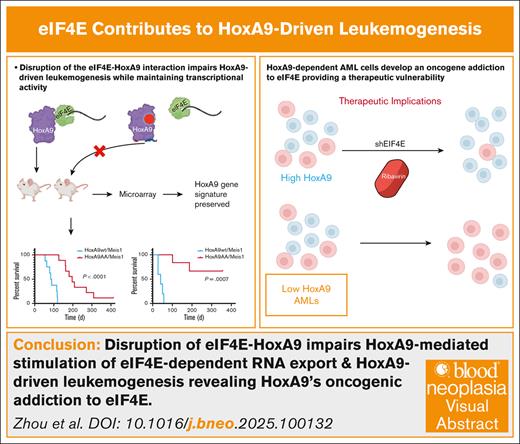
Visual Abstract
TO THE EDITOR:
More than 50% of patients with acute myeloid leukemia (AML) are characterized by twofold to eightfold elevation of homeobox A9 (HoxA9) protein which predicts poor outcomes.1-8 The pathogenic activities of HoxA9 are attributed to its transcriptional activity.7 However, HoxA9 acts beyond transcription but its relevance to leukemogenesis was unknown. For example, HoxA9 physically interacts with the eukaryotic translation initiation factor 4E (eIF4E), also dysregulated in AML.9 In many patients, eIF4E is highly elevated and nuclear-enriched corresponding to inappropriate elevation of eIF4E-dependent nuclear export of oncogenic messenger RNA (mRNAs), an activity tied to its oncogenic capacity.10-12 eIF4E has been targeted in AML clinical trials with ribavirin, a competitor of its normal ligand the m7G RNA cap.11,13,14 Ribavirin targeted eIF4E in patients corresponding to objective clinical responses, including complete remissions.11,13,14 Interestingly, eIF4E and HoxA9 interacted in vitro, in specimens from patients of primary AML, and HoxA9 stimulated eIF4E activities in mRNA export and translation in AML.9 However, the relevance to HoxA9-mediated leukemogenesis was not examined. Here, we demonstrated that HoxA9 relies on eIF4E for leukemogenesis revealing a new therapeutic vulnerability in HoxA9-dependent AML.
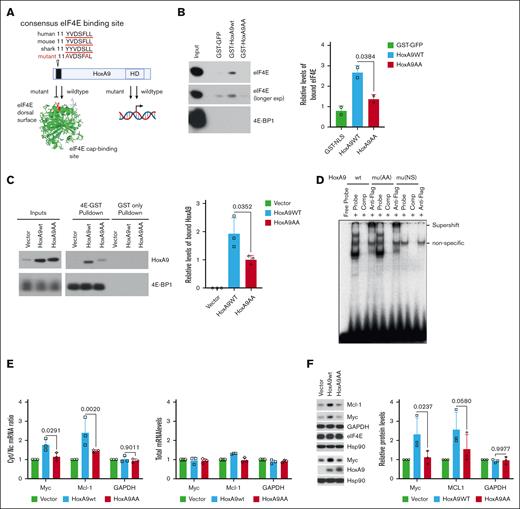
To disrupt the HoxA9-eIF4E axis, we targeted its conserved eIF4E-binding motif (11-YVDSFLL-17),9 mutating residues 11 and 16 to alanine (11-AVDSFAL-17, HoxA9AA; Figure 1A). Purified wildtype glutathione S-transferase (GST)–HoxA9 bound to endogenous eIF4E in K562 cell lysates, whereas HoxA9AA only exhibited background binding for GST (Figure 1B). No interaction was observed with the negative control, eIF4E binding-protein 1. In K562 cells stably overexpressing the respective HoxA9 constructs, purified GST-eIF4E bound to HoxA9 wild type (HoxA9wt), whereas HoxA9AA was much weaker (Figure 1C). HoxA9 and HoxA9AA proteins were produced at equivalent levels which were approximately threefold more than endogenous HoxA9 present in vector (Figure 1C, left panel). GST-eIF4E bound to eIF4E-bindingprotein 1 (4E-BP1), a positive control, but not GST (Figure 1C). Bait protein loading was equivalent (supplemental Figure 1A-B). HoxA9AA and HoxA9wt had comparable binding to cognate HoxA9 DNA-binding site by electrophoretic mobility shift assays (Figure 1D). Super-shift assays using FLAG antibody confirmed HoxA9wt and HoxA9AA complexed to DNA with equivalent affinity. The HoxA9 homeodomain N51S mutant did not bind DNA, as expected15 (Figure 1D). Taken together, HoxA9AA was impaired in eIF4E binding without impacting its association with cognate DNA.
