platelet transfusions among patients treated with tagraxofusp 12 μg/kg per day over time. (A) Platelet levels and platelet transfusions in the relapsed-refractory population. (B) Platelet levels and platelet transfusions in the treatment-naïve population. SD, standard deviation.
Key safety data for the overall population of 39 patients with MF are summarized in supplemental Table 2.
TEAEs leading to treatment discontinuation occurred in 8 (22%) patients, and included CLS (n = 2), pneumonia (n = 2), dyspnea, embolism, fatigue, hypoalbuminemia, Clostridium difficile infection, and pyrexia (n = 1 each). No patients had TEAEs leading to dose reduction. TEAEs that led to dose interruption occurred in 16 (44%) patients, with chills (n = 5), pyrexia (n = 5), and weight increase (n = 3) most commonly reported.
Five (14%) patients experienced a TEAE leading to death, none of which was treatment related, and included gastric perforation, septic shock, Clostridium difficile infection, intracranial hemorrhage, and pneumonia. These fatal TEAEs had an onset 11 to 22 days after the last dose of tagraxofusp.
Efficacy
The overall median follow-up was 44.7 months (range, 28.6-50.4). In the relapsed/refractory population, 1 patient achieved a PR (ORR 4%); 18 (72%) had stable disease; 4 (16%) had progressive disease; and 2 (8%) were not evaluable (NE). The 1 PR was per ELN/IWG-MRT at the end of treatment visit (day 119) based on total symptom score (TSS) reduction ≥50% (TSS50) at cycle 2 day 21 maintained through the end of treatment at cycle 5; the patient discontinued treatment due to nontreatment-related pneumonia.
Any spleen volume reduction (SVR) was observed in 6 (38%) of 16 patients with baseline splenomegaly and relapsed/refractory MF at any time, including 2 patients with ≥35% SVR (SVR35; Figure 2A). No SVR35 was observed at week 12 or week 24. The median time to best SVR response was 85 days (range, 15-1028). Seven patients with splenomegaly at baseline did not have any magnetic resonance imaging/computed tomography scan assessment postbaseline. No spleen response was observed by abdominal examination in any of these patients.
Change in spleen volume and tumor symptom score from baseline. (A) Waterfall plot of maximum percentage change in spleen volume at any time for patients with baseline splenomegaly treated with tagraxofusp 12 μg/kg per day (ITT population). (B) Waterfall plot of maximum percentage change in tumor symptom score at any time for patients treated with tagraxofusp 12 μg/kg per day (ITT population). ∗Seven patients are not shown as they were not evaluable because they did not undergo magnetic resonance imaging/computed tomography scan assessments postbaseline. No spleen response was observed by abdominal examination in any of these patients. Reduction of the line below left costal margin was observed in 2 patients: −29% and −14% (not clinically relevant). †Seven patients were not evaluable for changes in tumor symptoms because they did not have baseline and/or postbaseline tumor symptom scores.
Change in spleen volume and tumor symptom score from baseline. (A) Waterfall plot of maximum percentage change in spleen volume at any time for patients with baseline splenomegaly treated with tagraxofusp 12 μg/kg per day (ITT population). (B) Waterfall plot of maximum percentage change in tumor symptom score at any time for patients treated with tagraxofusp 12 μg/kg per day (ITT population). ∗Seven patients are not shown as they were not evaluable because they did not undergo magnetic resonance imaging/computed tomography scan assessments postbaseline. No spleen response was observed by abdominal examination in any of these patients. Reduction of the line below left costal margin was observed in 2 patients: −29% and −14% (not clinically relevant). †Seven patients were not evaluable for changes in tumor symptoms because they did not have baseline and/or postbaseline tumor symptom scores.
