Key Points
Belantamab mafodotin showed ORR 45%, PFS 3.8 months, and OS 17.2 months in relapsed/refractory multiple myeloma in a real-world setting.
ORR was 29% in heavily pretreated patients who had received prior BCMA-targeted therapy.
Visual Abstract
Belantamab mafodotin is an antibody-drug conjugate targeting B-cell maturation antigen (BCMA) for the treatment of multiple myeloma. To evaluate the efficacy and safety of belantamab mafodotin in a real-world setting in the United States, we assessed all patients treated with commercial belantamab mafodotin at the Memorial Sloan Kettering Cancer Center between 2020 and 2023. Ninety-four patients were identified; 57 had high-risk cytogenetics, 77 were triple-class refractory, the median prior lines of therapy was 6, and 18 patients had received prior BCMA-targeted treatment. The overall response rate (ORR) was 43%, and 21% achieved a very good partial response or better. The median progression-free survival (PFS) was 3.8 months, median overall survival (OS) was 17.2 months, and the median duration of response was 10.5 months. In patients with prior BCMA exposure (median prior lines of therapy = 9), the ORR was 29%, PFS was 2 months, and OS was 20.4 months. Sixty-one patients (65%) had any grade of ocular toxicity and 15 patients had grade 3 or more keratopathy. All keratopathy was reversible and resolved or reduced to grade 1 by the end of the follow-up. Most patients could continue on a reduced dose or with a longer dose interval while maintaining the clinical response. Eighteen patients had 1 or more infections, most of which were grade 1/2. In summary, belantamab mafodotin showed significant ORR, including those of patients with prior BCMA exposure. Ocular toxicity was similar to that in previous reports, and the risk of serious infections was low in this cohort of heavily pretreated patients with multiple myeloma.
Introduction
Belantamab mafodotin is an antibody-drug conjugate targeting B-cell maturation antigen (BCMA) for the treatment of relapsed/refractory multiple myeloma. BCMA is expressed in bone marrow plasma cells and plays an essential role in regulating B-cell maturation, proliferation, and survival.1-3 Overexpression of BCMA in multiple myeloma leads to activation of the NF-κB pathway as well as increased expression of genes involved in survival, growth, angiogenesis, metastasis, and immunosuppression.4 Belantamab mafodotin is a humanized anti-BCMA monoclonal antibody with a cleavable linker to the microtubule-disrupting agent monomethyl auristatin F.5 The single-agent activity of belantamab mafodotin was 32% in the DREAMM-2 phase 2 trial.6,7 Side effects from belantamab mafodotin mainly include ocular toxicity, keratopathy, and reduced best-corrected visual acuity (BCVA), which occurred in up to 70% of patients in the DREAMM-2 trial.6 These side effects can necessitate dose reduction and dose delays which may limit efficacy or lead to treatment discontinuation.
Belantamab mafodotin was the first of the BCMA-targeted drugs to be US Food and Drug Administration (FDA)–approved in the United States in 2020.8 The FDA approval in the United States was however withdrawn in November 2022 as belantamab mafodotin failed to show superiority over the control arm, pomalidomide, and dexamethasone, in the randomized phase 3 trial (DREAMM-3, ClinicalTrials.gov identifier: NCT04162210).9 Several clinical trials with belantamab mafodotin are ongoing, among them the phase 3 trial DREAMM7 (ClinicalTrials.gov identifier: NCT04246047) and DREAMM8 (ClinicalTrials.gov identifier: NCT04484623) which both reported favorable outcomes in the belantamab mafodotin vs comparator arms.10,11
As clinical trials have strict inclusion/exclusion criteria, the trial population does not necessarily reflect the entire population of patients with relapsed/refractory multiple myeloma. Several authors have published real-world studies of belantamab mafodotin; however, most studies included patients from Early or Expanded Access Programs that were in place before US and European Union approvals.12-21 The aim of this study was to assess the safety and efficacy, as well as dose modifications, in patients treated with commercially prescribed belantamab mafodotin in a real-world setting. We assessed the treatment patterns, response rates, and evaluated outcomes in patients who had received prior treatment with BCMA-targeted therapies.