Mutation in the HoxA9 eIF4E-binding site reduces eIF4E interaction and activity without modulating the DNA-binding activity of HoxA9. (A) Schematic depiction of the HoxA9 protein with consensus eIF4E-binding site (black bar) that is conserved from humans to sharks. Mutation of eIF4E-binding site was generated by alanine substitutions of the conserved Y and L and denoted HoxA9AA. HoxA9AA was generated using QuikChange site-directed mutagenesis (Agilent) and validated by sequencing. Also depicted is the W73A eIF4E mutation (red) that disrupts the eIF4E-HoxA9 interaction.9 Structure of cap-free eIF4E (pdb 2GPQ)16 was generated in Pymol. Both HoxA9AA and wildtype can bind DNA (1D). (B) Bacterially produced and purified GST- HoxA9wt, but not HoxA9AA or the GST control (GST-GFP), binds to endogenous eIF4E from K562 cell lysates. Right, quantification of 2 biological replicates. Equal loading of GST proteins is provided in supplemental Figure 1A. (C) Purified GST-eIF4E and GST were used in pulldowns from K562 cells stably expressing HoxA9AA and HoxA9wt from bicistronic vectors with GFP, with empty vector as control. Inputs are also shown. Right panel, quantification of 3 biological replicates. (D) Electrophoretic mobility assay showed HoxA9AA retains DNA-binding activity compared to HoxA9wt. DNA oligonucleotides containing a HoxA9 consensus binding motif described in Calvo et al.17 Super-shift reactions were performed with the anti-FLAG M2 antibody. “Comp” refers to cold competition reactions completed by using a 100-fold molar excess of nonradioactive labeled dsDNA probe. (E). HoxA9wt, but not HoxA9AA, promotes eIF4E-dependent mRNA export. Left panel: relative cyt/nuc ratio of MYC and MCL1 mRNAs in HoxA9 overexpressing cell lines or in GFP vector controls. Fractionation controls are shown in supplemental Figure 1C. Right panel: total RNAs levels do not change levels of target mRNAs or the housekeeping transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Three biological replicates are shown. (F) Immunoblot and quantitation of 3 biological replicates to assess protein expression of eIF4E-dependent RNA export targets in HoxA9wt/HoxA9AA stably overexpressing K562 cells relative to vector controls. Antibodies used for immunoblotting: mouse monoclonal anti-eIF4E (BD PharMingen), mouse monoclonal anti–β-actin (A5441, Sigma Aldrich), rabbit polyclonal anti-Mcl-1 (S19, sc819, Santa Cruz), rabbit polyclonal anti-Myc (ab32072, Abcam), mouse monoclonal anti-Hsp90 (sc-69703, Santa Cruz), rabbit polyclonal anti-GAPDH (sc-25778, Santa Cruz), rabbit monoclonal eIF4E binding-protein 1 (53H11, Cell Signaling), and rabbit polyclonal anti-HoxA9 (ab140631, Abcam). For panels B-C,E-F, means are shown; error bars indicate standard deviations, P values (student t test). Cyt, cytoplasmic; Exp, exposure; HD, homeodomain; nuc, nuclear; nonspecific band is noted on the EMSA (panel D).