Fifteen (60%) patients with relapsed/refractory MF had improvements in TSS (Figure 2B) at any time. TSS50 was observed in 6 (24%) patients at 12 weeks, 4 (16%) patients at 24 weeks, and in 10 (40%) patients at any time. The median time to TSS50 was 55 days (range, 23-239), and its median duration was 46 days (range, 15-989). The median time to best TSS response was 84 days (range, 20-274). Two patients with primary MF had both SVR35 and TSS50. A 69-year-old woman with primary MF (normal karyotype, no high-risk mutation) that relapsed after thalidomide/prednisone and ruxolitinib had SVR35 at cycle 28, lasting 82 days, and TSS50 at cycle 7, lasting 988 days (the only assessment was at end of treatment visit after week 24). A 72-year-old woman with primary MF (abnormal karyotype, no high-risk mutations) that relapsed after thalidomide and ruxolitinib had SVR35 at cycle 1, lasting 652 days, and TSS50 at cycle 10, lasting 64 days.
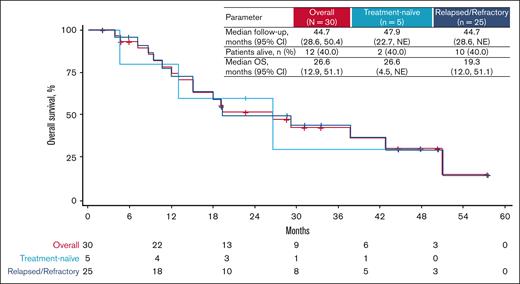
In the relapsed/refractory population, the 2 patients who were red blood cell (RBC) transfusion dependent at baseline did not achieve an anemia response by becoming transfusion independent for any 12-week period. Among transfusion-independent patients at baseline, 2 (8%) had an anemia response: 1 patient at cycle 4 day 21, and 1 patient at the end of treatment. Seventeen (74%) of 23 patients with relapsed/refractory disease became transfusion dependent for any 12-week period. This population had a median PFS of 15.1 months (range, 3.1-19.1) and median OS of 19.3 months (range, 12.0-51.1; Figure 3).
OS outcomes. Kaplan-Meier analysis of OS for all patients (red curve), patients who were treatment naïve (light blue curve), and relapsed or refractory patients (dark blue curve) treated with tagraxofusp 12 μg/kg per day. NE, not estimable.
OS outcomes. Kaplan-Meier analysis of OS for all patients (red curve), patients who were treatment naïve (light blue curve), and relapsed or refractory patients (dark blue curve) treated with tagraxofusp 12 μg/kg per day. NE, not estimable.
In the treatment-naïve population, 100% of patients achieved stable disease. Any SVR was observed in 1 (50%) of 2 patients who had splenomegaly at baseline (Figure 2A). Four (80%) patients had improvements in TSS at any time, including 2 (40%) with TSS50 (Figure 2B). The median time to TSS50 was 43 days (range, 22-64), and its median duration was 208 days (range, 99-317). The 1 patient who was RBC transfusion dependent at baseline did not achieve an anemia response. Three (75%) of 4 patients who were transfusion independent at baseline became transfusion dependent for any 12-week period. This population had a median PFS of 9.8 months (range, 4.5 to NE) and median OS of 26.6 months (range, 4.5 to NE; Figure 3).
Among patients treated with tagraxofusp 12 μg/kg per day, the presence or absence of monocytosis (defined as monocytes ≥0.5 × 109/L or ≥1.0 × 109/L) was not associated with any SVR, SVR35, any TSS reduction, or TSS50 (supplemental Figure 1A-B). Patients with monocytosis had similar OS to patients without monocytosis regardless of monocyte threshold definition (supplemental Figure 1C-D).
