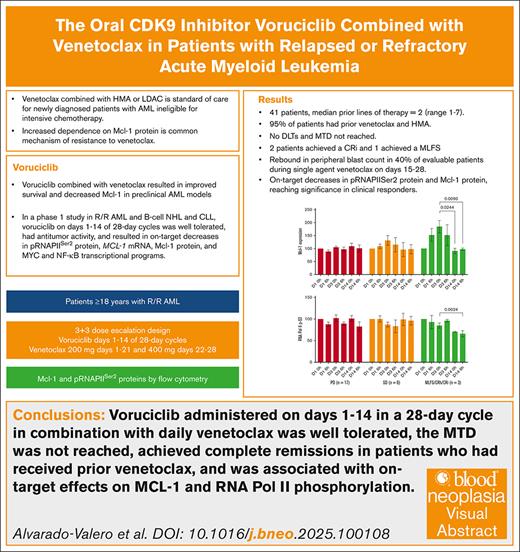
Key Points
Voruciclib combined with venetoclax was well tolerated and achieved objective responses in patients with AML and prior venetoclax.
Voruciclib decreased Mcl-1 protein expression and RNA polymerase II phosphorylation, demonstrating on-target effects.
Visual Abstract
The antiapoptotic protein myeloid cell leukemia 1 (Mcl-1) promotes cell survival in acute myeloid leukemia (AML), and its overexpression is associated with resistance to venetoclax. Voruciclib, an oral cyclin-dependent kinase 9 inhibitor, indirectly decreases Mcl-1 protein expression and has synergistic activity with venetoclax in AML preclinical models. We hypothesized that voruciclib in combination with venetoclax would induce responses in patients with AML with disease progression after venetoclax therapy. This dose-escalation study evaluated voruciclib administered on days 1 to 14 of 28-day cycles with venetoclax daily. The study enrolled 41 adult patients with AML after failure of previous standard therapies. Patients had a median of 2 (range, 1-7) previous lines of therapy, 19 patients (46%) had ≥3 previous lines of therapy, and 39 (95%) had previous venetoclax. No dose-limiting toxicities were reported in 7 dose levels evaluated. The most common adverse events were nausea (34%), febrile neutropenia (32%), diarrhea (22%), dyspnea (22%), hypokalemia (22%), and thrombocytopenia (22%). Antileukemic activity was observed in 10 (24%) patients, including 3 with complete marrow remission and 7 with stable disease lasting ≥3 months. We observed a rebound of circulating blasts during the 14 days of single-agent venetoclax dosing in 40% of evaluable patients. Mcl-1 protein expression and RNA polymerase II Ser-2 phosphorylation decreased on voruciclib. Overall, the combination of voruciclib with venetoclax was tolerable in patients with relapsed/refractory AML, had antileukemic activity, and showed on-target effects in heavily pretreated patients with disease progression after venetoclax. This trial was registered at www.ClinicalTrials.gov as #NCT03547115.
Introduction
B-cell leukemia/lymphoma 2 (Bcl-2) family of proteins are important regulators of apoptosis in normal and malignant hematopoietic cells, including in acute myeloid leukemia (AML).1,2 It has been established that overexpression of Bcl-2 protein is associated with resistance to chemotherapy and poor outcomes in AML.3-6 The Bcl-2 inhibitor venetoclax in combination with hypomethylating agents (HMA) or low-dose cytarabine has become the standard of care for patients with newly diagnosed AML ineligible for intensive chemotherapy.7-9 Furthermore, venetoclax is being evaluated in combination with chemotherapy or targeted therapies in treatment-naïve and relapsed AML, in both adult and pediatric populations.10-13 Despite high responses rates with venetoclax regimens, most patients will eventually experience disease progression. Salvage therapies after disease progression on venetoclax are rarely effective, with survival typically <6 months.14-18
The myeloid cell leukemia 1 (Mcl-1) protein, an antiapoptotic member of the Bcl-2 family, is dynamically regulated at both messenger RNA and protein levels. Inhibition of Mcl-1 is sufficient to induce apoptosis in AML, and patients with AML with lower MCL1 messenger RNA and protein expression levels have improved outcomes.19,20 Increased functional dependence on Mcl-1 protein is a common mechanism of resistance to venetoclax, which can be reversed by targeting Mcl-1.21-25 Several BH3-mimetics that directly inhibit Mcl-1 have been evaluated in the clinic, however, their development has been challenging because of on-target cardiovascular toxicity associated with Mcl-1 expression in cardiac tissues.26-28
Mcl-1 protein levels can also be decreased indirectly by inhibiting transcriptional cyclin-dependent kinase 7 and 9 (CDK7 and CDK9). CDK9/cyclin T, the catalytic core of the positive transcription elongation factor b, phosphorylate RNA polymerase II (RNAPII) at Ser-5 and Ser-2 sites to facilitate initiation and elongation of RNA transcription, respectively.29-33 Early studies with pan-CDK inhibitors in patients with various hematologic malignancies have shown antitumor activity but were associated with off-target toxicities.34 Preclinical studies have shown that specific CDK9 inhibition can decrease Mcl-1 protein expression in various tumor models.35,36 Preferential targeting of CDK9 could be a more effective and possibly safer anticancer therapy approach than either direct Mcl-1 inhibitors or pan-CDK inhibitors.
Voruciclib is an oral CDK inhibitor with greater binding affinity for CDK9 than CDK4, CDK6, and CDK1.37 In preclinical studies in AML, chronic lymphocytic leukemia, and diffuse large B-cell lymphoma models, voruciclib decreased MCL1 transcripts and proteins in a dose-dependent manner, with maximal effect plateauing at concentrations of 1 μM.37 Synergy of voruciclib with venetoclax was also observed, as evidenced by increased apoptosis, decreased tumor growth rate, and improved survival in murine xenograft models.38-40 Two phase 1 dose-escalation and expansion studies in patients with solid tumors evaluated voruciclib administered once daily continuously or on an intermittent dosing schedule on days 1 to 14 of 21-day cycles.41,42 The most common adverse events were nausea and diarrhea, which were generally manageable. The maximum tolerated dose (MTD) was 350 mg on continuous dosing and 600 mg on intermittent dosing.
A phase 1 dose-escalation study in 40 patients with relapsed or refractory (R/R) AML or B-cell malignancies found that voruciclib at doses up to 200 mg administered on days 1 to 14 of 28-day cycles was well tolerated, with no dose-limiting toxicities (DLTs).43 The 14 days on/14 days off voruciclib schedule was selected empirically after 2 DLT of interstitial pneumonitis were observed on continuous daily dosing at 100 mg.44 On-target effects were demonstrated by a decrease in MCL1 RNA expression, reduced phosphorylation on Ser-2 of RNA polymerase II (phosphorylated RNAPIISer2 [pRNAPIISer2]), and downregulation of MYC and nuclear factor κB (NF-κB) transcriptional gene sets.43,45 Clinical efficacy was modest, with 1 patient with AML achieving morphologic leukemia-free state (MLFS). Here, we report the results of a subsequent stage of this study that evaluated voruciclib in combination with venetoclax for R/R AML.