Mutation in the HoxA9 eIF4E-binding site reduces eIF4E interaction and activity without modulating the DNA-binding activity of HoxA9. (A) Schematic depiction of the HoxA9 protein with consensus eIF4E-binding site (black bar) that is conserved from humans to sharks. Mutation of eIF4E-binding site was generated by alanine substitutions of the conserved Y and L and denoted HoxA9AA. HoxA9AA was generated using QuikChange site-directed mutagenesis (Agilent) and validated by sequencing. Also depicted is the W73A eIF4E mutation (red) that disrupts the eIF4E-HoxA9 interaction.9 Structure of cap-free eIF4E (pdb 2GPQ)16 was generated in Pymol. Both HoxA9AA and wildtype can bind DNA (1D). (B) Bacterially produced and purified GST- HoxA9wt, but not HoxA9AA or the GST control (GST-GFP), binds to endogenous eIF4E from K562 cell lysates. Right, quantification of 2 biological replicates. Equal loading of GST proteins is provided in supplemental Figure 1A. (C) Purified GST-eIF4E and GST were used in pulldowns from K562 cells stably expressing HoxA9AA and HoxA9wt from bicistronic vectors with GFP, with empty vector as control. Inputs are also shown. Right panel, quantification of 3 biological replicates. (D) Electrophoretic mobility assay showed HoxA9AA retains DNA-binding activity compared to HoxA9wt. DNA oligonucleotides containing a HoxA9 consensus binding motif described in Calvo et al.17 Super-shift reactions were performed with the anti-FLAG M2 antibody. “Comp” refers to cold competition reactions completed by using a 100-fold molar excess of nonradioactive labeled dsDNA probe. (E). HoxA9wt, but not HoxA9AA, promotes eIF4E-dependent mRNA export. Left panel: relative cyt/nuc ratio of MYC and MCL1 mRNAs in HoxA9 overexpressing cell lines or in GFP vector controls. Fractionation controls are shown in supplemental Figure 1C. Right panel: total RNAs levels do not change levels of target mRNAs or the housekeeping transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Three biological replicates are shown. (F) Immunoblot and quantitation of 3 biological replicates to assess protein expression of eIF4E-dependent RNA export targets in HoxA9wt/HoxA9AA stably overexpressing K562 cells relative to vector controls. Antibodies used for immunoblotting: mouse monoclonal anti-eIF4E (BD PharMingen), mouse monoclonal anti–β-actin (A5441, Sigma Aldrich), rabbit polyclonal anti-Mcl-1 (S19, sc819, Santa Cruz), rabbit polyclonal anti-Myc (ab32072, Abcam), mouse monoclonal anti-Hsp90 (sc-69703, Santa Cruz), rabbit polyclonal anti-GAPDH (sc-25778, Santa Cruz), rabbit monoclonal eIF4E binding-protein 1 (53H11, Cell Signaling), and rabbit polyclonal anti-HoxA9 (ab140631, Abcam). For panels B-C,E-F, means are shown; error bars indicate standard deviations, P values (student t test). Cyt, cytoplasmic; Exp, exposure; HD, homeodomain; nuc, nuclear; nonspecific band is noted on the EMSA (panel D).
Next, we monitored the mutant’s impact on eIF4E-dependent mRNA export by measuring RNA content in nuclear and cytoplasmic compartments of K562 cells by quantitative reverse transcription polymerase chain reaction (RT-qPCR) (Figure 1E; supplemental Figure 1C).9,18,19 HoxA9wt stable overexpression stimulated export of MCL1 and MYC by approximately twofold vs vector, whereas HoxA9AA was inactive (Figure 1E). Consistently, HoxA9wt stimulated MYC and MCL1 production by more than twofold, whereas Hox9AA only produced marginal increases (Figure 1F). Neither HoxA9 construct altered levels of target mRNA consistent with arising from posttranscriptional effects (Figure 1E, lower panel). The negative control glyceraldehyde-3-phosphate dehydrogenase was insensitive to eIF4E, HoxA9wt or HoxA9AA (Figure 1E-F). Given HoxA9AA was deficient in binding and stimulation of eIF4E, we utilized it to explore the relevance of eIF4E for HoxA9-dependent leukemogenesis.
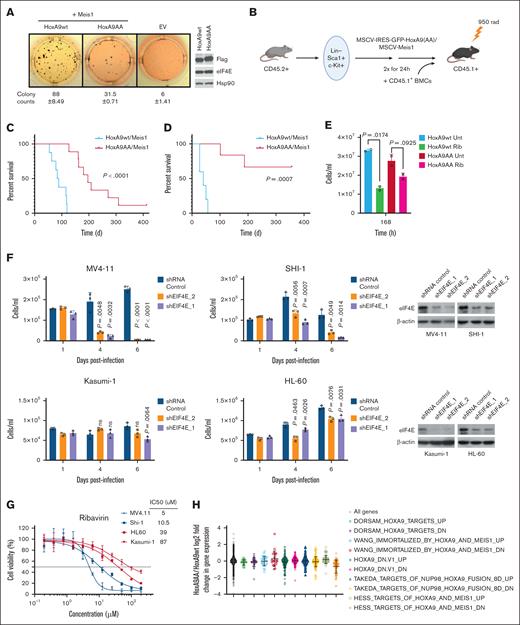
For this purpose, we utilized a well-established AML mouse model that employs co-overexpression of HoxA9 and MEIS1 based on their frequent co-overexpression in patients with poor prognosis AML.3,6,20 First, we monitored the ex vivo effects of the mutation on plating efficiency of sorted primary murine human stem and progenitor cells (HSPCs) Lin–/Sca-1+, c-Kit+ cells by performing serial replating assays in cytokine supplemented methylcellulose after retroviral transduction of freshly isolated murine HSPCs with HoxA9wt or HoxA9AA and Meis1. Importantly, the colony forming potential of HoxA9AA-transduced cells was reduced by 2.8-fold relative to HoxA9wt after 4 rounds of replating indicative of a major reduction in leukemic capacity (Figure 2A).