Methods
This was an observational study including all patients who received at least 1 dose of commercial belantamab mafodotin at the Memorial Sloan Kettering Cancer Center, New York, NY, between 1 October 2020 and 28 February 2023. A data search based on the hospital pharmacy administration records were used to identify patients treated with the commercial belantamab mafodotin. A manual assessment of patient electronic medical records was performed to obtain patient characteristics, including comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status, and multiple myeloma characteristics. Baseline information on prior lines of therapy, Revised International Staging System (R-ISS) stage, and cytogenetics were obtained. High-risk cytogenetics was defined as the presence of t(4;14), t(14;16), t(14;20), del 1p, gain or amplification of 1q, and del 17p. Information on treatment outcomes, adverse events, dose reductions and dose delays, and survival was obtained until the end of the follow-up on 29 February 2024.
All patients were included in the belantamab mafodotin Risk Evaluation and Mitigation Strategy (REMS) program and had ocular assessments at baseline and before each dose of belantamab mafodotin. Lubricating eye drops were prescribed to all patients as a prophylaxis. If patients had grade 2 or more keratopathy or a grade 2 or more reduction in BCVA, treatment was held until the keratopathy had returned to grade 1 or better.
Treatment responses were defined per the International Myeloma Working Group criteria.22,23 Descriptive statistics were used to assess patient characteristics, response rates, and rate of adverse events. Kaplan Meier curves were used to assess progression-free survival (PFS), overall survival (OS), and median duration of response. PFS was defined as the time from the start of belantamab mafodotin until disease progression or death. OS was defined as the time from the start of belantamab mafodotin until death from any cause. Adverse events were graded by the Common Terminology Criteria for Adverse Events v5 and the Keratopathy and Visual Acuity scale.6,24 Minimal residual disease (MRD) testing was performed in a subset of patients using 10-color multiparametric flow cytometry.25
R version 4.2.3. was used for statistical analyses. The study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board in accordance with the Declaration of Helsinki.
Results
Ninety-four patients with relapsed/refractory multiple myeloma were treated with at least 1 dose of commercial belantamab mafodotin were identified and included in the study. Forty-eight (51%) were women with a median age of 67 years (Table 1). Fifty-seven patients (61%) had the presence of high-risk cytogenetics. Fifteen patients had R-ISS stage I, 41 patients had R-ISS stage II, and 12 patients had R-ISS stage III. Information on the R-ISS stage was missing for 26 patients. Six patients had concomitant AL amyloidosis. The median follow-up in the whole cohort was 28.2 months.
Patient demographics
| . | Number . | % . |
|---|---|---|
| Total | 94 | 100 |
| Median age (y) | 67.5 | |
| Men | 46 | 49 |
| Women | 48 | 51 |
| High-risk cytogenetics∗ | 57 | 61 |
| ECOG performance status | ||
| 0 | 22 | 23 |
| 1 | 49 | 52 |
| 2 | 12 | 13 |
| 3 | 9 | 10 |
| R-ISS stage | ||
| I | 15 | 16 |
| II | 41 | 44 |
| III | 12 | 13 |
| Missing | 26 | 28 |
| Median number of prior lines | 6 | 6 |