Translational
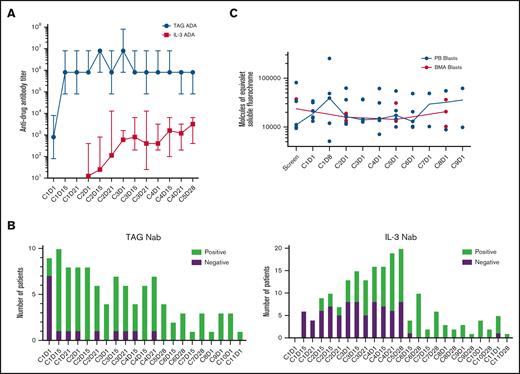
All patients in the ITT population had antitagraxofusp and anti–IL-3 ADA data assessed throughout the study. Ten had antitagraxofusp NAb data, and 29 had anti–IL-3 NAb data available for analysis. Twenty-nine patients had baseline samples for testing antitagraxofusp and anti–IL-3 ADA. Overall, at baseline, 28 (97%) patients were antitagraxofusp antibody positive, and 1 (3%) was antitagraxofusp antibody negative. After cycle 5, 13 patients had samples available, and all (100%) were antitagraxofusp antibody positive. Similarly at baseline, 7 (24%) patients were anti–IL-3 antibody positive, and 22 (76%) were anti–IL-3 antibody negative. After cycle 5, 15 patients had samples available; 14 (93%) were anti–IL-3 antibody positive. During treatment, patients generated ADA with similar frequency and kinetics. Median antitagraxofusp ADA titers increased by 3 logs after 1 cycle of treatment, and were sustained during tagraxofusp treatment. Median anti–IL-3 titers increased by 1 log after 2 cycles of tagraxofusp treatment, and by 2 logs after 5 cycles of treatment (Figure 4A).
Frequency of ADAs, detection of NAbs, and relative expression of CD123 on blasts. (A) Median anti-TAG and anti–IL-3 ADA titers during 5 cycles of tagraxofusp treatment, N = 30. (B) Presence or absence of anti-TAG NAb (n = 10) and anti–IL-3 NAb (n = 29) over 11 cycles of tagraxofusp treatment. (C) The relative expression of CD123 on blasts in the peripheral blood (PB) and BMA, n = 7 with a range of posttreatment samples from 3 to 10. TAG, tagraxofusp.
Frequency of ADAs, detection of NAbs, and relative expression of CD123 on blasts. (A) Median anti-TAG and anti–IL-3 ADA titers during 5 cycles of tagraxofusp treatment, N = 30. (B) Presence or absence of anti-TAG NAb (n = 10) and anti–IL-3 NAb (n = 29) over 11 cycles of tagraxofusp treatment. (C) The relative expression of CD123 on blasts in the peripheral blood (PB) and BMA, n = 7 with a range of posttreatment samples from 3 to 10. TAG, tagraxofusp.
When patients tested positive for ADA, they were subsequently tested for NAb. Of the 10 patients tested for antitagraxofusp NAb, 9 (90%) developed antitagraxofusp NAb by the end of cycle 1, and 1 (10%) developed antitagraxofusp NAb by the end of cycle 5. Twenty-nine patients were assessed for anti–IL-3 NAb; 7 (24%) remained negative for anti–IL-3 NAb at the end of treatment, and 22 (76%) developed anti–IL-3 NAb by the end of cycle 6 (Figure 4B).
Among the 10 patients in whom both tagraxofusp and IL-3 NAbs were tested and who were evaluable for symptom response, 6 developed both tagraxofusp and IL-3 NAbs: 4 achieved a TSS50 response despite NAb development, while in 2 patients, symptoms worsened after an initial minimal symptom score reduction. In the remaining 4 patients, only tagraxofusp NAbs were detected by the end of cycle 1, with no IL-3 NAbs detected. Two patients had symptom score increases, and 2 achieved TSS50 (supplemental Table 3). Both patients with SVR35 developed tagraxofusp and IL-3 NAbs before achieving SVR35; 1 of these patients maintained response despite persistence of NAbs (supplemental Table 4).
The expression of CD123 on blasts was assessed in the peripheral blood and bone marrow aspiration (BMA) of 7 patients (12 μg/kg per day) with pretreatment and posttreatment samples available (Figure 4C). There was no observed loss or reduction of CD123 expression on the surface of blasts during treatment, and blasts in the peripheral blood and BMA had comparable levels of CD123 expression.