Methods
Patients
Patients aged ≥18 years with a diagnosis of AML were eligible if they had disease progression after previous standard therapies, no previous CDK9 inhibitor exposure, Eastern Cooperative Oncology Group performance status score of 0 or 1, and adequate renal and hepatic function. Further details on eligibility criteria are available in the protocol (see protocol in the supplemental Data).
Study design
Dose escalation followed a 3+3 design with the option to enroll in a 12-patient expansion cohort at a biologically effective dose, defined as a dose with a DLT in ≤1 patient, an overall response rate (ORR) of ≥30%, and mean trough plasma concentrations of 1 to 1.5 μM. All DLTs were assessed in cycle 1 and included grade ≥3 nonhematologic toxicity, grade ≥3 tumor lysis syndrome regardless of prophylaxis, grade ≥3 nonhematologic laboratory abnormalities, febrile neutropenia, and protracted myelosuppression, if they were considered not related to AML (details provided in the protocol in the supplemental Data). The MTD was defined as the dose level with ≤1 DLT in up to 6 evaluable patients and 1 dose level lower than the dose at which ≥2 DLTs were reported.
Voruciclib was administered orally once a day on days 1 to 14 of 28-day cycles and continued until disease progression or intolerability. Because of possible induction of cytochrome P450 and P-glycoprotein (P-gp) by voruciclib, venetoclax was administered at the prescribing information recommended reduced dose of 200 mg on days 1 to 21 (ie, during voruciclib dosing and for 7 half-lives after completing voruciclib) and then 400 mg daily on days 22 to 28 of the cycle. In cycle 1, venetoclax dose was ramped up on days 1 to 3 (50, 100, and 200 mg) and voruciclib was started on day 3. Venetoclax dose modifications followed the product labeling recommendation.
Clinical assessments
The schedule of patient visits and safety assessments is found in the protocol (see protocol in the supplemental Data). Tumor lysis syndrome monitoring was specified and treatment, if required, followed institutional guidelines. Use and type of antimicrobial prophylaxis was not mandated and left to the investigator’s choice. Azoles were not allowed in cycle 1 because of inhibition of cytochrome P450 enzymes and the potential for drug interaction. Azoles were allowed in later cycles and required administering voruciclib and venetoclax at lower doses. Disease response assessment was performed using the 2017 European LeukemiaNet response criteria and reported based on the investigator’s assessment.46 The ORR was defined as the combination of complete response (CR), CR with incomplete hematologic recovery (CRi), and MLFS.
Correlative studies
Pharmacodynamic analyses were performed on longitudinal blood samples collected before, and 4 to 6 hours after dose on days 1, 3, and 14 of cycle 1. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll gradient and resuspended in ammonium-chloride-potassium lysing buffer (Life Technologies) for red blood cell lysis. Cells were viably frozen in fetal bovine serum with 10% dimethyl sulfoxide and stored in liquid nitrogen until use. Mcl-1 and pRNAPIISer2 proteins in the PBMCs were quantified by flow cytometry (details in the supplemental Data).
Analyses
All statistical analyses were descriptive and performed in the intent-to-treat population. Ratios were calculated with their 95% confidence intervals. Voruciclib and venetoclax pharmacokinetic parameters were estimated by noncompartmental analysis.47 Correlative study analyses were performed in GraphPad Prism.
Study conduct and oversight
The study was registered in www.ClinicalTrials.gov as #NCT03547115 and conducted in accordance with applicable regulatory requirements and in accordance with the updated Declaration of Helsinki and International Conference on Harmonization good clinical practice guidelines. The protocol was approved by each study site’s independent ethics committee, and all patients provided written informed consent before enrolling in the study.
Results
Patients
Forty-one patients were enrolled between November 2022 and April 2024. The median age was 69 years (range, 34-89) and baseline Eastern Cooperative Oncology Group performance status was 1 in 76% of patients (Table 1). Nineteen patients had received ≥3 previous lines of therapy (median, 2 [range, 1-7]), with 95% of patients having received previous venetoclax, 95% previous HMAs, 54% previous anthracyclines, and 20% previous allogeneic stem cell transplantation (SCT). The median bone marrow blast count was 28% (range, 2%-77%; Table 2). Most common types of AML were AML with myelodysplasia-related changes (42.5%), acute myelomonocytic leukemia (17.5%), and AML with mutated RUNX1 (12.5%). Adverse cytogenetics were reported in 75% of the patients, including 25% with complex karyotype, 32% with monosomy 7, and 17% with deletion 5. The most common adverse molecular abnormalities were ASXL1 in 39% of patients, TP53 in 24%, and RUNX1 in 22%.