| Prior high dose melphalan with ASCT | 72 | 77 |
| Prior treatment | ||
| Exposure to IMiD | 94 | 100 |
| Exposure to proteasome inhibitor | 94 | 100 |
| Exposure to anti-CD38 antibody | 94 | 100 |
| Triple-class refractory | 77 | 82 |
| Penta-refractory | 36 | 38 |
| Exposure to BCMA therapy | 18 | 19 |
| CAR T therapy | 12 | 13 |
| BCMA-directed bispecific antibody | 6 | 6 |
| Belantamab mafodotin | 1 | 1 |
| Exposure by drug | ||
| Lenalidomide | 88 | 94 |
| Pomalidomide | 84 | 89 |
| Thalidomide | 26 | 28 |
| Bortezomib | 91 | 97 |
| Carfilzomib | 82 | 87 |
| Ixazomib | 32 | 34 |
| Daratumumab | 94 | 100 |
| Isatuximab | 9 | 10 |
| Elotuzumab | 26 | 28 |
| Selinexor | 11 | 12 |
| . | Number . | % . |
|---|---|---|
| Total | 94 | 100 |
| Median age (y) | 67.5 | |
| Men | 46 | 49 |
| Women | 48 | 51 |
| High-risk cytogenetics∗ | 57 | 61 |
| ECOG performance status | ||
| 0 | 22 | 23 |
| 1 | 49 | 52 |
| 2 | 12 | 13 |
| 3 | 9 | 10 |
| R-ISS stage | ||
| I | 15 | 16 |
| II | 41 | 44 |
| III | 12 | 13 |
| Missing | 26 | 28 |
| Median number of prior lines | 6 | 6 |
| Prior high dose melphalan with ASCT | 72 | 77 |
| Prior treatment | ||
| Exposure to IMiD | 94 | 100 |
| Exposure to proteasome inhibitor | 94 | 100 |
| Exposure to anti-CD38 antibody | 94 | 100 |
| Triple-class refractory | 77 | 82 |
| Penta-refractory | 36 | 38 |
| Exposure to BCMA therapy | 18 | 19 |
| CAR T therapy | 12 | 13 |
| BCMA-directed bispecific antibody | 6 | 6 |
| Belantamab mafodotin | 1 | 1 |
| Exposure by drug | ||
| Lenalidomide | 88 | 94 |
| Pomalidomide | 84 | 89 |
| Thalidomide | 26 | 28 |
| Bortezomib | 91 | 97 |
| Carfilzomib | 82 | 87 |
| Ixazomib | 32 | 34 |
| Daratumumab | 94 | 100 |
| Isatuximab | 9 | 10 |
| Elotuzumab | 26 | 28 |
| Selinexor | 11 | 12 |
ASCT, autologous stem cell transplant; IMiD, immunomodulatory drug.
High risk cytogenetics was defined as 1 or more of the following cytogenetic aberrations: t(4;14), t(14;16), t(14;20), del 1p, del 17p, and gain/amp 1q.
Twenty-two patients had ECOG performance status 0, 49 patients had ECOG 1, 12 patients had ECOG 2, 9 had ECOG 3, and ECOG performance status was missing for 2 patients. Seventeen patients had atrial fibrillation, 15 had diabetes mellitus, 7 had coronary artery disease, 5 had congestive heart failure, and 1 had cardiomyopathy. Fifty-eight patients had creatinine clearance (CrCl) >60 mL/min, 28 patients had CrCl between 30 and 59 mL/min, and 8 patients had chronic kidney disease with CrCl <30 mL/min, of which 1 was on hemodialysis. Six patients had cataracts, 1 patient had a prior retinal hemorrhage, 3 patients had glaucoma, 1 patient had bilateral keratopathy, 1 patient had bilateral dry eyes, and 1 patient was blind at baseline.
Patients had been treated with a median of 6 (range, 2-14) prior lines of therapy, and all patients (100%) had received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody. Seventy-seven patients (82%) were triple-class refractory, and 36 (38%) were penta-refractory. Seventy-two patients had undergone high-dose melphalan with autologous stem cell transplantation; of these, 27 had 2 autologous transplants, 3 patients had 3 autologous transplants, and 7 patients had an allogeneic stem cell transplant before starting belantamab mafodotin. Twelve patients started treatment in the US Expanded Access Program in 2020 with belantamab mafodotin before receiving the commercial drug.