Discussion
Findings of this phase 1/2 trial evaluating tagraxofusp monotherapy in patients with MF suggest that targeting CD123 may be a potential therapeutic strategy for this challenging MPN. In our trial, there were no DLTs at the dose levels tested, and tagraxofusp at the 12 μg/kg per day RP2D had a manageable safety profile, with no new safety signals and limited cumulative hematologic toxicity observed. There were no CLS events after cycle 1, and resolution of CLS occurred in all patients.
Regarding the classical MF end points of SVR and TSS reduction that were designed to measure responses with JAKi, we found modest but demonstrable evidence of clinical activity for tagraxofusp 12 μg/kg per day in this cohort of high-risk patients. Of patients with baseline splenomegaly, 2 relapsed/refractory patients (13%) had SVR35 at any time, and any reduction of spleen volume was observed in 38% of relapsed/refractory patients and in 50% of patients who were treatment naïve. In the patients with relapsed/refractory MF, 60% had any TSS improvements, 40% had TSS50 at any time, and the median OS was 19.3 months. Two patients with relapsed/refractory MF had both SVR35 and TSS50. Despite translational data demonstrating stable expression of CD123 in both peripheral and BMA blasts following tagraxofusp treatment, the safety findings suggest that targeting MF with a CD123-targeted therapy is associated with minimal hematologic toxicity.
NAb analysis showed that all patients developed NAb against tagraxofusp and IL-3, the majority after 1 cycle of treatment. The presence of NAb may have limited clinical relevance regarding symptom or spleen responses, given these were achieved or maintained irrespective of NAb development.
The median OS of 19.3 months in patients with relapsed/refractory MF treated with tagraxofusp in our study shows a marginal benefit when compared with historical median survival reported for patients after discontinuing ruxolitinib (OS, 11.1-16.0 months).6-8,25-27 When our study was initiated in 2014, the treatment landscape for MF was sparse, with ruxolitinib as the only approved JAKi. Since then, newer generation JAKi and other novel therapies have demonstrated prolonged survival rates (median OS of 30 months with imetelstat,28,29 and >34 months with momelotinib)30,31 in patients with relapsed/refractory MF. Contextualizing these findings is challenging due to lack of standardized criteria for ruxolitinib discontinuation and high heterogeneity in identifying relapsed/refractory/JAKi-intolerant patients, as well as the need to interpret comparisons across different trials with caution.
The relapsed/refractory population in our study represented a difficult-to-treat, unfit population. Patients were heavily pretreated, with a median of 2 (range 1-8) prior therapies (all were previously treated with JAKi, some therapies also including hypomethylating agents and allogeneic SCT), and 43.5 months median time since diagnosis. Moreover, 40% had high-risk molecular mutations, and the majority (56%) had platelet counts <100 × 109/L, with 36% of them having <50 × 109/L. Overall, treatment-related grade 3 to 4 thrombocytopenia was observed in 8%, platelets counts were stable throughout the study, and patients receiving platelet transfusions did not increase after the third cycle. These observations are clinically relevant because thrombocytopenia has been consistently associated with high-risk features and poorer outcomes, and is an independent risk factor for survival in MF,26 with several risk category models assigning 1 to 2 points for thrombocytopenia. Furthermore, this profile compares favorably with approved JAKi agents. Indeed, hematologic toxicity and thrombocytopenia are common AEs observed during JAKi treatment, and frequently the reason for discontinuation in patients treated with ruxolitinib. While survival data are unavailable for pacritinib, the only drug approved for patients with thrombocytopenic MF, our findings in this difficult-to-treat population warrant further investigation of tagraxofusp.
RBC transfusional burden increased throughout the study in both populations. While the role of tagraxofusp cannot be ruled out, this observation might be related to the evolution of the disease itself. Indeed, treatment-related anemia was reported in 6% of patients.
