TO THE EDITOR:
The B-cell lymphoma 2 (BCL2) antagonist venetoclax is approved to treat patients with chronic lymphocytic leukemia (CLL) in first line and at relapse.1 Its application has led to an increasing clinical occurrence of venetoclax resistance.2,3 The investigation of this phenomenon in in vivo settings critically depends on the availability of appropriate mouse models. So far, B-cell non-Hodgkin lymphoma (CLL/B-NHL) mouse models, that allow translation of potential resistance driving genetic findings from clinical observation, are rare. The Eμ:T-cell leukemia/lymphoma 1 (TCL1) model is widely used in CLL research including preclinical drug testing, but has been hampered by reduced dependence on BCL2, due to protection by other antiapoptotic proteins not targeted by venetoclax such as mantel-cell lymphoma (MCL1) and B-cell lymphoma-extra large (BCL-XL).4,5 Therefore, to shift balance of antiapoptotic BCL2 proteins to BCL2 dependence we compared Eμ:TCL1; Cd19Cre (TC) mice to a new mouse model by cross-breeding TC mice with mice with conditional (Cd19Cre-driven, prevented by loxp-flanked STOP cassette in the Rosa26 locus) human BCL2 knockin6 and characterized the resulting TCB mouse model.
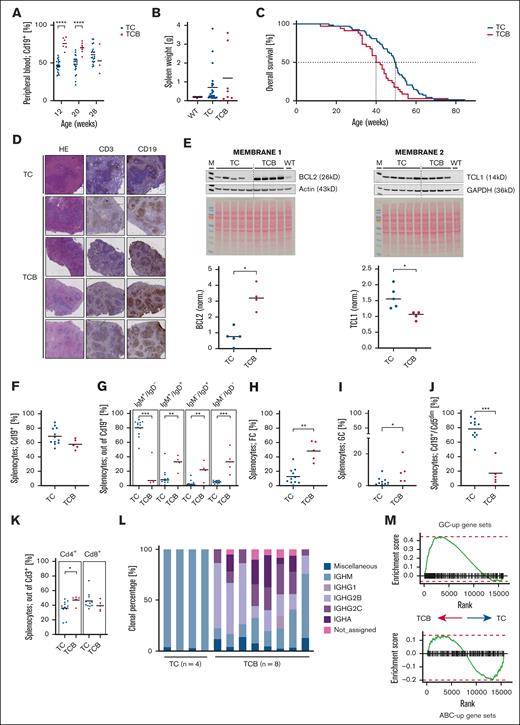
We first monitored leukocyte and B lymphocyte counts in the peripheral blood in TC vs TCB mice longitudinally. Although TCB mice initially exhibited higher leukocyte counts (white blood cells, week 12: TC, 14.2 × 109/L vs TCB, 92.2 × 109/L; P < .0001) with higher proportion of B cells in TCB vs TC mice (percentage of B cells at 12 weeks in TC 45.9% vs TCB 75.7%) in the peripheral blood (Figure 1A; supplemental Figure 1A), splenomegaly was not statistically different between both models (Figure 1B; supplemental Figure 1B), indicating that the TCB mouse model resembles a leukemic course of disease. Disease kinetics translate into a significantly shorter median overall survival of TCB mice in comparison to TC mice (TC, 50 weeks vs TCB, 41 weeks; log-rank P = .0028) (Figure 1C). Enforced BCL2 expression thus augmented the mortality of TC mice by ∼18%, which is comparable to shortened overall survival observed in TC mice with conditional deletion of Ataxia-teleangiectasie mutated (ATM) or p53.7 Immunohistochemistry revealed a remaining follicular structure in spleen tissue in TCB mice while follicular structure starts to dissolve in TC mice at week 30 (Figure 1D). Cell sizes were at comparable ranges between TC and TCB mice as determined by flow cytometry (supplemental Figure 1C). To determine that BCL2 and TCL1 cooperate in the TCB model, the protein amount was measured in purified B cells. Immunoblot analyses show significantly higher expression of BCL2 in TCB mice (P = .0159) (Figure 1E), whereas TCL1 expression is higher in TC vs TCB mice (P = .0259).