Eighteen patients (20%) had received 1 or more BCMA-targeted agents before starting belantamab mafodotin, including bispecific antibodies (n = 6), chimeric antigen receptor (CAR) T cells (n = 12), and trial therapy with belantamab mafodotin (n = 1). Of these 18 patients, 11 had bone marrow or soft tissue biopsies before starting belantamab mafodotin, 9 were positive for BCMA on immunohistochemistry, and 2 were indeterminate because of limited plasma cell infiltration in the bone marrow. Three patients received bispecific antibodies targeting GPRC5D or FCRH5 before starting belantamab mafodotin.
Patients received a median of 4 cycles (range, 1-54) of belantamab mafodotin. For nonresponders, the median number of cycles was 2, whereas patients who responded had a median of 9 cycles of belantamab mafodotin. The median time to the best response was 50 days (1.7 months). Most patients (95%, n = 89) received single-agent treatment, whereas 5 patients received belantamab mafodotin in combination with other antimyeloma agents. Agents that were combined off-label with belantamab mafodotin were bortezomib, carfilzomib, pomalidomide, cyclophosphamide, and 1 with low-dose cytarabine.
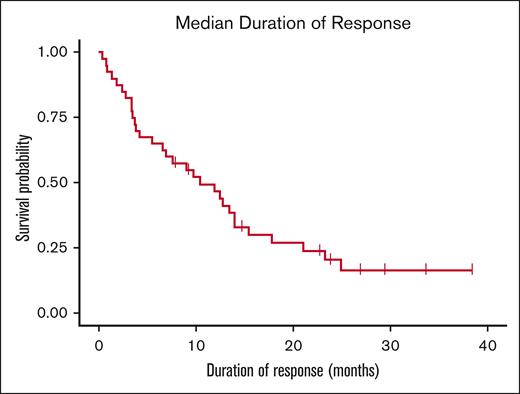
The overall response rate (ORR) was 43% (40/94) in all patients. The median PFS was 3.8 months, and the median OS was 17.2 months (Figure 1). Seventeen patients achieved partial response (PR); 21 patients achieved very good partial response (VGPR), and 2 patients achieved complete response (CR) (Table 2). Of the patients who achieved VGPR or better, 7 patients had bone marrow biopsies for assessment of MRD; 3 were MRD-positive and 4 were MRD-negative. The median duration of response for patients in the ORR cohort was 10.2 months (Figures 2 and 3). Twenty-five patients had stable disease and 29 patients had progressive disease. Of the 6 patients with concomitant amyloid light chain (AL) amyloidosis, 4 patients responded to belantamab mafodotin (3 PR/1 VGPR), whereas 2 patients did not respond. In a separate analysis of patients not included in the 2020 Expanded Access Program, the ORR was 39%; 14 patients achieved PR, 17 achieved VGPR, and 1 patient achieved CR. At the end of follow-up in February 2024, 4 patients continued therapy with belantamab mafodotin.
Response rates and rates of ocular adverse events in relapsed/refractory multiple myeloma treated with belantamab mafodotin in a real-world setting
| Patient outcomes . | Number . | % . |
|---|---|---|
| Response rates | ||
| ORR | 40 | 43 |
| PR | 17 | 18 |
| VGPR | 21 | 22 |
| CR | 2 | 2 |
| SD | 25 | 27 |
| PD | 29 | 31 |
| Ocular adverse events | ||
| Keratopathy | 60 | 64 |
| Grade 1 | 28 | 30 |
| Grade 2 | 17 | 18 |
| Grade 3 | 13 | 14 |
| Grade 4 | 2 | 2 |
| Reduced BCVA | 46 | 49 |
| Grade 1 | 23 | 24 |
| Grade 2 | 14 | 15 |
| Grade 3 | 3 | 3 |
| Grade 4 | 1 | 1 |
| Grade missing | 4 | 4 |
| Total | 94 | 100 |
| Patient outcomes . | Number . | % . |
|---|---|---|
| Response rates | ||
| ORR | 40 | 43 |
| PR | 17 | 18 |
| VGPR | 21 | 22 |
| CR | 2 | 2 |
| SD | 25 | 27 |
| PD | 29 | 31 |
| Ocular adverse events | ||
| Keratopathy | 60 | 64 |
| Grade 1 | 28 | 30 |
| Grade 2 | 17 | 18 |
| Grade 3 | 13 | 14 |
| Grade 4 | 2 | 2 |
| Reduced BCVA | 46 | 49 |
| Grade 1 | 23 | 24 |
| Grade 2 | 14 | 15 |
| Grade 3 | 3 | 3 |
| Grade 4 | 1 | 1 |
| Grade missing | 4 | 4 |
| Total | 94 | 100 |
PD, progressive disease; SD, stable disease.
