Visual Abstract
TO THE EDITOR:
FMS-like tyrosine kinase 3 (FLT3) mutations, including internal tandem duplication (ITD) and tyrosine kinase domain, are frequently observed in acute myeloid leukemia (AML). The prognosis of relapsed and/or refractory (R/R) FLT3-ITD AML is poor, even after allogeneic hematopoietic stem cell transplantation (HSCT).1 Recent studies have shown that FLT3 inhibitor maintenance improves outcomes in both newly diagnosed and R/R AML. The QuANTUM-R study demonstrated improved survival with quizartinib maintenance therapy,2 and the MORPHO study also demonstrated an improvement in relapse-free survival (RFS) in patients with measurable residual disease (MRD) positivity with gilteritinib maintenance therapy.3 Despite a 2-year planned duration in these trials, after discontinuation, relapse has been reported, and the optimal maintenance duration remains uncertain.
To address relapse risk in R/R FLT3–mutated AML after HSCT, we previously reported that low-dose and early-initiation gilteritinib maintenance (40 mg per day), initiated at a median of 36 days after HSCT, improved outcomes even in patients with complications such as graft-versus-host disease (GVHD) or thrombocytopenia.4 In the report, the median maintenance duration was 6 months, but long-term data were limited. Therefore, we report extended follow-up to evaluate long-term outcomes in patients with R/R FLT3–mutated AML receiving gilteritinib maintenance.
After a median follow-up of 36.4 months (range, 3.3-92.5), we retrospectively analyzed 25 patients with R/R FLT3–mutated AML, who underwent allogeneic HSCT at our center between 1 January 2011 and 30 April 2022 (Table 1). Fourteen received gilteritinib maintenance after HSCT (gil group) and 11 did not (nongil group). Graft sources were comparable, although cord blood transplantation (CBT) was more frequent in the gil group (42.9% vs 27.3%). The median CD34+ cell dose in the cord blood was 1.06 × 106 in the gil group vs 1.29 × 106 in the nongil group (P = .439). FLT3 inhibitors were more commonly used as bridging therapy before HSCT in the gil group (n = 11, 78.6%) than in the nongil group (n = 2, 18.2%; P = .050). None received frontline FLT3 inhibitors during the initial AML treatment. However, 4 patients received quizartinib during the R/R phase, including reinduction (n = 2), bridging (n = 1), and post-HSCT maintenance (n = 1). The reasons for not administering the gilteritinib maintenance therapy in 11 patients in the nongil group were because it was before the approval of gilteritinib in the health insurance system in Japan (n = 4) and based on the physician’s assessment of the risks and benefits of maintenance therapy (n = 7).
Patient characteristics
| Factors . | All patients . | With gilteritinib . | Without gilteritinib . | P value . |