We did not collect data on MF treatments after patients withdrew from the study, which limited our knowledge of factors with potential impact on survival. Most patients had their last visit between March 2016 and August 2020, with only 5 leaving the trial between March 2021 and January 2022. At that time, these patients had few options after failure of ruxolitinib, and likely enrolled in a clinical trial. Based on these considerations, our data on symptom score reduction, stated spleen size changes, and OS highlight the emerging need for alternative primary end points in clinical trials in MF to expand upon the use of SVR and TSS, and better evaluate impact on disease modification.4,10
Tagraxofusp monotherapy treatment was well tolerated, with mild hematologic toxicity that was not cumulative for all patients and platelet stabilization for those with baseline thrombocytopenia. This BM-sparing effect supports tagraxofusp as a safe and promising combination partner for MF where the risk of cumulative myelotoxicity, including thrombocytopenia, increases with many available combination partners. Furthermore, preclinical studies showed both in vitro and in vivo synergistic activity for tagraxofusp in combination with ruxolitinib or pacritinib,17,18 suggesting a role for tagraxofusp in combination with JAKi for MF.18 A single-arm trial (NCT06414681) is testing the combination of tagraxofusp and pacritinib in patients with IPSS/DIPSS/DIPSS-plus intermediate-2 or higher-risk MF previously treated with an approved JAKi or who are not candidates for such therapy.
The presence of monocytosis has been shown to have a dose-dependent adverse prognostic effect in primary MF.32-34 However, in our study population, the presence or absence of monocytosis did not have any impact on OS or clinical responses.
In summary, results from this phase 1/2 trial suggest that tagraxofusp monotherapy has modest clinical activity in patients with MF. As monotherapy for MF, tagraxofusp is well tolerated, with no new safety signals, and has a manageable and predictable safety profile, consistent with its mechanism of action. Combination strategies are being explored to better understand the potential of tagraxofusp in the treatment of MF.
Acknowledgments
The authors thank the study participants, investigators, and site staff for making this study possible.
This study was funded by the Menarini Group. Medical writing and editing assistance was provided by Monica Nicosia and Claire Gilmore of Phillips Group Oncology Communications, Inc, and funded by the Menarini Group.
Authorship
Contribution: A.Y. and N.P. contributed to the conception and design of the study; R.L. and A.G. analyzed the data; and all authors acquired the data, contributed to data interpretation, manuscript writing, editing, and content review, and approved the final draft of the manuscript.
Conflict-of-interest disclosure: A.Y. has served in consulting/advisory roles for AbbVie, Acceleron Pharma, Apellis, Blueprint Medicines, CTI BioPharma, Gilead, Incyte, Karyopharm Therapeutics Inc, Notable Labs, Novartis, Pfizer, PharmaEssentia, Protagonist, and Servier. H.A. has received research funding from Incyte; reports consulting/advisory roles with GlaxoSmithKline (GSK), Incyte, Karyopharm, and PharmaEssentia; has received honoraria from GSK, Incyte, PharmaEssentia, and Sobi; and reports travel accommodations/expenses support from Incyte and Karyopharm. V.G. has received research funding from AbbVie (payments made to the institution); reports consulting/advisory roles with AbbVie, Bristol Myers Squibb (BMS), Celgene, Daiichi Sankyo, GSK, and Pfizer; reports honoraria from BMS, Celgene, GSK, and Novartis; reports participation in a data safety and monitoring board (DSMB) or advisory board for Daichii Sankyo and GSK; and has stock ownership with Syndax. E.S.W. has served in an advisory/consulting role with AbbVie, Blueprint, Daiichi Sankyo, Immunogen, Kite, Kura, Novartis, Qiagen, Rigel, Ryvu, Schrödinger, Servier, Stemline, Syndax, and Takeda; reports speaking role with Astellas, Dava, and Pfizer; reports participation in DSMB for AbbVie and Gilead; and reports role as a section editor with UpToDate. M.M.P. has