Swimmer plot of treatment response to belantamab mafodotin. Patients who had previous exposure to BCMA-targeted therapies are marked with an asterisk. LOT, line of therapy; POD, progression of disease.
Swimmer plot of treatment response to belantamab mafodotin. Patients who had previous exposure to BCMA-targeted therapies are marked with an asterisk. LOT, line of therapy; POD, progression of disease.
Among the 18 patients who had prior exposure to BCMA-targeted therapy, the ORR was 29%, and 5 patients had a PR or better (4 VGPR and 1 CR). Two patients had VGPR at the start of belantamab mafodotin therapy and continued to be in remission after starting belantamab mafodotin. Four patients had stable disease, whereas 7 patients had progressive disease. The median number of prior lines of therapy in the BCMA-exposed group was 9. The median time from prior BCMA CAR T-cell or BCMA bispecific therapy to starting belantamab mafodotin was 17.6 months and 9.3 months, respectively. The median time from the last BCMA treatment of any kind to starting belantamab mafodotin was 10.7 months. The median PFS was 2 months and the OS was 20.4 months in this group. There was no significant difference in the median PFS (P = .86; Figure 4) and OS between patients who were BCMA-exposed and those who were BCMA-naïve.
PFS in patients who had 1 or more prior BCMA-targeted therapy vs BCMA therapy–naïve patients.
PFS in patients who had 1 or more prior BCMA-targeted therapy vs BCMA therapy–naïve patients.
Sixty-one patients (65%) had any grade of ocular toxicity per the Keratopathy and Visual Acuity scale; 28 patients had grade 1, 17 had grade 2, 13 had grade 3, and 2 patients had grade 4 keratopathy. Twenty-nine patients had the presence of corneal microcysts (information missing in 13 patients). Forty-six patients (49%) experienced a decline in BCVA; 23 patients had grade 1, 14 had grade 2, 3 had grade 3, and 1 patient had a grade 4 decline in BCVA (grade missing in 4 patients). All patients had resolution or reduction to grade 1 keratopathy by the end of follow-up.
All but 1 patient were started on belantamab mafodotin dose of 2.5 mg/kg every 3 weeks, whereas 1 patient was started on a dose of 1.9 mg/kg. The dosage was reduced from 2.5 mg/kg to 1.9 mg/kg in 28 patients, whereas 33 patients had dose delays. Twenty patients had both dose delays and dose reductions, most of which were due to ocular adverse events but also due to baseline cytopenia, infections, and patient preference. Most patients who had dose modifications due to ocular events were able to continue therapy at a lower dose with a maintained response. Ten patients discontinued the treatment due to keratopathy.
There were 26 reported infections in 18 patients; 13 patients had 1 infection and 5 patients had 2 or more infections during belantamab treatment. There were 14 grade 1/2 and 12 grade 3/4 infections. There were 15 bacterial infections and 9 viral infections, of which 4 were COVID-19 infection. All infections were treated and resolved, and there were no deaths due to infections in this patient cohort. Eighty-nine patients were on prophylaxis with acyclovir or valacyclovir, 3 were on entecavir prophylaxis, and 2 were on letermovir prophylaxis. Two patients were on secondary prophylaxis for invasive fungal infections with isavuconazonium and voriconazole. Seventeen patients were on pneumocystis jiroveci prophylaxis with either sulfamethoxazole and trimethoprim, atovaquone, or pentamidine. Five patients were on prophylaxis with monthly intravenous immunoglobulin (IVIG) owing to a history of repeated infections.