|---|---|---|---|---|
| n = 25 . | n = 14 . | n = 11 . | ||
| Age, y (median [range]) | 52 (25-72) | 50 (32-72) | 53 (25-70) | .94 |
| Transplantation years before 2018, n (%) | 6 (24%) | 2 (14.3) | 4 (36.4) | .35 |
| Conventional karyotype, n (%) | ||||
| Normal karyotype | 17 (68.0) | 10 (71.4) | 7 (63.6) | 1.00 |
| FLT3-ITD/TKD, n (%) | ||||
| ITD | 20 (80.0) | 11 (78.6) | 9 (81.8) | 1.00 |
| TKD | 3 (12.0) | 2 (14.3) | 1 (9.1) | |
| Both | 2 (8.0) | 1 (7.1) | 1 (9.1) | |
| Prior administration of FLT3-inhibitor before HSCT, n (%) | ||||
| Yes | 19 (76.0) | 14 (100) | 5 (45.5) | .003 |
| AML status at HSCT, n (%) | ||||
| CR, MRD-negative | 6 (24.0) | 2 (14.3) | 3 (27.3) | .28 |
| CR, MRD-positive | 12 (48.0) | 10 (71.4) | 4 (36.4) | |
| Non-CR | 6 (24.0) | 2 (14.3) | 4 (36.4) | |
| No. of HSCT, n (%) | ||||
| First HSCT | 19 (76.0) | 11 (78.6) | 8 (72.7) | .79 |
| Second HSCT | 5 (20.0) | 2 (14.3) | 3 (27.3) | |
| Third HSCT | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| Conditioning intensity, n (%) | ||||
| MAC | 17 (68.0) | 9 (64.3) | 8 (72.7) | 1 |
| HCT-CI (%)∗ | ||||
| 0 | 4 (20.0) | 2 (16.7) | 2 (25.0) | .88 |
| 1 | 11 (55.0) | 6 (50.0) | 5 (62.5) | |
| 2 | 2 (10.0) | 1 (8.3) | 1 (12.5) | |
| ≥3 | 3 (15.0) | 3 (27.3) | 0 (0.0) | |
| Donor selection, n (%) | ||||
| 8 of 8 matched related | 5 (20.0) | 3 (21.4) | 2 (18.2) | .85 |
| 8 of 8 matched unrelated | 3 (12.0) | 1 (7.1) | 2 (18.2) | |
| 7 of 8 mismatched unrelated | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| ≤ 6 of 8 haploidentical | 7 (28.0) | 3 (21.4) | 4 (36.4) | |
| Cord blood | 9 (36.0) | 6 (42.9) | 3 (27.3) | |
| CD34+cells/CB unit (median 106cells [range]) | 1.11 (0.80-1.83) | 1.06 (0.80-1.83) | 1.29 (0.96-1.31) | .44 |
| Bridging chemotherapy before HSCT, n (%) | ||||
| Gilteritinib | 10 (40.0) | 8 (57.1) | 2 (18.2) | .05 |
| Quizartinib | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| Gilteritinib + α† | 2 (8.0) | 2 (14.3) | 0 (0.0) | |
| Intensive chemotherapy | 6 (24.0) | 2 (14.3) | 4 (36.4) | |
| Low-dose chemotherapy | 4 (20.0) | 1 (7.1) | 3 (27.3) | |
| None | 2 (10.0) | 0 (0.0) | 2 (18.2) | |
| Post-HSCT status at day 28-45, n (%) | ||||
| CR, MRD-negative | 8 (32.0) | 3 (21.4) | 5 (45.5) | .14 |
| CR, MRD-positive | 16 (64.0) | 11 (78.6) | 5 (45.5) | |
| CR, not MRD-evaluated | 1 (4.0) | 0 (0.0) | 1 (9.1) |
| Factors . | All patients . | With gilteritinib . | Without gilteritinib . | P value . |
|---|---|---|---|---|
| n = 25 . | n = 14 . | n = 11 . | ||
| Age, y (median [range]) | 52 (25-72) | 50 (32-72) | 53 (25-70) | .94 |
| Transplantation years before 2018, n (%) | 6 (24%) | 2 (14.3) | 4 (36.4) | .35 |
| Conventional karyotype, n (%) | ||||
| Normal karyotype | 17 (68.0) | 10 (71.4) | 7 (63.6) | 1.00 |