Lymphoid disease of Eμ:TCL1 mice without or with enforced B-cell–specific BCL2 expression. Heterozygous Eμ:TCL1; CD19Cre (TC) controls and Eμ:TCL1; CD19Cre; BCL2 (TCB) animals were compared. (A) The time course of the percentages of total and CLL-like B cells in the peripheral blood of TC and TCB mice was measured by flow cytometric quantification of Cd19+ leukocytes. (B) Spleen weights of wild type (WT) (n = 10), TC (n = 23), and TCB (n = 7) mice at an age of 30 weeks. (C) In cohorts of TC (n = 68) and TCB (n = 34) mice the median overall survival was 50 or 41 weeks, respectively (P = .0028). (D) Immunohistochemistry of exemplarily 1 representative TC vs 4 TCB spleens in hematoxylin and eosin (HE), CD3, and CD19 staining reveals follicular structure in TC and TCB mice at 30 weeks. (E) Lysates were generated from B cells from TC (n = 5) and TCB (n = 4) mice and splenocytes from 1 WT B6 mouse at the age of 28 to 30 weeks. Immunoblots were generated for the BCL2, antiactin, TCL1, and Glyceraldehyd-3-phosphate-dehydrogenase (GAPDH). Coomassie stainings are provided for both membranes as controls of protein quality and loading. TCL1 protein level is higher in TC mice (∗P = 0259); whereas BCL2 protein level is higher in TCB mice. (F) Splenocytes of 30-week-old TC (n = 11) and TCB (n = 5) mice were analyzed by flow cytometry and the relative amounts of different B-cell developmental stages were quantified. Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated/CD19+ population was gated. (G) Debris and doublets were excluded from singlets. Viable cells were defined via forward scatter intensity/ side scatter intensity (FS INT/SS INT). Green fluorescent protein (GFP+) population was gated. The total B-cell population (GFP+) was gated in the IgM vs IgD plot defining the subpopulations. (H) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated defining the B cells. Defining the GFP+/CD93– population out of viable cells. The total GFP+/CD93– population was gated in the CD23/CD21/35 plot. Follicular cells are defined as CD93–/CD23+/CD21/35+ B cells (GFP+). (I) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated defining the B cells. The total GFP+ population was gated in the Cd95 vs GL7 plot. Germinal center cells are defined as CD95+/GL7+ B cells (GFP+). (J) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. The total viable population was gated in the GFP vs CD5 plot defining the subpopulations. (K) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. CD3+ population was gated defining the T cells. The total CD3+ population was gated in the CD4 vs CD8 plot defining the CD4+ and CD8+ subpopulation of the CD3+ T cells. (L) Clonal percentages of IgH isotypes were determined by Ig sequencing of splenic B lymphocytes from individual animals. (M) Bulk RNA sequencing of splenic B cells was performed and evaluated as previously described for diffuse large B-cell lymphoma (DLBCL) models, using the same reference gene sets.8 The enrichment of upregulated regulated activated B-cell (ABup) and germinal center (GCup) gene sets defined by 3´ RNA sequencing from WT animals was determined by comparing the transcriptional profiles of TCB and TC lymphoma. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. Welch unpaired 2-tailed t test performed for panels A,B,E,F,G,H,I,J,K; log-rank test performed for panel C.
Lymphoid disease of Eμ:TCL1 mice without or with enforced B-cell–specific BCL2 expression. Heterozygous Eμ:TCL1; CD19Cre (TC) controls and Eμ:TCL1; CD19Cre; BCL2 (TCB) animals were compared. (A) The time course of the percentages of total and CLL-like B cells in the peripheral blood of TC and TCB mice was measured by flow cytometric quantification of Cd19+ leukocytes. (B) Spleen weights of wild type (WT) (n = 10), TC (n = 23), and TCB (n = 7) mice at an age of 30 weeks. (C) In cohorts of TC (n = 68) and TCB (n = 34) mice the median overall survival was 50 or 41 weeks, respectively (P = .0028). (D) Immunohistochemistry of exemplarily 1 representative TC vs 4 TCB spleens in hematoxylin and eosin (HE), CD3, and CD19 staining reveals follicular structure in TC and TCB mice at 30 weeks. (E) Lysates were generated from B cells from TC (n = 5) and TCB (n = 4) mice and splenocytes from 1 WT B6 mouse at the age of 28 to 30 weeks. Immunoblots were generated for the BCL2, antiactin, TCL1, and Glyceraldehyd-3-phosphate-dehydrogenase (GAPDH). Coomassie stainings are provided for both membranes as controls of protein quality and loading. TCL1 protein level is higher in TC mice (∗P = 0259); whereas BCL2 protein level is higher in TCB mice. (F) Splenocytes of 30-week-old TC (n = 11) and TCB (n = 5) mice were analyzed by flow cytometry and the relative amounts of different B-cell developmental stages were quantified. Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated/CD19+ population was gated. (G) Debris and doublets were excluded from singlets. Viable cells were defined via forward scatter intensity/ side scatter intensity (FS INT/SS INT). Green fluorescent protein (GFP+) population was gated. The total B-cell population (GFP+) was gated in the IgM vs IgD plot defining the subpopulations. (H) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated defining the B cells. Defining the GFP+/CD93– population out of viable cells. The total GFP+/CD93– population was gated in the CD23/CD21/35 plot. Follicular cells are defined as CD93–/CD23+/CD21/35+ B cells (GFP+). (I) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated defining the B cells. The total GFP+ population was gated in the Cd95 vs GL7 plot. Germinal center cells are defined as CD95+/GL7+ B cells (GFP+). (J) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. The total viable population was gated in the GFP vs CD5 plot defining the subpopulations. (K) Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. CD3+ population was gated defining the T cells. The total CD3+ population was gated in the CD4 vs CD8 plot defining the CD4+ and CD8+ subpopulation of the CD3+ T cells. (L) Clonal percentages of IgH isotypes were determined by Ig sequencing of splenic B lymphocytes from individual animals. (M) Bulk RNA sequencing of splenic B cells was performed and evaluated as previously described for diffuse large B-cell lymphoma (DLBCL) models, using the same reference gene sets.8 The enrichment of upregulated regulated activated B-cell (ABup) and germinal center (GCup) gene sets defined by 3´ RNA sequencing from WT animals was determined by comparing the transcriptional profiles of TCB and TC lymphoma. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; and ∗∗∗∗P < .0001. Welch unpaired 2-tailed t test performed for panels A,B,E,F,G,H,I,J,K; log-rank test performed for panel C.
To further characterize and to compare both models flow cytometrical immune phenotyping was performed. Both models show comparable B-cell proportions in their spleens (Figure 1F-G), with immune-phenotypic differences between TC and TCB mice as CD5 expression is significantly weaker in TCB mice (Figure 1J; supplemental Figure 1D). B cells from TC mice exhibit predominantly an immature IgM+ IgD– and Cd19+/Cd5dim CLL-like phenotype, while TCB mice show a class-switched oligoclonal disease with mainly IgM+ IgD+ or IgM– IgD– Cd19+/Cd5– germinal center (GC) phenotype (Figure 1G-J; supplemental Figure 1D-G), reminiscent of the VavP-BCL2 model; however, our TCB mouse model offers further leukemic aspects by showing leukocytosis (supplemental Figure 1A).9 Interestingly, T-cell ratio shifts toward CD4+ in TCB mice with higher Cd4+ counts in TCB mice (P = .0263) with comparable CD8+ counts in both models (Figure 1K).
To confirm the clonal character of these class-switched B cells, variable, diversity, joining (VDJ) hypermutation analysis and immunoglobulin (Ig) sequencing of splenic B cells identified besides known IgM clones also a class switch to other isotypes and (oligo)clonal Ig rearrangements in TCB mice (Figure 1L; supplemental Figures 1H and 2A). As VDJ hypermutation is absent in two-third of CLL cases, we determined VDJ hypermutation in TC vs TCB mice. Sequence homology below 98% was defined as hypermutated. These analyses revealed differences between TC and TCB mice; whereas all TC mice showed an unmutated sequence, TCB mice showed both mutated and unmutated sequences (supplemental Figure 1H). Transcriptomic profiling using previously described methodology and evaluation,8 revealed positive normalized enrichment scores for GC upregulated gene sets in TCB mice but no positive association for activated B-cell gene sets (Figure 1M; supplemental Figure 2B) supporting our observations that B cells from TCB mice exhibit a GC gene expression pattern. Accordingly, unsupervised clustering of predefined gene sets assigned to distinct GC B-cell populations8 clearly distinguished transcriptome patterns of these populations in TCB and TC mice (supplemental Figure 2C). Taken together, TCB mice exhibited earlier onset of more aggressive, leukemic disease, with oligoclonal B lymphocytes of largely GC phenotype with both VDJ unmutated and hypermutated cases. Thus, B-cell–specific human BCL2 expression in Eμ:TCL1 mice did not result in transformation of CLL-like cells as the expression of myristoylated AKT serine/threonine kinase (Myr-Akt),10 but rather in a oligoclonal lymphoma population comparable to the double-transgenic Eμ:TCL1xMyc mice.11,12 In our query of publicly available data sets, notably, cancer cell lines and primary germinal center–derived B-cell lymphomas exhibited high coexpression of TCL1A and BCL2, including B-cell acute lymphoblastic leukemia (B-ALL), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL) (supplemental Figure 2D-F). We hypothesize that the immune phenotype of our model is caused by expression of both TCL1 and BCL2, as TCL1 is frequently expressed in GC-type lymphoma with coexpression of BCL213,14 (Figure 1E). Increased survival capacity of B cells due to TCL1 and BCL2 expression might cause prolonged GC reactions.