Demographics and baseline characteristics
| n (%) . | 50 mg qod (n = 6) . | 50 mg (n = 3) . | 100 mg (n = 4) . | 150 mg (n = 4) . | 200 mg (n = 4) . | 250 mg (n = 4) . | 300 mg (n = 16) . | Total (N = 41) . |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Female | 4 (66.7) | 0 | 1 (25.0) | 2 (50.0) | 2 (50.0) | 3 (75.0) | 6 (37.5) | 18 (43.9) |
| Male | 2 (33.3) | 3 (100.0) | 3 (75.0) | 2 (50.0) | 2 (50.0) | 1 (25.0) | 10 (62.5) | 23 (56.1) |
| Race | ||||||||
| Asian | 2 (33.3) | 0 | 0 | 0 | 0 | 0 | 2 (12.5) | 4 (9.8) |
| Black or African American | 0 | 0 | 0 | 2 (50.0) | 0 | 0 | 1 (6.3) | 3 (7.3) |
| White | 4 (66.7) | 3 (100.0) | 3 (75.0) | 2 (50.0) | 3 (75.0) | 4 (100.0) | 12 (75.0) | 31 (75.6) |
| Age | ||||||||
| Median (range), y | 70.5 (64-87) | 73 (63-82) | 62 (57-89) | 55 (52-74) | 66.5 (34-82) | 67.5 (59-78) | 71 (41-78) | 69 (34-89) |
| ≥65 years | 5 (83.3) | 2 (66.7) | 1 (25.0) | 1 (25.0) | 2 (50.0) | 3 (75.0) | 11 (68.8) | 25 (61.0) |
| ECOG performance status | ||||||||
| 0 | 1 (16.7) | 1 (33.3) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 4 (25.0) | 9 (22.0) | |
| 1 | 5 (83.3) | 2 (66.7) | 3 (75.0) | 3 (75.0) | 3 (75.0) | 3 (75.0) | 12 (75.0) | 31 (75.6) |
| Previous lines of therapy | ||||||||
| 1 | 1 (16.7) | 2 (66.7) | 3 (75.0) | 0 | 1 (25.0) | 0 | 4 (25.0) | 11 (26.8) |
| 2 | 1 (16.7) | 1 (33.3) | 0 | 0 | 2 (50.0) | 2 (50.0) | 5 (31.3) | 11 (26.8) |
| ≥3 | 4 (66.7) | 0 | 1 (25.0) | 4 (100.0) | 1 (25.0) | 2 (50.0) | 7 (43.8) | 19 (46.3) |
| Median (range) | 3 (1-7) | 1 (1-2) | 1 (1-5) | 3 (3-4) | 2 (1-4) | 2.5 (2-5) | 2 (1-5) | 2 (1-7) |
| Type of previous therapies | ||||||||
| Venetoclax | 6 (100.0) | 3 (100.0) | 4 (100.0) | 3 (75.0) | 4 (100.0) | 3 (75.0) | 16 (100.0) | 39 (95.1) |
| HMA | 5 (83.3) | 3 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 15 (93.7) | 39 (95.1) |
| Allogeneic SCT | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0 | 3 (18.7)) | 8 (19.5) |
| Anthracyclines | 4 (66.7) | 1 (33.3) | 2 (50.0) | 4 (100.0) | 3 (75.0) | 2 (50.0) | 6 (14.6) | 22 (53.7) |
| Mutation-targeted agent | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (50.0) | 3 (18.7) | 6 (14.6) |
| n (%) . | 50 mg qod (n = 6) . | 50 mg (n = 3) . | 100 mg (n = 4) . | 150 mg (n = 4) . | 200 mg (n = 4) . | 250 mg (n = 4) . | 300 mg (n = 16) . | Total (N = 41) . |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Female | 4 (66.7) | 0 | 1 (25.0) | 2 (50.0) | 2 (50.0) | 3 (75.0) | 6 (37.5) | 18 (43.9) |
| Male | 2 (33.3) | 3 (100.0) | 3 (75.0) | 2 (50.0) | 2 (50.0) | 1 (25.0) | 10 (62.5) | 23 (56.1) |
| Race | ||||||||
| Asian | 2 (33.3) | 0 | 0 | 0 | 0 | 0 | 2 (12.5) | 4 (9.8) |
| Black or African American | 0 | 0 | 0 | 2 (50.0) | 0 | 0 | 1 (6.3) | 3 (7.3) |
| White | 4 (66.7) | 3 (100.0) | 3 (75.0) | 2 (50.0) | 3 (75.0) | 4 (100.0) | 12 (75.0) | 31 (75.6) |
| Age | ||||||||
| Median (range), y | 70.5 (64-87) | 73 (63-82) | 62 (57-89) | 55 (52-74) | 66.5 (34-82) | 67.5 (59-78) | 71 (41-78) | 69 (34-89) |
| ≥65 years | 5 (83.3) | 2 (66.7) | 1 (25.0) | 1 (25.0) | 2 (50.0) | 3 (75.0) | 11 (68.8) | 25 (61.0) |
| ECOG performance status | ||||||||
| 0 | 1 (16.7) | 1 (33.3) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 4 (25.0) | 9 (22.0) | |
| 1 | 5 (83.3) | 2 (66.7) | 3 (75.0) | 3 (75.0) | 3 (75.0) | 3 (75.0) | 12 (75.0) | 31 (75.6) |
| Previous lines of therapy | ||||||||
| 1 | 1 (16.7) | 2 (66.7) | 3 (75.0) | 0 | 1 (25.0) | 0 | 4 (25.0) | 11 (26.8) |
| 2 | 1 (16.7) | 1 (33.3) | 0 | 0 | 2 (50.0) | 2 (50.0) | 5 (31.3) | 11 (26.8) |
| ≥3 | 4 (66.7) | 0 | 1 (25.0) | 4 (100.0) | 1 (25.0) | 2 (50.0) | 7 (43.8) | 19 (46.3) |
| Median (range) | 3 (1-7) | 1 (1-2) | 1 (1-5) | 3 (3-4) | 2 (1-4) | 2.5 (2-5) | 2 (1-5) | 2 (1-7) |
| Type of previous therapies | ||||||||
| Venetoclax | 6 (100.0) | 3 (100.0) | 4 (100.0) | 3 (75.0) | 4 (100.0) | 3 (75.0) | 16 (100.0) | 39 (95.1) |
| HMA | 5 (83.3) | 3 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 4 (100.0) | 15 (93.7) | 39 (95.1) |
| Allogeneic SCT | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0 | 3 (18.7)) | 8 (19.5) |
| Anthracyclines | 4 (66.7) | 1 (33.3) | 2 (50.0) | 4 (100.0) | 3 (75.0) | 2 (50.0) | 6 (14.6) | 22 (53.7) |
| Mutation-targeted agent | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (50.0) | 3 (18.7) | 6 (14.6) |
ECOG, Eastern Cooperative Oncology Group; qod, every other day dosing.
Disease characteristics
| Characteristics . | N = 41 . |
|---|---|
| Baseline bone marrow blasts, median (range), % | 28 (2-77) |
| AML type (n = 40),∗ n (%) | |
| AML with myelodysplasia-related changes | 17 (42.5) |
| Acute myelomonocytic leukemia | 7 (17.5) |
| AML with RUNX-1 mutation | 5 (12.5) |
| AML with mutated NPM1 | 2 (5) |
| AML without maturation | 2 (5) |
| Pure erythroleukemia | 2 (5) |
| AML with maturation | 2 (5) |
| Other | 3 (7.5) |
| 2017 ELN risk category (n = 41), n (%) | |
| Adverse | 30 (73) |
| Intermediate | 7 (17) |
| Favorable | 4 (10) |
| Cytogenetics (n = 40),∗ n (%) | |
| Monosomy 7 or deletion 7 | 13 (32.5) |
| Complex karyotype | 10 (25) |
| Deletion 5 | 7 (17.5) |
| Molecular mutations (n = 36),∗ n (%) | |
| ASXL1 | 14 (39) |
| TP53 | 10 (24) |
| RUNX1 | 8 (22) |
| SRSF2 | 7 (19) |
| GATA2 | 5 (14) |
| STAG2 | 4 (11) |
| U2AF1 | 4 (11) |
| BCOR | 3 (8) |
| SF3B1 | 3 (8) |
| EXH2 | 3 (8) |
| Characteristics . | N = 41 . |
|---|---|
| Baseline bone marrow blasts, median (range), % | 28 (2-77) |
| AML type (n = 40),∗ n (%) | |
| AML with myelodysplasia-related changes | 17 (42.5) |
| Acute myelomonocytic leukemia | 7 (17.5) |
| AML with RUNX-1 mutation | 5 (12.5) |
| AML with mutated NPM1 | 2 (5) |
| AML without maturation | 2 (5) |
| Pure erythroleukemia | 2 (5) |
| AML with maturation | 2 (5) |
| Other | 3 (7.5) |
| 2017 ELN risk category (n = 41), n (%) | |
| Adverse | 30 (73) |
| Intermediate | 7 (17) |
| Favorable | 4 (10) |
| Cytogenetics (n = 40),∗ n (%) | |
| Monosomy 7 or deletion 7 | 13 (32.5) |
| Complex karyotype | 10 (25) |
| Deletion 5 | 7 (17.5) |
| Molecular mutations (n = 36),∗ n (%) | |
| ASXL1 | 14 (39) |
| TP53 | 10 (24) |
| RUNX1 | 8 (22) |
| SRSF2 | 7 (19) |
| GATA2 | 5 (14) |
| STAG2 | 4 (11) |
| U2AF1 | 4 (11) |
| BCOR | 3 (8) |
| SF3B1 | 3 (8) |
| EXH2 | 3 (8) |
ELN, European LeukemiaNet.