| FLT3-ITD/TKD, n (%) | ||||
| ITD | 20 (80.0) | 11 (78.6) | 9 (81.8) | 1.00 |
| TKD | 3 (12.0) | 2 (14.3) | 1 (9.1) | |
| Both | 2 (8.0) | 1 (7.1) | 1 (9.1) | |
| Prior administration of FLT3-inhibitor before HSCT, n (%) | ||||
| Yes | 19 (76.0) | 14 (100) | 5 (45.5) | .003 |
| AML status at HSCT, n (%) | ||||
| CR, MRD-negative | 6 (24.0) | 2 (14.3) | 3 (27.3) | .28 |
| CR, MRD-positive | 12 (48.0) | 10 (71.4) | 4 (36.4) | |
| Non-CR | 6 (24.0) | 2 (14.3) | 4 (36.4) | |
| No. of HSCT, n (%) | ||||
| First HSCT | 19 (76.0) | 11 (78.6) | 8 (72.7) | .79 |
| Second HSCT | 5 (20.0) | 2 (14.3) | 3 (27.3) | |
| Third HSCT | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| Conditioning intensity, n (%) | ||||
| MAC | 17 (68.0) | 9 (64.3) | 8 (72.7) | 1 |
| HCT-CI (%)∗ | ||||
| 0 | 4 (20.0) | 2 (16.7) | 2 (25.0) | .88 |
| 1 | 11 (55.0) | 6 (50.0) | 5 (62.5) | |
| 2 | 2 (10.0) | 1 (8.3) | 1 (12.5) | |
| ≥3 | 3 (15.0) | 3 (27.3) | 0 (0.0) | |
| Donor selection, n (%) | ||||
| 8 of 8 matched related | 5 (20.0) | 3 (21.4) | 2 (18.2) | .85 |
| 8 of 8 matched unrelated | 3 (12.0) | 1 (7.1) | 2 (18.2) | |
| 7 of 8 mismatched unrelated | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| ≤ 6 of 8 haploidentical | 7 (28.0) | 3 (21.4) | 4 (36.4) | |
| Cord blood | 9 (36.0) | 6 (42.9) | 3 (27.3) | |
| CD34+cells/CB unit (median 106cells [range]) | 1.11 (0.80-1.83) | 1.06 (0.80-1.83) | 1.29 (0.96-1.31) | .44 |
| Bridging chemotherapy before HSCT, n (%) | ||||
| Gilteritinib | 10 (40.0) | 8 (57.1) | 2 (18.2) | .05 |
| Quizartinib | 1 (4.0) | 1 (7.1) | 0 (0.0) | |
| Gilteritinib + α† | 2 (8.0) | 2 (14.3) | 0 (0.0) | |
| Intensive chemotherapy | 6 (24.0) | 2 (14.3) | 4 (36.4) | |
| Low-dose chemotherapy | 4 (20.0) | 1 (7.1) | 3 (27.3) | |
| None | 2 (10.0) | 0 (0.0) | 2 (18.2) | |
| Post-HSCT status at day 28-45, n (%) | ||||
| CR, MRD-negative | 8 (32.0) | 3 (21.4) | 5 (45.5) | .14 |
| CR, MRD-positive | 16 (64.0) | 11 (78.6) | 5 (45.5) | |
| CR, not MRD-evaluated | 1 (4.0) | 0 (0.0) | 1 (9.1) |
CR, complete remission; HCT-CI, hematopoietic cell transplant–comorbidity index; MAC, myeloablative conditioning regimen; TKD, tyrosine kinase domain.
n = 20.
Gilteritinib with 3 + 7 (n =1) and gilteritinib with venetoclax and azacitidine (n = 1).
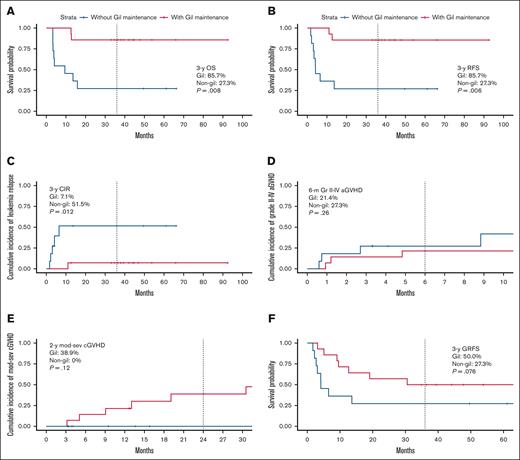
Regarding the survival outcomes, the 3-year overall survival was 85.7% (95% confidence interval [CI], 69.2-100.0) in the gil group and 27.3% (95% CI, 10.4-71.6) in the nongil group (P =.008, Figure 1A). Similarly, the 3-year RFS was 85.7% (95% CI, 69.2-100.0) in the gil group and 27.3% (95% CI, 10.4-71.6) in the nongil group (P =.006, Figure 1B). The 3-year cumulative incidence of relapse was 7.1% (95% CI, 0.0-19.7) in the gil group and 51.5% (95% CI, 5.1-75.2) in the nongil group (P =.012, Figure 1C).