received research funding from Epigenetix, Kura Oncology, Polaris, Solu Therapeutics, and Stemline Pharmaceuticals; and reports consulting/advisory role with CTI Pharmaceuticals and AstraZeneca. G.J.S. has received research funding from AbbVie, Actinium, Actuate, Agios, AlloVir, AltruBio, Amgen, Aptevo, Arog, Astellas, AVM Biotechnology, BMS/Celgene, Bio-Path, Biomea, BioSight, Celator, Cellectis, Cellularity, Constellation, Cogent, Cullinan, Daiichi Sankyo, Deciphera, Delta-Fly, Fate, Forma, Fujifilm, Gamida, Genentech-Roche, GlycoMimetics, Geron, Gilead, lmmunogen, lmmune-Onc, Incyte, Janssen, Jazz, Karyopharm, Kite/Gilead, Kronos Bio, Kura, Loxo, Marker, Mateon, Novartis, Onconova, Ono-UK, Orca, Pfizer, PrECOG, REGiMMUNE, Rigel, Samus, Sangamo, Sellas, Stemline, Syros, Takeda, Tolero, and Trovagene; reports consulting/advisory roles for BMS, Curios, Daiichi, and Novartis; has received honoraria on the speakers’ bureau for AbbVie, Agios, Amgen, Astellas, Blueprint Medicines, BMS Celgene, Karyopharm, GSK, Kite (Gilead), Jazz, Rigel, Seattle Genetics, and Stemline; has served as a board or advisory committee member for Agios, Autolus, AVM Biotechnology, BMS, Gamida, Gilead, GSK, Incyte, Novartis, Orca, Rigel, and Stemline; and reports stock ownership in Amgen, BMS, and Janssen/J&J. M.T. has served in a consulting/advisory role with the avapritinib national advisory board for mastocytosis. T.I.M. has served as a consultant for Menarini-Stemline; reports patent, royalties, or other intellectual property with Oxford University Press; and reports stock or stock options with Saga Diagnostics. R.L., A.G., and I.G. report employment with Menarini Group. N.P. has received research grants from the United States Department of Defense, National Institutes of Health, and National Cancer Institute; reports consultant/scientific advisor board/speaking role for AbbVie, Aplastic Anemia and MDS International Foundation, Aptitude Health, Astellas Pharma US, Blueprint Medicines, BMS Pharmaceuticals, CancerNet, CareDx, Celgene, Cimeio Therapeutics AG, ClearView Healthcare Partners, CTI BioPharma, Curio Science, Dava Oncology, EUSA Pharma, Harborside Press, Imedex, Immunogen, Intellisphere, Karyopharm, Magdalen Medical Publishing, Medscape, Menarini Group, MorphoSys, Neopharm, Novartis Pharmaceuticals, OncLive, Pacylex, Patient Power, PeerView Institute for Medical Education, Pharma Essentia, and Physician Education Resource; has served on board of directors/management for Dan’s House of Hope; has served in leadership role for American Society of Hematology Committee on Communications and American Society of Clinical Oncology Cancer.Net Editorial Board; and reports licenses with Karger Publishers.
Correspondence: Abdulraheem Yacoub, Department of Internal Medicine, University of Kansas Cancer Center, 2650 Shawnee Mission Pkwy, Westwood, KS 66205; email: ayacoub@kumc.edu; and Naveen Pemmaraju, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 428, Houston, TX 77030; email: npemmaraju@mdanderson.org.
References
Author notes
A.Y. and H.A. are joint lead authors and contributed equally to this study.
The data that support the results of this study may be requested for products and the relevant indications that have been authorized by the regulatory authorities in Europe/the United States (or, if not, 2 years have elapsed since the study completion). The Menarini Group will review requests individually to determine whether (1) the requests are legitimate and relevant and meet sound scientific research principles, (2) the requests are within the scope of the participants’ informed consent, and (3) the request is compliant with any applicable law and regulation and with any contractual relationship that Menarini Group and its affiliates and partners have in place with respect to the study and/or the relevant product. Prior to making data available, requestors will be required to agree in writing to certain obligations, including without limitation, compliance with applicable privacy and other laws and regulations. Proposals should be directed to medicalinformation@menarinistemline.com.
The full-text version of this article contains a data supplement.