Reduction of the HoxA9-eIF4E interaction impairs leukemogenesis. (A) Colonies of primary lin–Sca-1+c-Kit+ (LSK) cells transduced with bicistronic vectors for HoxA9-IRES-GFP or HoxA9AA-IRES-GFP and Meis-IRES-YFP and corresponding vector controls after 4 rounds of serial replating in cytokine supplemented semisolid medium. The mean number of colonies and the standard deviation are shown below done in 3 replicates. Right, western blot of HoxA9 proteins from leukemic mouse cells, using the FLAG antibody, Hsp90 is provided as a loading control. (B) Schematic of murine transplantation model. (C) Kaplan-Meyer plot of mice transplanted with HoxA9/Meis1 (n = 10) or HoxA9AA/Meis1 (n = 14) transduced LSK cells. (D) Kaplan-Meyer plot after secondary transplants of HoxA9wt/Meis1 or HoxA9AA/Meis1 leukemic bone marrow cells into secondary recipient mice (n = 5 per group). Cells were analyzed by flow cytometry (supplemental Figure 2A-D). Serial replating assays were as in.21 (E) Response curve of murine leukemic bone marrow subjected to pharmacological inhibition of eIF4E with ribavirin. Clinically achievable ribavirin concentration (10 uM) after 168 hours in technical triplicates and biological duplicates. 2 cell lines generated from mouse primary cell lines (HoxA9wt and HoxA9AA) compared to untreated cells. (F) Impact of genetic reduction of eIF4E on proliferation in human AML cell lines based on HoxA9 levels. Cells with HoxA9-dependency (MV411 and SHI-1) were more sensitive to 2 different short hairpin RNAs (shRNAs) to eIF4E than those with low levels of HoxA9 (Kasumi-1 and HL-60) (left panels). eIF4E western blots confirm shRNA mediated knockdown in each cell type and actin is provided for loading (right panels). Experiments were carried out 3 times for each hairpin. Means and standard deviations are shown. For panels E-F, means are provided, error bars indicate standard deviation, P values shown (Student t test). (G) Sensitivity to pharmacological targeting of eIF4E with high-HoxA9 cells (blue) were more sensitive to ribavirin than low-HoxA9 cells (red). IC50s provided in inset. Experiments were carried out in 3 replicates. (H) Microarray analysis of fold change in gene expression of HoxA9AA/Meis1 over HoxA9wt/Meis1 leukemic mouse cells demonstrated highly similar transcriptional signatures. Bone marrow cells from 3 HoxA9wt/Meis1and 3 HoxA9AA/Meis1 mice were harvested with ≥97% GFP+ cells in the leukemic bone marrow. The expression levels of HoxA9-regulated gene sets, selected from Gene Set Enrichment Analysis (GSEA) database, were analyzed for overlapping genes in our microarray data set, and mean log2 fold change was determined for each gene set. EV, empty vector; IC50, 50% inhibitory concentration.