The survival outcomes and river plot with or without gilteritinib maintenance therapy. (A) OS from transplantation (3-year OS; 85.7% [95% CI, 69.2-100.0] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P =.008). (B) RFS from transplantation (3-year OS; 85.7% [95% CI, 69.2-100.0] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P =.006). (C) CIR from transplantation (3-year CIR; 7.1% [95% CI, 0.0-19.7] in the gil group vs 51.5% [95% CI, 5.1-75.2] in the nongil group; P = .012). (D) Grade 2 to 4 acute GVHD (6-month grade 2-4 acute GVHD; 21.4% [95% CI, 0.0-40.2] in the gil group vs 27.3% [95% CI, 0.0-49.4] in the nongil group; P = .31). (E) Moderate-to-severe chronic GVHD (2-year moderate-to-severe chronic GVHD; 38.9% [95% CI, 4.8-60.8] in the gil group vs 0% [95% CI, 0-0] in the nongil group; P = .12). (F) GRFS from transplantation (3-year GRFS; 50% [95% CI, 29.6-84.4] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P = .076). (G) The river plot is shown. The top river is the gil group, whereas the bottom river is the nongil group. CIR, cumulative incidence of relapse; GRFS, GVHD-free, relapse-free survival; OS, overall survival.
The survival outcomes and river plot with or without gilteritinib maintenance therapy. (A) OS from transplantation (3-year OS; 85.7% [95% CI, 69.2-100.0] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P =.008). (B) RFS from transplantation (3-year OS; 85.7% [95% CI, 69.2-100.0] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P =.006). (C) CIR from transplantation (3-year CIR; 7.1% [95% CI, 0.0-19.7] in the gil group vs 51.5% [95% CI, 5.1-75.2] in the nongil group; P = .012). (D) Grade 2 to 4 acute GVHD (6-month grade 2-4 acute GVHD; 21.4% [95% CI, 0.0-40.2] in the gil group vs 27.3% [95% CI, 0.0-49.4] in the nongil group; P = .31). (E) Moderate-to-severe chronic GVHD (2-year moderate-to-severe chronic GVHD; 38.9% [95% CI, 4.8-60.8] in the gil group vs 0% [95% CI, 0-0] in the nongil group; P = .12). (F) GRFS from transplantation (3-year GRFS; 50% [95% CI, 29.6-84.4] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P = .076). (G) The river plot is shown. The top river is the gil group, whereas the bottom river is the nongil group. CIR, cumulative incidence of relapse; GRFS, GVHD-free, relapse-free survival; OS, overall survival.
The cumulative incidence of grade 2 to 4 and grade 3 to 4 acute GVHD (aGVHD) at 6 months were similar in both groups (grade 2-4 aGVHD; 21.4% [95% CI, 0.0-40.2] in the gil group vs 27.3% [95% CI, 0.0-49.4] in the nongil group, P =.31, Figure 1D, and grade 3-4 aGVHD; 0% [95% CI, 0-0] in the gil group vs 9.1% [95% CI, 0.0-24.6] in the nongil group, P =.26). However, the cumulative incidence of moderate-to-severe chronic GVHD at 2 years was higher in the gil group (38.9%; 95% CI, 4.8-60.8) than the nongil group (0%; 95% CI, 0-0; P =.12, Figure 1E). Despite the high incidence of chronic GVHD in the gil group, the 3-year GVHD-free, RFS was 50% (95% CI, 29.6-84.4) in the gil group and 27.3% (95% CI, 10.4-71.6) in the nongil group (P =.076, Figure 1F).