Sixty-five patients discontinued treatment due to disease progression, and 10 patients stopped due to ocular toxicity as the only reason. Three patients relocated during the study period and were lost to follow-up. Ten patients stopped belantamab treatment as they were switched to other treatments, such as CAR T-cell therapy, without disease progression per the International Myeloma Working Group criteria. One patient discontinued treatment due to a new diagnosis of myelodysplastic syndrome. Sixty patients had passed away at the end of the follow-up on 29 February 2024.
Thirty-three patients had 1 or more BCMA-targeted therapies after discontinuation of belantamab mafodotin. Eighteen patients were treated with BCMA CAR T-cell therapy, of which 3 achieved CR, 4 achieved VGPR, 5 achieved PR, and 6 had no response to subsequent BCMA CAR T-cell therapy. Furthermore, 18 patients were treated with a BCMA-targeted bispecific antibodies after belantamab mafodotin, 4 achieved a VGPR or better, 3 achieved a PR, 2 had stable disease, and 9 had no response to the BCMA-targeted bispecific antibody.
Discussion
In this heavily pretreated population of patients with multiple myeloma, the ORR was 43% which is slightly higher than in DREAMM-2.6 The PFS was 3.8 months, the median duration of response was 10.5 months, and the median OS was 17.2 months. Interestingly, patients with a prior BCMA exposure had an ORR of 29%. The rate of ocular toxicity, 65% keratopathy, was also comparable to previous reports.6 Dose reductions and delays were common; however, most patients were able to continue on reduced doses of belantamab mafodotin and maintain a clinical response.
Patients with triple-class refractory multiple myeloma have traditionally had poor OS ranging from 8 to 13 months.26,27 In recent years, novel therapies, many targeting BCMA, have led to significantly improved outcomes in multiple myeloma.28,29 Belantamab mafodotin was the first of the BCMA-targeted agents to be FDA-approved in 2020 based on the DREAMM-2 phase 2 study.6 Belantamab mafodotin was however withdrawn from the United States and European Union after the publication of the DREAMM-3 trial where belantamab failed to show superiority over the control arm, pomalidomide/dexamethasone.9 Conversely, recently reported results from 2 phase 3 trials with 3-drug combination treatments, DREAMM-7 and DREAMM-8 showed favorable outcomes in the belantamab mafodotin treatment arms.10,11 In DREAMM-7 (ClinicalTrials.gov identifer: NCT04246047), patients treated with belantamab mafodotin, bortezomib, and dexamethasone had longer PFS, 28.2 months, compared with those treated with daratumumab, bortezomib, and dexamethasone, PFS was 13.4 months.10 In DREAMM-8 (ClinicalTrials.gov identifer: NCT04484623), patients treated with belantamab mafodotin, pomalidomide, and dexamethasone had an ORR of 77% and the 12-month estimated PFS was 71% compared with patients treated with bortezomib, pomalidomide, and dexamethasone, ORR 72% and 12 month estimated PFS 51%.11 Furthermore, there are ongoing trials and efforts to mitigate the ocular toxicities through dose modifications and combination therapies, particularly combinations with pomalidomide and/or the gamma-secretase inhibitor nirogacestat are promising (ClinicalTrials.gov identifers: NCT04126200 and NCT05556798).30,31
The ORR in our study was 43%, which is higher than the 32% ORR reported in the DREAMM-2 trial and similar to other real-world studies.12-21 Real-world data have been reported from the Spanish, French, Italian, and Israeli Expanded Access Programs, as well as single-center studies from the Mayo Clinic and a few other US centers, as summarized in Table 3. The patient cohorts were similar to ours in terms of median prior lines, triple-class exposure, and refractoriness.6 In a real-world setting, patients tended to be less fit and had more comorbidities than those treated in clinical trials. Our patient cohort included patients with multiple comorbidities, some with ECOG 3, and chronic kidney disease. Encouragingly, the PFS in our study was in line with the published DREAMM-2 and real-world studies, whereas the OS was longer.