Results not available in some patients.
Exposure and patient disposition
Voruciclib was evaluated at 7 dose levels ranging from 50 mg every other day to 300 mg daily, including 12 patients in an expansion cohort at 300 mg. All patients have discontinued voruciclib: 48% due to progressive disease (PD), 27% due to an adverse event, 10% due to withdrawal of consent, 10% due to investigator’s decision, and 5% due to study closure (supplemental Figure 1). The median duration of therapy was 5.7 weeks (range, 0.6-41.1). Voruciclib dose was reduced in 5 (12%) patients per protocol because of concomitant azole administration. Dose escalation was stopped at 300 mg without reaching the MTD, because this dose achieved plasma concentrations considered sufficient for target inhibition based on the preclinical models and significant decreases in Mcl-1 protein expression at the doses studied.
Safety
No DLTs were reported with voruciclib and venetoclax at the doses and schedule evaluated. The most common any-grade adverse events were nausea (34%), febrile neutropenia (32%), diarrhea (22%), dyspnea (22%), hypokalemia (22%), and thrombocytopenia (22%), with incidence by dose level shown in Table 3. The most common nonhematologic grade 3 to 4 adverse events were febrile neutropenia (29%), lung infection (7%), acute respiratory failure (5%), hypokalemia (5%), and muscle weakness (5%), with incidence by dose shown in supplemental Table 1. An adverse event led to treatment discontinuation in 11 patients; infection in 8, and hemorrhage in 3. Seven (17%) patients had an adverse event with a fatal outcome, 5 because of an infection (pulmonary aspergillosis, Pneumocystis jirovecii pneumonia, respiratory syncytial virus pneumonia, Escherichia coli sepsis, and febrile neutropenia in 1 patient each) and 2 because of hemorrhage (1 cerebral and 1 subdural). Both patients who died from an opportunistic infection (ie, aspergillosis and pneumocystis pneumonia) had a previous allogeneic stem cell transplant. A serious adverse event was reported in 28 patients (68%), of which 7 (17%) were considered by the investigator to be drug-related, including 4 cases of febrile neutropenia and 1 case each of sepsis, pharyngitis, and fever. No tumor lysis syndrome was observed with either drug.
Adverse events in 5% or more of patients
| n (%) . | 50 mg qod (n = 6) . | 50 mg (n = 3) . | 100 mg (n = 4) . | 150 mg (n = 4) . | 200 mg (n = 4) . | 250 mg (n = 4) . | 300 mg (n = 16) . | Total (N = 41) . |
|---|---|---|---|---|---|---|---|---|
| Nausea | 0 | 0 | 2 (50.0) | 3 (75.0) | 2 (50.0) | 2 (50.0) | 5 (31.3) | 14 (34.1) |
| Febrile neutropenia | 0 | 1 (33.3) | 2 (50.0) | 2 (50.0) | 0 | 1 (25.0) | 7 (43.8) | 13 (31.7) |
| Diarrhea | 1 (16.7) | 0 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 4 (25.0) | 9 (22.0) |
| Dyspnea | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 0 | 1 (25.0) | 4 (25.0) | 9 (22.0) |
| Hypokalemia | 0 | 0 | 2 (50.0) | 1 (25.0) | 2 (50.0) | 1 (25.0) | 3 (18.8) | 9 (22.0) |
| Platelet count decreased | 0 | 1 (33.3) | 1 (25.0) | 3 (75.0) | 1 (25.0) | 1 (25.0) | 2 (12.5) | 9 (22.0) |
| Anemia | 0 | 0 | 2 (50.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | 1 (6.3) | 7 (17.1) |
| Vomiting | 0 | 0 | 1 (25.0) | 0 | 0 | 2 (50.0) | 4 (25.0) | 7 (17.1) |
| Cough | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0 | 1 (6.3) | 6 (14.6) |
| Fatigue | 0 | 0 | 0 | 3 (75.0) | 1 (25.0) | 0 | 2 (12.5) | 6 (14.6) |
| Stomatitis | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 2 (12.5) | 6 (14.6) |
| Arthralgia | 0 | 0 | 2 (50.0) | 0 | 0 | 0 | 2 (12.5) | 4 (9.8) |
| Dizziness | 1 (16.7) | 0 | 0 | 1 (25.0) | 0 | 0 | 2 (12.5) | 4 (9.8) |
| Hypotension | 1 (16.7) | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 1 (6.3) | 4 (9.8) |
| Anxiety | 1 (16.7) | 0 | 2 (50.0) | 0 | 0 | 0 | 0 | 3 (7.3) |
| Contusion | 2 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (6.3) | 3 (7.3) |
| COVID-19 | 1 (16.7) | 0 | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 3 (7.3) |
| Fall | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | 2 (12.5) | 3 (7.3) |
| Gingival bleeding | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (25.0) | 1 (6.3) | 3 (7.3) |
| Hemorrhoids | 0 | 0 | 0 | 1 (25.0) | 1 (25.0) | 0 | 1 (6.3) | 3 (7.3) |
| Lung infection | 0 | 0 | 0 | 0 | 0 | 0 | 3 (18.8) | 3 (7.3) |
| Neutrophil count decreased | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 2 (12.5) | 3 (7.3) |
| Oropharyngeal pain | 1 (16.7) | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (6.3) | 3 (7.3) |
| White blood cell count decreased | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (12.5) | 3 (7.3) |
| n (%) . | 50 mg qod (n = 6) . | 50 mg (n = 3) . | 100 mg (n = 4) . | 150 mg (n = 4) . | 200 mg (n = 4) . | 250 mg (n = 4) . | 300 mg (n = 16) . | Total (N = 41) . |