A river plot illustrating the clinical course of all patients is shown in Figure 1G. The median duration of gilteritinib maintenance in the gil group was 31.5 months (range, 1.5-52.7). Ten patients (71.4%) discontinued therapy, with a median duration of 26.5 months (range, 1.5-36.5), significantly shorter than that of the 4 patients who continued treatment (36.3 months; range, 32.2-52.7; P =.024). Reasons for discontinuation included physician decision (n = 5), complications (n = 4), and relapse (n = 1). Complications were pelvic fracture, gastrointestinal symptoms, an increase in creatine kinase, and pancytopenia (n = 1 each). The median time from discontinuation to last follow-up was 16.4 months (range, 0.2-60.6), with 1 relapse (patient number 14), who died from progressive AML after donor lymphocyte infusion. Among 10 pre-HSCT MRD-positive patients, the median therapy duration was 32.9 months (range, 10.3-37.2), and none relapsed during or after discontinuation.
We conducted a retrospective, extended-cohort, 3-year follow-up study of low-dose, early-start gilteritinib maintenance therapy with a median duration of 31.5 months. Although our study is not a prospective study, comparable long-term prospective data on our unique treatment approach are lacking. Two key findings emerged. First, none of the patients in the gil group relapsed after >2 years of maintenance therapy.
In previous clinical trials on maintenance therapy using FLT3 inhibitors, the planned treatment duration was typically 1 to 2 years.2,3,5,6 However, on the basis of RFS data from these studies, some MRD-positive patients before HSCT relapsed after stopping FLT3 inhibitor maintenance. Based on these previous reports, we aimed to continue maintenance therapy for over 2 years when feasible. In this extended-cohort study, the median duration of maintenance therapy was 31.5 months in the entire gil group and 36.3 months in those who continued therapy. Even among those who discontinued treatment, the median duration was 26.5 months. Only 1 patient relapsed; notably, most in the discontinuation group, including some pre-HSCT MRD-positive patients, remained relapse-free after a median of 16.4 months after discontinuation. Our data needs careful consideration based on several clinical factors, including the retrospective nature, the relatively high proportion of mismatched CBT, the higher proportion of patients not being in remission, and potential reverse causation bias. Of note, our gil group included more patients with CBT than those in the nongil group (42.9% vs 27.3%). Therefore, further studies are warranted to evaluate the efficacy and appropriate duration of gilteritinib maintenance therapy in patients who have not undergone CBT. However, on the basis of our overall results, 2 years of maintenance therapy may not be sufficient, and maintenance therapy for >2 years or up to 3 years may be preferable.
Another finding was the higher incidence of chronic GVHD in the gil group. Prior studies reported ∼10% higher rates in FLT3 inhibitor groups vs placebo (29.4% in the quizartinib group vs 19.8% in the placebo group, P value not described in the QuANTUM-First trial7; and 52.2% in the gilteritinib group vs 42.4% in the placebo group, P =.181 in the MORPHO trial3). Chronic GVHD is a long-term post-HSCT complication. Although survivor bias—relapse preceding chronic GVHD in nonmaintenance patients—must be considered, our data also showed that moderate-to-severe chronic GVHD at 2 years was numerically higher in the gil group (38.9%; 95% CI, 4.8-60.8) than in the nongil group (0%; 95% CI, 0-0; P =.12). Although the biological mechanism was not elucidated, previous studies have shown that sorafenib8 and gilteritinib9 maintenance therapy induced a graft-versus-leukemia effect by inducing interleukin-15 secretion from residual AML cells. Conversely, interleukin-15 has been implicated in chronic GVHD development in a mouse model model10 through activation of the JAK1/STAT3 (signal transducer and activator of transcription protein 3) and JAK3/STAT5 pathways.11 To continue gilteritinib maintenance therapy safely, control of chronic GVHD, in addition to the adverse events of gilteritinib itself, is necessary. Discovering the association between gilteritinib therapy and chronic GVHD, as well as the ideal duration of gilteritinib maintenance, are important clinical aspects that can only be addressed in a prospective controlled clinical trial.