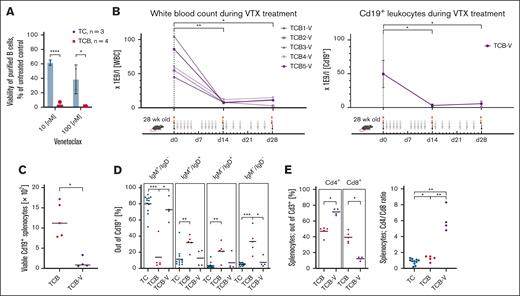
As our main goal was to establish a novel model for investigation of BCL2-inhibitors in vivo, we next examined the viability of B cells isolated from TC and TCB mice in vitro when exposed to venetoclax (Figure 2A). Viability was determined by bioluminescence adenosine triphosphate (ATP) measurements after 24 hours in vitro treatment with 10 or 100 nM venetoclax. Treatment with venetoclax led to a significantly stronger reduction of B-cell viability from TCB than TC mice (Figure 2A) indicating that overexpression of BCL2 potentiates susceptibility to venetoclax.3,5 Based on these observations, we initiated in vivo treatment of TCB mice with venetoclax for 4 weeks by oral gavage (Figure 2B). Strikingly, the elevated leukocyte counts and the amount of CD19+ B cells in the peripheral blood and the spleens of TCB mice were efficiently reduced to normal levels after venetoclax treatment (Figure 2B-C) indicating that TCB mice are sensitive toward venetoclax not only in vitro but also in vivo. Furthermore, the remaining B cells represented mainly IgM+/IgD– but also IgM+/IgD+ and IgM– phenotypes (Figure 2D). Notably, among splenic T lymphocytes, venetoclax treatment increased the CD4/CD8 ratio 4.5-fold by further normalizing the balance of these T-cell subpopulations (Figure 2E), similarly as in the adoptive transfer Eμ:TCL1 model.15 Although our observation here are limited to the early effects of venetoclax treatment in the TCB mouse model, future experiments will be extended to longer follow-ups to investigate selection pressure of venetoclax based treatments. In summary, overexpression of BCL2 in the autochthonous Eμ:TCL1 model resulted in a venetoclax-responsive leukemia model. This model will allow to study the therapeutic effects of venetoclax and the role of different proapoptotic/antiapoptotic molecules, to determine networks of genes, which mediate venetoclax resistance or to identify drugs which act synergistically with venetoclax, such as Bruton tyrosine kinase inhibitors (ibrutinib, acalabrutinib, and noncovalent binding Bruton tyrosine kinase inhibitors).15,16 Finally, our autochthonous model can also be used to investigate effects on cells of the tumor microenvironment.17
Increased sensitivity of TCB mice to venetoclax. (A) The viability of murine splenocytes (purified B cells) relative to corresponding untreated samples was assessed via annexin V binding or adenosine triphosphate (ATP) content after overnight in vitro treatment with 10 or 100 nM venetoclax, respectively. P values refer to the comparison of isolated B splenocytes from TC and TCB mice. (B) Five 28-week-old TCB mice were subjected to intragastric (p.o.) administration of venetoclax (200 mg/kg) on the indicated days for 4 weeks. Their white blood cell counts and Cd19+ population (day 0 n = 4; day 14 n = 5; day 28 n = 4; day 0 to day 14, ∗P = .0159; day 0 to day 28, ∗P= .0286) were longitudinally followed during the treatment period. There is a significant reduction of white blood cell count in TCB mice within 14 days of venetoclax (VTX) treatment (day 0 to day 14, ∗∗P = .0079; day 0 to day 28, ∗P = .159). Numbers of B cells were calculated as follows: white blood cells × (% of B cells of viable cells per 100). After 28 days of treatment all mice were sacrificed and immunophenotyped. (C) Viable splenocytes were counted and multiplied by the proportion of B cells in the spleen. The absolute numbers of CD19+ splenocytes between untreated and venetoclax treated TCB mice were compared. (D) Splenocytes of 30-week-old TC (n = 12), TCB (n = 5), and TCB-V (n = 4) mice were analyzed by flow cytometry and the relative amounts of different B-cell developmental stages were quantified. Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated. The total B-cell population (GFP+) was gated in the IgM vs IgD plot defining the subpopulations. (E) Among splenic T cells from untreated or venetoclax-treated mice, subpopulations with CD4 or CD8 expression were distinguished by flow cytometry. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Welch unpaired 2-tailed t test performed for panels A-E.