|---|---|---|---|---|---|---|---|---|
| Nausea | 0 | 0 | 2 (50.0) | 3 (75.0) | 2 (50.0) | 2 (50.0) | 5 (31.3) | 14 (34.1) |
| Febrile neutropenia | 0 | 1 (33.3) | 2 (50.0) | 2 (50.0) | 0 | 1 (25.0) | 7 (43.8) | 13 (31.7) |
| Diarrhea | 1 (16.7) | 0 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 4 (25.0) | 9 (22.0) |
| Dyspnea | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 0 | 1 (25.0) | 4 (25.0) | 9 (22.0) |
| Hypokalemia | 0 | 0 | 2 (50.0) | 1 (25.0) | 2 (50.0) | 1 (25.0) | 3 (18.8) | 9 (22.0) |
| Platelet count decreased | 0 | 1 (33.3) | 1 (25.0) | 3 (75.0) | 1 (25.0) | 1 (25.0) | 2 (12.5) | 9 (22.0) |
| Anemia | 0 | 0 | 2 (50.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | 1 (6.3) | 7 (17.1) |
| Vomiting | 0 | 0 | 1 (25.0) | 0 | 0 | 2 (50.0) | 4 (25.0) | 7 (17.1) |
| Cough | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 1 (25.0) | 0 | 1 (6.3) | 6 (14.6) |
| Fatigue | 0 | 0 | 0 | 3 (75.0) | 1 (25.0) | 0 | 2 (12.5) | 6 (14.6) |
| Stomatitis | 2 (33.3) | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 2 (12.5) | 6 (14.6) |
| Arthralgia | 0 | 0 | 2 (50.0) | 0 | 0 | 0 | 2 (12.5) | 4 (9.8) |
| Dizziness | 1 (16.7) | 0 | 0 | 1 (25.0) | 0 | 0 | 2 (12.5) | 4 (9.8) |
| Hypotension | 1 (16.7) | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 1 (6.3) | 4 (9.8) |
| Anxiety | 1 (16.7) | 0 | 2 (50.0) | 0 | 0 | 0 | 0 | 3 (7.3) |
| Contusion | 2 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (6.3) | 3 (7.3) |
| COVID-19 | 1 (16.7) | 0 | 0 | 1 (25.0) | 1 (25.0) | 0 | 0 | 3 (7.3) |
| Fall | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | 2 (12.5) | 3 (7.3) |
| Gingival bleeding | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (25.0) | 1 (6.3) | 3 (7.3) |
| Hemorrhoids | 0 | 0 | 0 | 1 (25.0) | 1 (25.0) | 0 | 1 (6.3) | 3 (7.3) |
| Lung infection | 0 | 0 | 0 | 0 | 0 | 0 | 3 (18.8) | 3 (7.3) |
| Neutrophil count decreased | 0 | 0 | 1 (25.0) | 0 | 0 | 0 | 2 (12.5) | 3 (7.3) |
| Oropharyngeal pain | 1 (16.7) | 0 | 1 (25.0) | 0 | 0 | 0 | 1 (6.3) | 3 (7.3) |
| White blood cell count decreased | 0 | 0 | 0 | 0 | 1 (25.0) | 0 | 2 (12.5) | 3 (7.3) |
Efficacy
Two patients achieved CRi. One was a patient with diagnosis of acute myelomonocytic leukemia secondary to a chronic stage who was treated with voruciclib at the 200-mg dose level for 1 cycle and then was transitioned to haploidentical allogeneic SCT, and he remains in remission. The second was a patient with AML without maturation treated with voruciclib at the 100-mg dose level, who achieved a CRi after 1 cycle and remained in remission for 6 months until disease progression. One patient with AML with myelodysplasia-related changes was administered voruciclib at 200 mg, achieved a MLFS after 2 cycles, and continued therapy for 9 months until study closure. Details on the responders, including genomic profile, are included in supplemental Table 2. The ORR was 7.3% in the total population and 9.7% in 32 patients administered voruciclib doses of ≥100 mg. In addition, 7 patients had stable disease (SD) that lasted at least 3 months.
In 21 patients who had at least 1 bone marrow biopsy obtained after starting voruciclib, there was a decrease in bone marrow blast count in 6 (29%) patients. Circulating blasts count were measurable in 24 patients at baseline and at different time points in cycle 1; in the remaining 17 patients circulating blasts were <100 × 109/L at baseline. There was a decrease in blast count in 18 of 24 patients (75%) on day 14 of cycle 1, at the end of the combination of voruciclib and venetoclax dosing. On day 28, after 14 days of single-agent venetoclax dosing there was a rebound in blast count in 8 of 18 patients (44%) as shown in supplemental Figure 2.
Pharmacokinetics
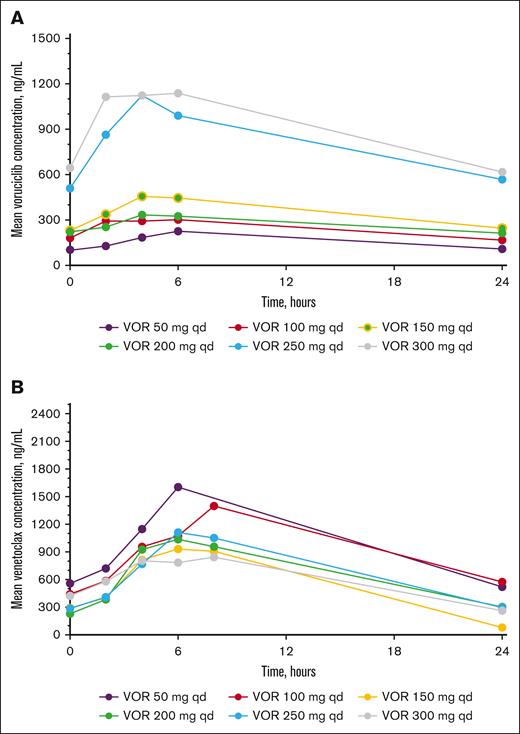
Voruciclib and venetoclax multiple dose plasma concentration profiles are shown in Figure 1A-B, respectively, and all pharmacokinetic parameters for voruciclib and venetoclax are presented in supplemental Tables 3 and 4, respectively. After oral administration of 50 to 300 mg of voruciclib, drug absorption was gradual, and maximum plasma concentrations were typically observed at 4 hours after administration. Taking into consideration the limited sample size in each cohort and the heterogenous patient population, voruciclib multiple dose exposures were generally dose proportional in the dose range studied. The observed twofold accumulation of voruciclib after multiple once-daily dosing is consistent with the previously reported half-life of ∼28 hours.41 After oral administration of venetoclax at 200 mg, maximum plasma concentrations were generally observed around 4 to 6 hours after dose, and venetoclax plasma exposures were generally comparable in all cohorts.