In conclusion, our retrospective study provides 3-year follow-up data of low-dose, early-start gilteritinib maintenance therapy in patients with R/R FLT3–mutated AML. Although our data needs careful interpretation due to the selection bias and therefore, differences in patient background, our novel findings on the efficacy of maintenance therapy for >2 years and the impact of maintenance therapy on the onset of chronic GVHD could contribute to the development of treatment plans for patients with R/R FLT3–mutated AML. However, further large-scale, prospective, randomized trials are required to determine whether our unique low-dose, early-start gilteritinib maintenance therapy for >2 years reduces late relapse or relapse after the discontinuation of maintenance therapy.
Acknowledgments: The authors would like to thank the patients with hematologic malignancy, their families, and the medical staff of the Department of Hematology and Oncology of Okayama University Hospital. They also thank Editage for English language editing. The graphical abstract was created with BioRender.com.
Contribution: T.T. conceived and designed the study, collected data, performed the statistical analysis, wrote the manuscript, and provided patient care; K.-i.M. designed and supervised the research and edited the manuscript; T.K. collected data and provided patient care; Y.M. supervised this study and edited the manuscript; and all authors reviewed and approved the manuscript.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the institutional review board (approval no. 2502-010). This is a retrospective observational study and obtaining written consent was not mandatory. All participants did not provide any denial to this study documents that were made available to them in an opt-out manner on the web. The opt-out method was approved by the institutional review board.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshiki Terao, Department of Hematology and Oncology, Okayama University Hospital, 2-5-1, Shikata-cho, Kita-ku, Okayama 700-8558, Japan; email: tarao.toshiki.0127@gmail.com.
References
Author notes
The data sets generated during and/or analyzed during the current study are available on reasonable request from the corresponding author, Toshiki Terao (tarao.toshiki.0127@gmail.com).


![The survival outcomes and river plot with or without gilteritinib maintenance therapy. (A) OS from transplantation (3-year OS; 85.7% [95% CI, 69.2-100.0] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P =.008). (B) RFS from transplantation (3-year OS; 85.7% [95% CI, 69.2-100.0] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P =.006). (C) CIR from transplantation (3-year CIR; 7.1% [95% CI, 0.0-19.7] in the gil group vs 51.5% [95% CI, 5.1-75.2] in the nongil group; P = .012). (D) Grade 2 to 4 acute GVHD (6-month grade 2-4 acute GVHD; 21.4% [95% CI, 0.0-40.2] in the gil group vs 27.3% [95% CI, 0.0-49.4] in the nongil group; P = .31). (E) Moderate-to-severe chronic GVHD (2-year moderate-to-severe chronic GVHD; 38.9% [95% CI, 4.8-60.8] in the gil group vs 0% [95% CI, 0-0] in the nongil group; P = .12). (F) GRFS from transplantation (3-year GRFS; 50% [95% CI, 29.6-84.4] in the gil group vs 27.3% [95% CI, 10.4-71.6] in the nongil group; P = .076). (G) The river plot is shown. The top river is the gil group, whereas the bottom river is the nongil group. CIR, cumulative incidence of relapse; GRFS, GVHD-free, relapse-free survival; OS, overall survival.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/2/4/10.1016_j.bneo.2025.100141/2/m_bneo_neo-2025-000687-gr1g.jpeg?Expires=1767696094&Signature=VBqclL8bw3cnrREogmrtEA8WlIW8Evl9cpYKtpt7FSBGewv26JsJQJlOx6YmJ30EyBSAF0S3QU2VEvXyyVPxert1lX7Pq5AEVhBBxj5UjHgXWvpw0ZkGn8e--7rmrD~klfHYWjUdHbL8tjRwVlNMgkJuEXWq8n0xOHDATLZPqJXCqjYQ-wzkO1AQYQJmmvyEtGsv~wvl6NJ2Ax0KZn3ovShmTT7Faa85EmDdn-p8tyc~L7e~z5ubIbvvzFQEMqaIWDBFu6GvNMT18K~E61q3-0fKRhtlP2I3ooZDwZCbPCoTB0RiN3xmjIdBfvqR-oG4ytLdHbGl~C502tlLsh6vVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)