Summary of belantamab mafodotin real-world studies
| Author . | Country/setting . | Number of patients . | EAP . | ORR (%) . | PFS (mo) . | OS (mo) . | Ocular toxicity . |
|---|---|---|---|---|---|---|---|
| Alegre et al12 | Spain, multicenter | 33 | Yes | 42.2 | 3 | 13 | Keratopathy (51.5%) Patient-reported vision-related symptoms (45.5%) |
| Atieh et al13 | Kansas, US single center | 35 | Partly | 43 | 4.9 | 10.7 | 86% |
| de la Rubia et al14 | Spain, multicenter | 156 | Yes | 40 | 3.6 | 11 | 87.9% grade ≥3 was 33.7% |
| Iula et al15 | Italy, 4 hematology centers | 28 | No | 40 | 3 | 8 | Keratopathy 32% |
| Ntanasis-Stathopoulos et al17 | Greece | 27 | Yes | 52 | 2 | 16 | Keratopathy 33% |
| Mohan et al16 | Wisconsin and Arkansas, 2 US academic institutions | 56 | No | 44.6 | 3.6 | 10 | 71.4% Grade 3 keratopathy 54% |
| Roussel et al18 | France, multicenter | 184 | Yes | 32.7 | 2.4 | 8.8 | 56% Grade 3 keratopathy 27% |
| Shragai et al19 | Israel, multicenter | 106 | Yes | 45.5 | 4.7 | 14.5 | 68.4% Grade 3 keratopathy 40.5% |
| Talbot et al20 | France, multicenter | 104 | Yes | 38.1 | 3.5 | 9.3 | 37% |
| Vaxman et al21 | One US academic institution, 3 centers | 36 | No | 33 | 2 | 6.5 | 44% |
| Author . | Country/setting . | Number of patients . | EAP . | ORR (%) . | PFS (mo) . | OS (mo) . | Ocular toxicity . |
|---|---|---|---|---|---|---|---|
| Alegre et al12 | Spain, multicenter | 33 | Yes | 42.2 | 3 | 13 | Keratopathy (51.5%) Patient-reported vision-related symptoms (45.5%) |
| Atieh et al13 | Kansas, US single center | 35 | Partly | 43 | 4.9 | 10.7 | 86% |
| de la Rubia et al14 | Spain, multicenter | 156 | Yes | 40 | 3.6 | 11 | 87.9% grade ≥3 was 33.7% |
| Iula et al15 | Italy, 4 hematology centers | 28 | No | 40 | 3 | 8 | Keratopathy 32% |
| Ntanasis-Stathopoulos et al17 | Greece | 27 | Yes | 52 | 2 | 16 | Keratopathy 33% |
| Mohan et al16 | Wisconsin and Arkansas, 2 US academic institutions | 56 | No | 44.6 | 3.6 | 10 | 71.4% Grade 3 keratopathy 54% |
| Roussel et al18 | France, multicenter | 184 | Yes | 32.7 | 2.4 | 8.8 | 56% Grade 3 keratopathy 27% |
| Shragai et al19 | Israel, multicenter | 106 | Yes | 45.5 | 4.7 | 14.5 | 68.4% Grade 3 keratopathy 40.5% |
| Talbot et al20 | France, multicenter | 104 | Yes | 38.1 | 3.5 | 9.3 | 37% |
| Vaxman et al21 | One US academic institution, 3 centers | 36 | No | 33 | 2 | 6.5 | 44% |
EAP, expanded access program.