Mean VOR (A) and venetoclax (B) plasma concentration-time profiles on cycle 1, day 14 after multiple dose administration. VOR, voruciclib; qd, once daily.
Mean VOR (A) and venetoclax (B) plasma concentration-time profiles on cycle 1, day 14 after multiple dose administration. VOR, voruciclib; qd, once daily.
Voruciclib decreases Mcl-1 protein expression and RNAPII phosphorylation
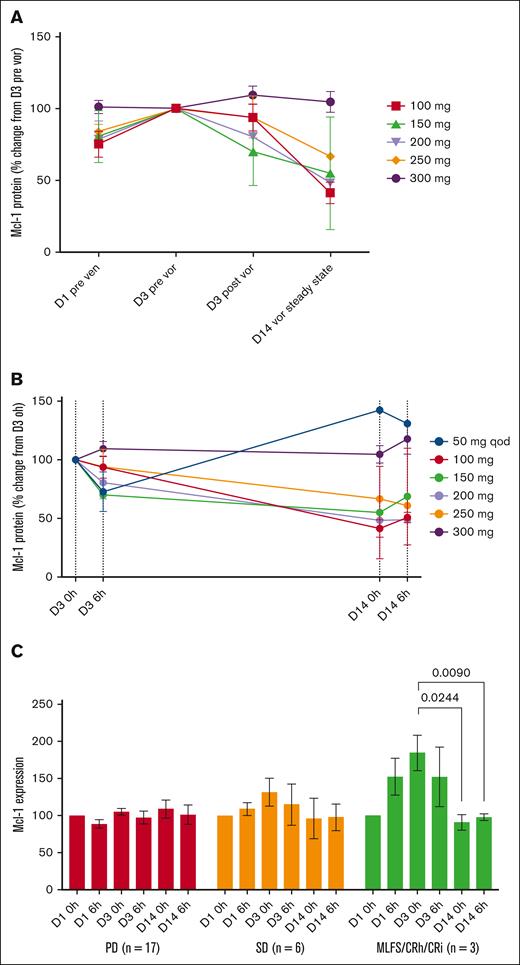
Mcl-1 protein in PBMCs increased initially during single-agent venetoclax dose ramp-up on days 1 to 3, and then decreased during voruciclib and venetoclax administration on day 14 relative to day 3 (Figure 2A). Decreases in Mcl-1 values were observed primarily for voruciclib doses of 100 to 250 mg (Figure 2B). For all doses combined, there was an 0.80-fold decrease (P < .05) in Mcl-1 protein levels from day 3 before initiating voruciclib dosing to day 14 (supplemental Figure 3A).
Mcl-1 protein expression in cycle 1 by dose level. Relative mean (standard error) change to baseline (A), mean values over time during VOR administration (B), and change in relative mean values by clinical response (C). CRh, complete response with partial hematologic recovery; qod, every other day dosing.
Mcl-1 protein expression in cycle 1 by dose level. Relative mean (standard error) change to baseline (A), mean values over time during VOR administration (B), and change in relative mean values by clinical response (C). CRh, complete response with partial hematologic recovery; qod, every other day dosing.
Mcl-1 protein values increased during venetoclax ramp-up and then significantly decreased relative to baseline while on voruciclib in patients achieving a complete marrow remission, numerically decreased but not attaining statistical significance in patients with SD lasting ≥3 months, and were unchanged in patients with primary refractory disease (Figure 2C). This observation raises the question of whether an increase in expression of Mcl-1 with venetoclax treatment could serve as a biomarker of response to voruciclib, a hypothesis that can be tested with larger numbers in a future study. To explore differences in Mcl-1 expression dynamics, we compared Mcl-1 levels on day 3 of venetoclax and before starting voruciclib dosing between patients who subsequently achieved a CRi/MLFS, SD, and PD. Compared with the PD group, there was significant (P < .0001) increase in Mcl-1 expression in the CR group and a trend toward increase in Mcl-1 expression in the SD group (supplemental Figure 4). There was no association between baseline Mcl-1 levels and subsequent clinical response (supplemental Figure 5).
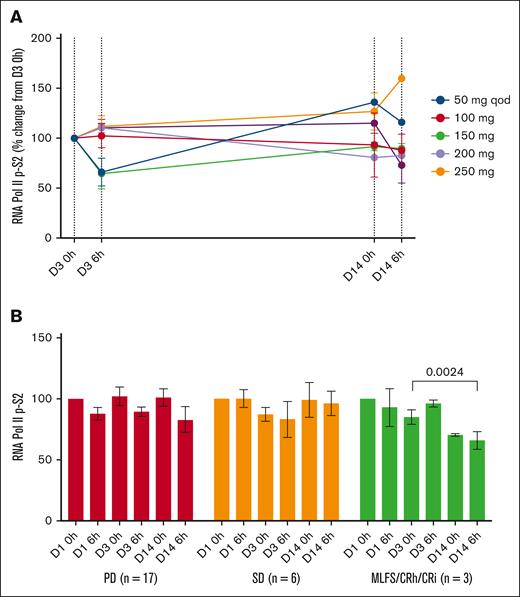
Reduced levels of pRNAPIISer2 were observed in PBMCs after voruciclib treatment (Figure 3A), with significant decreases in clinical responders, similar to what was observed with Mcl-1 protein expression (Figure 3B). Across dose levels, there was an 0.81-fold decrease in pRNAPIISer2 levels (supplemental Figure 3B).
Change in pRNAPIISer2 in cycle 1. Mean change by dose level (A) and by disease response (B). CRh, complete remission with partial hematologic recovery; qod, every other day dosing.
Change in pRNAPIISer2 in cycle 1. Mean change by dose level (A) and by disease response (B). CRh, complete remission with partial hematologic recovery; qod, every other day dosing.