Increased sensitivity of TCB mice to venetoclax. (A) The viability of murine splenocytes (purified B cells) relative to corresponding untreated samples was assessed via annexin V binding or adenosine triphosphate (ATP) content after overnight in vitro treatment with 10 or 100 nM venetoclax, respectively. P values refer to the comparison of isolated B splenocytes from TC and TCB mice. (B) Five 28-week-old TCB mice were subjected to intragastric (p.o.) administration of venetoclax (200 mg/kg) on the indicated days for 4 weeks. Their white blood cell counts and Cd19+ population (day 0 n = 4; day 14 n = 5; day 28 n = 4; day 0 to day 14, ∗P = .0159; day 0 to day 28, ∗P= .0286) were longitudinally followed during the treatment period. There is a significant reduction of white blood cell count in TCB mice within 14 days of venetoclax (VTX) treatment (day 0 to day 14, ∗∗P = .0079; day 0 to day 28, ∗P = .159). Numbers of B cells were calculated as follows: white blood cells × (% of B cells of viable cells per 100). After 28 days of treatment all mice were sacrificed and immunophenotyped. (C) Viable splenocytes were counted and multiplied by the proportion of B cells in the spleen. The absolute numbers of CD19+ splenocytes between untreated and venetoclax treated TCB mice were compared. (D) Splenocytes of 30-week-old TC (n = 12), TCB (n = 5), and TCB-V (n = 4) mice were analyzed by flow cytometry and the relative amounts of different B-cell developmental stages were quantified. Debris and doublets were excluded from singlets. Viable cells were defined via FS INT/SS INT. GFP+ population was gated. The total B-cell population (GFP+) was gated in the IgM vs IgD plot defining the subpopulations. (E) Among splenic T cells from untreated or venetoclax-treated mice, subpopulations with CD4 or CD8 expression were distinguished by flow cytometry. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Welch unpaired 2-tailed t test performed for panels A-E.
Acknowledgments: The authors thank Olaf Merkel and Julia Claasen for excellent technical assistance. The authors also thank the Cologne Center for Genomics for performing the RNA sequencing.
This work was supported by an Fonds zur Förderung der wissenschaftlichen Forschung Grant P 34977 (A.E.); by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Sonderforschungsbereich-Geschäftszeichen, 455784452, projects A01 (H.C.R.), A06 and Z02 (L.P.F.), and project B01 (M.H.); and by a project grant (A05) by the Center for Molecular Medicine Cologne (L.P.F. and M.H.).
Contribution: I.H. and L.P.F. developed the study concept and wrote the initial manuscript; I.H., D.A., L.B., and G. Knittel performed the experiments; G. Knittel and D.A. provided bioinformatic analyses; H.C.R., H.K., M.H., A.E., G. Krause, B.C., J.L., A.d.P.G., and L.P.F. provided data interpretation and intellectual input, and revised the original manuscript; and H.K. provided BCL2 knockin mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lukas P. Frenzel, Department I of Internal Medicine, University of Cologne, Joseph-Stelzmann-Str 26, 50937 Cologne, Germany; email: lukas.frenzel@uk-koeln.de.
References
Author notes
A.d.P.G and L.P.F. contributed equally to this work.
The transcriptome sequencing data are available at the Sequence Read Archive (project ID PRJNA1190676): https://dataview.ncbi.nlm.nih.gov/object/PRJNA1190676?reviewer=ikesgoldoqq0l1qbfa1ia394vg.
All data generated or analyzed during this study are included in this published article and its `supplementary information files. A section on materials and methods for all experiments performed is provided as a supplementary document.
The full-text version of this article contains a data supplement.