Reduction of the HoxA9-eIF4E interaction impairs leukemogenesis. (A) Colonies of primary lin–Sca-1+c-Kit+ (LSK) cells transduced with bicistronic vectors for HoxA9-IRES-GFP or HoxA9AA-IRES-GFP and Meis-IRES-YFP and corresponding vector controls after 4 rounds of serial replating in cytokine supplemented semisolid medium. The mean number of colonies and the standard deviation are shown below done in 3 replicates. Right, western blot of HoxA9 proteins from leukemic mouse cells, using the FLAG antibody, Hsp90 is provided as a loading control. (B) Schematic of murine transplantation model. (C) Kaplan-Meyer plot of mice transplanted with HoxA9/Meis1 (n = 10) or HoxA9AA/Meis1 (n = 14) transduced LSK cells. (D) Kaplan-Meyer plot after secondary transplants of HoxA9wt/Meis1 or HoxA9AA/Meis1 leukemic bone marrow cells into secondary recipient mice (n = 5 per group). Cells were analyzed by flow cytometry (supplemental Figure 2A-D). Serial replating assays were as in.21 (E) Response curve of murine leukemic bone marrow subjected to pharmacological inhibition of eIF4E with ribavirin. Clinically achievable ribavirin concentration (10 uM) after 168 hours in technical triplicates and biological duplicates. 2 cell lines generated from mouse primary cell lines (HoxA9wt and HoxA9AA) compared to untreated cells. (F) Impact of genetic reduction of eIF4E on proliferation in human AML cell lines based on HoxA9 levels. Cells with HoxA9-dependency (MV411 and SHI-1) were more sensitive to 2 different short hairpin RNAs (shRNAs) to eIF4E than those with low levels of HoxA9 (Kasumi-1 and HL-60) (left panels). eIF4E western blots confirm shRNA mediated knockdown in each cell type and actin is provided for loading (right panels). Experiments were carried out 3 times for each hairpin. Means and standard deviations are shown. For panels E-F, means are provided, error bars indicate standard deviation, P values shown (Student t test). (G) Sensitivity to pharmacological targeting of eIF4E with high-HoxA9 cells (blue) were more sensitive to ribavirin than low-HoxA9 cells (red). IC50s provided in inset. Experiments were carried out in 3 replicates. (H) Microarray analysis of fold change in gene expression of HoxA9AA/Meis1 over HoxA9wt/Meis1 leukemic mouse cells demonstrated highly similar transcriptional signatures. Bone marrow cells from 3 HoxA9wt/Meis1and 3 HoxA9AA/Meis1 mice were harvested with ≥97% GFP+ cells in the leukemic bone marrow. The expression levels of HoxA9-regulated gene sets, selected from Gene Set Enrichment Analysis (GSEA) database, were analyzed for overlapping genes in our microarray data set, and mean log2 fold change was determined for each gene set. EV, empty vector; IC50, 50% inhibitory concentration.
Next, we evaluated the eIF4E contribution to HoxA9-induced leukemogenesis in vivo. CD45.2+ Lin–/Sca-1+, c-Kit+ cells overexpressing HoxA9wt-IRES-GFP or HoxA9AA-IRES-GFP and MEIS1-IRES-YFP were transduced into lethally irradiated CD45.1+ recipient mice (Figure 2B). Transduction efficiency was similar for HoxA9wt and HoxA9AA, 22.9% and 24.5%, respectively (supplemental Figure 2A). Both groups showed comparable engraftment with similar kinetics based on fractions of CD45.2 and green fluorescent protein (GFP) positive peripheral blood cells at 4 weeks posttransplantation (supplemental Figure 2B). Both eventually developed myeloid leukemia with 97% to 99% CD45.2+/GFP+ in the bone marrow (Figure 2C). Diseased mice exhibited myeloid (Mac1/Gr-1) but not B- or T-lymphoid markers and maturation profiles were indistinguishable between groups (supplemental Figure 2C-D).
There were highly significant differences in latency of disease onset and penetrance between HoxA9wt and HoxA9AA groups. All animals (n = 10) in the HoxA9wt group succumbed to aggressive leukemia within 120 days (median, 90 days), corresponding to full penetrance of HoxA9wt/Meis1 leukemia (Figure 2C). By contrast, the median latency in the HoxA9AA/Meis1 group (n = 14) was 280 days. Two HoxA9AA/Meis1 mice never developed leukemia. One mouse had a low frequency of GFP positive cells in peripheral blood and bone marrow when sacrificed 413 days after transplantation. The second had detectable GFP+ peripheral blood cells until day 175 after transplantation with no remaining GFP+ cells in the bone marrow at sacrifice (day 190). HoxA9AA protein levels were marginally higher than HoxA9wt in mouse leukemic cells indicating that reduced leukemia is not a result of lower HoxA9AA production nor due to differences in eIF4E levels between groups (Figure 2A, right panel).
In secondary recipients, retransplantation of bone marrow cells from leukemic animals demonstrated an even more pronounced difference in latency and penetrance (Figure 2D). Although secondary recipients transplanted with 5/5 HoxA9wt/Meis1 developed myeloid leukemia and died within 60 days after transplantation, only 2 of 5 in the HoxA9AA/Meis1 group developed disease even a year after transplantation. The remaining 3 recipients had no detectable GFP+ cells in peripheral blood or bone marrow. Thus, the eIF4E interaction is continuously required for maintenance of leukemic transformation by HoxA9. Furthermore, eIF4E-dependence is not overcome by leukemic evolution.