Discussion
Despite the recent introduction of targeted therapies with activity in AML, ∼20% of patients receiving initial therapy do not achieve a CR/CRi, and ∼30% of responders will relapse, with a median time to progression of ∼7 months. Regardless of treatment intensity, salvage regimens achieve overall responses in ∼35% to 45% of patients, but median overall survival after first relapse is only ∼6 months.48 Allogeneic SCT at the time of second remission remains the only option with curative potential, with an estimated 2-year survival rate of 22.5% to 35%.49
As single agents, approved therapies targeting AML with mutations in FLT3, IDH1, and IDH2 achieve ORRs ranging from 25% to 35%, with median overall survival of <1 year in patients with R/R AML who were mostly venetoclax-naive.50-53 Similarly, menin inhibitors targeting KMT2A and/or NPM1 have achieved ORRs ranging from 23% to 29% in patients with R/R AML, some of whom were previously treated with venetoclax.54-57
Therapies not targeting specific mutations and administered at relapse in patients not previously treated with venetoclax have benefited only a small proportion of patients. For example, in a randomized study, the hedgehog pathway inhibitor, glasdegib, in combination with low-dose cytarabine resulted in a response rate of 17% and median overall survival of 8.8 months.58 In 2 single-center retrospective analyses, venetoclax in combination with HMAs or low-dose cytarabine yielded a CR rate (CR + CRi) of 32% and 34%, respectively, and a median overall survival of 5.5 and 6.1 months, respectively.59,60 Similarly, in a randomized phase 3 trial comparing guadecitabine with investigator treatment choice of high-intensity chemotherapy or low-intensity treatment, the complete remission rates (CR + CRi) were 27% and 14% respectively, with median overall survival of 6.4 and 5.4 months, respectively.61
In patients with R/R AML with no actionable mutations and with previous exposure to venetoclax, outcomes are dismal, with CR rates (CR + CR with partial hematologic recovery/CRi) of <15% and median survival of <6 months.14-18 There is a clear need for new therapies with different mechanisms of action for R/R AML, particularly for patients previously treated with venetoclax and whose leukemia has no actionable mutations or has progressed on mutation-targeted therapies.
Our hypothesis in combining voruciclib with venetoclax was that reversal of a common mechanisms of resistance to venetoclax through indirect inhibition of Mcl-1 could restore susceptibility to venetoclax after failure of previous venetoclax. Furthermore, Mcl-1 inhibition by itself could result in an antileukemia effect comparable with what is observed with Bcl-2 inhibition in AML. We considered that a response rate (CR + CRi) of ≥20% in R/R AML would be meaningful and support further development.
The voruciclib schedule of 14 days on/14 days off per cycle had been shown to be safe up to a dose of 200 mg in the single-agent dose-escalation part of the study. This schedule was selected empirically based on the voruciclib mean half-life of at least 24 hours, with the first 14 days aimed at achieving steady-state plasma concentrations for at least 10 days, then ∼7 days for drug clearance from plasma and tissue, followed by 7 days free of drug exposure.
In vitro studies suggest that voruciclib is a substrate of P-gp and may inhibit this transporter at high concentrations, although there was no clear relationship to concentration. In vitro studies have shown that cytochrome (CYP) P450 CYP3A4 and other CYP enzymes are involved in voruciclib metabolism and that voruciclib is potentially an inhibitor of CYP3A4. Venetoclax is a substrate and inhibitor of P-gp and is metabolized by CYP3A4.62 Because of the potential for inhibition of P-gp and CYP3A4 by voruciclib, the dose of venetoclax was reduced by 50% according to its package insert and patients enrolled in this study received venetoclax 200 mg once daily on days 1 to 21 of each cycle and then 400 mg for the rest of the cycle. Mutual pharmacokinetic drug interaction between voruciclib and venetoclax was assessed by collecting pharmacokinetic samples for both drugs at steady state when maximal interaction, if it were to occur, is likely to be observed. Voruciclib multiple dose plasma exposures at the 300 mg dose level in patients with AML enrolled in this study were comparable with historical single-agent dose normalized data in patients with solid tumors, indicating that venetoclax did not affect voruciclib pharmacokinetics. Venetoclax plasma exposures observed in this study were also generally consistent with historical single-agent data in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma indicating that voruciclib did not affect venetoclax pharmacokinetics.63,64 Based on historical comparisons and because pharmacokinetic results from this study are in line with expectations, we concluded that concomitant administration of voruciclib and venetoclax does not result in clinically relevant pharmacokinetic interactions.
Voruciclib at doses up to 300 mg in combination with venetoclax was tolerable in patients with R/R AML, and no DLTs were observed. The MTD was not identified, as we stopped dose escalation at 300 mg, a dose that achieved plasma concentrations higher than needed for antileukemic effect based on preclinical studies.40 The most common adverse events were those typically observed in patients with relapsed AML, primarily febrile neutropenia, sepsis, pulmonary infection, and hemorrhage. Although 27% of the patients discontinued study drugs because of adverse events, these events, such as infection and hemorrhage, were considered disease-related although a contribution of the study drugs to myelosuppression could not be ruled out. Two patients, both with a history of allogeneic SCT, developed opportunistic infections that were fatal.
Pharmacodynamic studies demonstrated the anticipated on-target effect at doses ranging from 100 to 250 mg. There was an increase in Mcl-1 protein between days 1 and 3, during single-agent venetoclax dose ramp-up, suggesting rapid increase of Mcl-1 expression with venetoclax therapy, followed by a decrease in Mcl-1 between days 3 and 14 while on voruciclib. Interestingly, these changes were more pronounced in the 3 patients with CRi or MLFS, and not in patients with SD and PD, potentially reflecting underlying resistance mechanisms or differences in disease biology. We hypothesize that sensitivity to venetoclax is a critical determinant of response to the voruciclib and venetoclax combination. This is supported by our observation that clinical responders exhibited a significant increase in Mcl-1 levels after venetoclax exposure, indicative of functional Bcl-2 inhibition and compensatory upregulation of Mcl-1. Voruciclib may potentiate this response by decreasing Mcl-1 levels, thereby enhancing apoptotic priming.
Although we saw a decrease in pRNAPIISer2, the effect was less prominent, perhaps because of a single sampling time, ∼6 hours after voruciclib dosing. Assessment of pRNAPIISer2 at multiple time points and days after dosing may have uncovered greater decreases. Because we did not measure CDK9 target engagement in patient samples, we cannot exclude reduced potency due to incomplete or short-lived CDK9 inhibition with voruciclib.
We did not see a decrease in Mcl-1 or pRNAPIISer2 at the 50-mg dose level, most likely because plasma concentrations achieved at this were suboptimal. In the 300-mg dose cohort, we did not observe an increase in Mcl-1 levels after venetoclax exposure, nor did we detect a reduction in Mcl-1 levels with voruciclib, as seen at the 100- to 250-mg dose levels. This cohort also lacked clinical responses. These observations may reflect differences in disease biology among patients in this cohort, with intrinsic resistance mechanisms potentially unrelated to Mcl-1 predominating. Another possibility is that higher doses of voruciclib could induce cellular stress responses that activate survival pathways, such as NF-κB or MAPK, which mitigate the effects of venetoclax and voruciclib. These findings underscore the importance of further investigation into dose-dependent effects and patient-specific resistance mechanisms.
It is important to note that, unlike most CDK9 inhibitors that are administered intravenously, voruciclib is orally administered. This mode of administration introduces variability in pharmacokinetics across patients, potentially influencing the magnitude and timing of pharmacodynamic effects. By contrast, other CDK9 inhibitors that are administered intravenously achieve more predictable time to maximal plasma concentration and allow for uniform sample collection relative to peak drug exposure.