Given these findings, we examined the sensitivity of HoxA9-dependent cells to therapeutic targeting of eIF4E. Treatment with a clinically achievable dose of ribavirin, 10 uM,11,13,14 reduced proliferation in HoxA9wt leukemic murine cells approximately threefold with only modest impacts on HoxA9AA cells suggestive of an oncogene addiction to eIF4E in the HoxA9wt group (Figure 2E). To explore this in a human context, we compared human AML cells with established HoxA9 elevation and dependency (MV4-11, SHI-1) to cells characterized by reduced HoxA9 sensitivity and low levels (Kasumi-1, HL60) confirming expression differences by droplet polymerase chain reaction (∼10 mRNA copies per cell MV4-11 and SHI-1 vs <0.1 copies per cell for Kasumi and HL60) (https://www.proteinatlas.org/ENSG00000078399-HOXA9/cell+line).22-25 Genetic reduction of eIF4E with 2 different short hairpin RNAs reduced eIF4E levels in both groups (Figure 2F). Proliferation of HoxA9-dependent cells was reduced up to 10-fold by eIF4E knockdown relative to short hairpin RNA controls. Reduction in low-HoxA9 cells only marginally impacted growth, and indeed, HL60 cells continued to proliferate (Figure 2F). Ribavirin treatment displayed similar trends with HoxA9-dependent cells approximately eightfold more sensitive to ribavirin than low-HoxA9 cells as seen by comparison of 50% inhibitory concentrations, which were in clinically achievable ranges (Figure 2G).11,13,14 Thus, high-HoxA9 cells develop an oncogene dependency to eIF4E providing a new therapeutic vulnerability.
To examine the impact on transcription, we analyzed our primary leukemic murine cells and observed that both HoxA9AA and HoxA9wt induced the expected HoxA9 gene signature (GSE288783). A comparison of HoxA9wt and HoxA9AA transcriptomes revealed only minor differences with 163 downregulated and 247 upregulated genes of 23 589 transcripts observed (>1.5 fold, P <.05) (Figure 2H). Genes in HoxA9-related pathways were not altered (Figure 2H). In all, the reduced leukemogenic capacity of HoxA9AA is not caused by driving an alternative transcriptional program.
In conclusion, we discovered a posttranscriptional mechanism underpinning HoxA9-mediated leukemogenesis. HoxA9AA elicited a highly similar transcriptional program and bound DNA indistinguishably from HoxA9wt; however, this mutation no longer stimulated eIF4E, which correlated to substantially extended disease latency and reduced penetrance in mice. eIF4E interaction plays an essential role in induction and maintenance of HoxA9-dependent leukemogenesis. Strategies to directly target HoxA9 in patients remain elusive. We discovered a new therapeutic vulnerability whereby high-HoxA9 AML patients could benefit from eIF4E inhibitors like ribavirin.
All animal studies were approved by the Beth Israel Deaconess Medical Center/Harvard Medical School and were used in accordance with protocols approved by the institutional animal care and use committee.
Acknowledgments: The authors are grateful for assistance from Laurent Volpon for purified protein production.
This work was supported by the National Institutes of Health (RO1CA098571 and RO1CA080728 to K.L.B.B.; 1P01HL131477-6 A1 to D.G.T.); the Canada Research Chair programme (K.L.B.B.); and the AGA/Jenzabar Foundation (D.G.T.).
Contribution: F.Z., C.B., L.F., Y.M., B.C.-K., K.L.B.B., and D.G.T. designed the experiments; B.C.-K., C.B., L.F., and Y.M. performed experiments; and F.Z., C.B., L.F., B.C.-K., K.L.B.B., and D.G.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel G. Tenen, Harvard Stem Cell Institute, Center for Life Science, Room 437, Boston, MA; email: dtenen@bidmc.harvard.edu; and Katherine L. B. Borden, Department of Pharmacology and Robert H. Lurie Cancer Center, Northwestern University, Chicago, IL. 60611; email: katherine.borden@northwestern.edu.
References
Author notes
F.Z. and B.C.-K. are joint first authors.
K.L.B.B. and D.G.T. are joint last authors.
Microarray data were deposited in the Gene Expression Omnibus (GSE288783).
The full-text version of this article contains a data supplement.