Antileukemic activity in this heavily pretreated patient population enrolled in the study was limited, with a CR + CRi + MLFS rate of 7% in the intent-to-treat population, and 10% in patients administered biologically relevant voruciclib doses of ≥100 mg. This rate is lower than our target and may reflect the highly refractory nature of disease in patients enrolled in the study, including a high proportion with TP53 overexpression or refractory disease after multiple previous therapies. Another reason could be the 14-day interval without voruciclib in each cycle leading to blast rebound and subsequent disease escape.
Early-generation small-molecule pan-CDK inhibitors such as flavopiridol/alvocidib and dinaciclib have shown activity in hematologic malignancies, as single agents or in combination with chemotherapy. However, their development has been challenged by toxicities due to off-target effects, including a high incidence of serious adverse events and discontinuations because of toxicities.65-67 In addition, tumor lysis syndrome has been reported in chronic lymphocytic leukemia and AML, requiring prophylactic measures and careful monitoring.68,69 Because the pan-CDK inhibitors were overly toxic, they are not suitable for combination with venetoclax in hematologic malignancies.
Given the compelling scientific rationale of indirectly targeting Mcl-1, numerous other CDK9 inhibitors are in early stages of development, although published data at this time are limited, particularly in AML.70 The infusional CDK9 inhibitor AZD4573 was evaluated in a phase 1 study for patients with various hematologic malignancies and resulted in a high rate of tumor lysis syndrome (TLS) in AML, and the development of this product has been discontinued.71 Another infusional CDK9 inhibitor, GFH-009, is being studied in various hematologic malignancies and solid tumors, with no responses observed in 14 patients with relapsed or refractory AML enrolled in a phase 1 dose-escalation study.72 A subsequent study evaluated GFH-009 (renamed SLS009) in combination with venetoclax and azacitidine in R/R AML with previous exposure to venetoclax, which resulted in a CR + CRi rate of 17% in 29 evaluable patients, and notably a response rate of 56% in 9 patients with ASXL1 mutation.73 In a phase 2a study in 28 patients with R/R AML, of whom 62.5% had previous exposure to venetoclax and azacitidine, the oral CDK9 inhibitor, QHRD107, in combination with venetoclax plus azacitidine achieved a CR + CRi rate of 22.2% in 18 evaluable patients, including 3 patients with a TP53 mutation.74 Based on these 2 studies, it is conceivable that the addition of azacitidine to a CDK9 inhibitor plus venetoclax has led to a higher response rate. The orally administered CDK9 inhibitor, fadraciclib, has been evaluated as a single agent in solid tumors and hematologic malignancies, and is now being evaluated in combination with venetoclax in AML.75 Some of these other studies have been evaluating various administration schedules with the CDK9 inhibitors to optimize efficacy while maintaining tolerability. We believe that careful evaluation of dose and schedule informed by rigorous pharmacokinetic and correlative pharmacodynamic analyses, as performed in our study, are helpful to understand the potential benefits and risks of each of this drug class.
In summary, our phase 1 study defined a dose schedule of voruciclib in combination with venetoclax that is well tolerated at doses that achieve pharmacodynamic evidence of decreased RNAPIISer2 phosphorylation and inhibition of MCL-1 transcription. Although the efficacy we observed is limited, we believe that the favorable safety and pharmacodynamic properties of this combination warrant further exploration to determine whether a more dose-intensive schedule could increase the efficacy of the combination without negatively affecting the otherwise favorable nature of this rational drug combination for patients with AML.
Acknowledgments
The authors thank the patients for their participation in the study. Ingrid Koo contributed to manuscript preparation.
M.S.D. is supported by a National Institutes of Health award (R01CA266298). N.J.-S. is supported by a FLAIR award from Pan-Mass Challende (PMC) Team FLAMES.
The study was designed with input from the investigators and sponsored by MEI Pharma (San Diego, CA). Data were verified by the sponsor, analyzed by sponsor statisticians, and interpreted by academic authors and sponsor representatives.
Authorship
Contribution: Y.A.-V., R.G.G., and M.S.D. were responsible for protocol development; Y.A.-V., R.J.C., S.N.D., M.K., K.H.B., S.A., M.M.A.M., and V.R.B. were responsible for patient management and data collection; M.T., R.G.G., S.E.W., Y.A.-V., M.S.D., and N.J.-S. were responsible for study analyses; P.R. was responsible for pharmacokinetic analyses; M.S.D., N.J.-S., and S.E.W. were responsible for correlative studies; and all authors contributed to data collection, data review and interpretation, trial procedures, and manuscript preparation, and had final responsibility for content and the decision to submit for publication.
Conflict-of-interest disclosure: Y.A.-V. has received research funding from Jazz, BerGenBio, MEI Pharma, Astex, Sun Pharma, FibroGen, and Daiichi Sankyo; and has received consultancy fees from CytomX and Sun Pharma. S.N.D. has received consultancy fees from Pfizer, Rigel, and Kite/Gilead. K.H.B. served on the advisory board of Novartis. S.A. has received consultancy fees from AbbVie, Daiichi Sankyo, and Servier; and has received research funding from Incyte, AltruBio, and Actinium Pharmaceutical. M.M.A.M. has received consultancy fees from, and was on the advisory board of, Hasna Biopharma, CareDx, National Marrow Donor Program, Incyte, Gilead, NexImmune, and Stemline Therapeutics; and has received research funding from Gilead and NexImmune. V.R.B. participates in the safety monitoring committee for Protagonist; serves as a member of the National Comprehensive Cancer Network Acute Myeloid Leukemia Panel, as an associate editor for the journal, Current Problems in Cancer, and as a contributor for British Medical Journal Best Practice; received consulting fees from Jazz, Imugene, Sanofi, and Taiho; reports research funding (institutional) from Cynata Therapeutics, MEI Pharma, Actinium Pharmaceutical, Sanofi US Services, AbbVie, Pfizer, Incyte, Jazz, and the National Marrow Donor Program; and receives drug support (institutional) from Chimerix for a trial. P.R. is a consultant to MEI Pharma. M.T. and S.E.W. are employees of MEI Pharma. R.G.G. is an employee of MEI Pharma and holds equity shares in MEI Pharma. M.S.D. has received institutional research funding from AbbVie, AstraZeneca, Ascentage Pharma, Genentech, MEI Pharma, Novartis, and Surface Oncology; and received personal consulting income from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, Bristol Myers Squibb, Eli Lilly, Galapagos, Genentech, Genmab, Janssen, Merck, MEI Pharma, Nuvalent, Secura Bio, TG Therapeutics, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Yesid Alvarado-Valero, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: yalvarad@mdanderson.org.
References
Author notes
Original data are available on request from the coauthor, Richard G. Ghalie (rghalie@meipharma.com).
The full-text version of this article contains a data supplement.