In this cohort, 18 patients had prior BCMA-targeted therapy, including CAR T-cell therapy and bispecific antibodies. This is one of the largest cohorts, to our knowledge, of patients treated with belantamab mafodotin and prior BCMA exposure. These patients were particularly heavily pretreated with a median of 9 prior lines of therapy. In this cohort, the ORR was 29% and there was no significant difference in PFS between patients who had been exposed to BCMA-targeted drugs and those who were BCMA-naïve. One of the mechanisms of resistance to BCMA-targeted therapies is antigen escape either through biallelic deletion or mutations in BCMA (TNFRSF17).32-34 For most patients with prior BCMA exposure in this study who underwent a biopsy before starting belantamab mafodotin, there was no report of BCMA antigen loss. Of the 33 patients who had 1 or more BCMA-targeted therapies after belantamab mafodotin, 18 patients responded with PR or better. Although this should be interpreted with caution, the results indicate that a significant number of patients can respond to repeat treatment with BCMA-targeted therapy.
Compliance with the REMS program was high; all patients had baseline ocular assessments as well as before each dose of belantamab mafodotin. The rate of ocular adverse events was 65%, which is comparable to that in the DREAMM-2 study.6 Ocular adverse events were managed through dose holds and dose reductions.24 Ten patients discontinued belantamab mafodotin due to ocular events; however, most patients who developed ocular toxicity could continue belantamab mafodotin at a reduced dose or with a longer dose interval. There are ongoing efforts to minimize the ocular side effects through treatment combinations and alternating dosing schedules such as in DREAMM-14 (ClinicalTrials.gov identifer: NCT05064358).35 Moreover, belantamab without mafodotin is being investigated in the ongoing DREAMM-20 trial (ClinicalTrials.gov identifer: NCT05714839).36
Overall, monotherapy with belantamab mafodotin does not induce the same high response rates as other BCMA-targeted therapies, such as CAR T cells or bispecific antibodies.37-40 Furthermore, belantamab mafodotin is associated with significant ocular adverse events and necessitates regular ocular monitoring. In contrast, belantamab mafodotin is associated with a lower risk of neutropenia, infections, and other potentially serious adverse events associated with CAR T cells and bispecific antibody therapy.41 Here, 20% of patients had infection of any grade, 13% were grade 3/4 infections and there was no death from infections in this cohort. With increasing knowledge on how to prevent adverse events from various BCMA-targeted therapies, for example, with optimal dosing and prophylactic measures, these can safely be administered in both academia and community settings.
The strength of this study is the large, well-defined cohort of patients with relapsed/refractory multiple myeloma treated with commercial belantamab mafodotin, with a long follow-up period. There was a high compliance with ocular assessments and the belantamab mafodotin REMS program. Limitations include the retrospective single-center design, which does not capture as detailed information on adverse events as in clinical trials.
In summary, the response rates to belantamab mafodotin in this study and other real-world studies were similar to or better than those in the DREAMM-2 study, whereas the rate of ocular toxicities was comparable. Importantly, clinically significant responses were observed in patients with prior BCMA exposure. In addition, >50% of patients treated with another BCMA-targeted therapy after belantamab mafodotin responded to subsequent BCMA therapy. Furthermore, belantamab mafodotin was associated with lower rates of infections in relation to BCMA-targeted bispecific antibodies and CAR T-cell therapy.41 Belantamab mafodotin can thus be an option for heavily pretreated patients, where a lower risk of infection is favored. These results are in line with previously published response rates and suggest a continued role of belantamab mafodotin, especially in light of recent combination treatment trials and alternative dosing schedules.
Acknowledgments
Funding support for this publication was provided by a Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748) and GlaxoSmithKline (GSK). GSK was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for the final content and interpretation.
Authorship
Contribution: M.H. designed the study; M.H., A.D., and D.N. analyzed and interpreted the data; M.H. wrote the manuscript; and all authors provided critical input on the results and gave final approval of the manuscript.
Conflict-of-interest disclosure: M.H. reports research funding from AbbVie, Bristol Myers Squibb (BMS), Daiichi Sankyo, Cosette Pharmaceuticals, GlaxoSmithKline (GSK), Johnson & Johnson, and The Binding Site; and has received honoraria for consultancy/participation in advisory boards for BMS, Janssen, and GSK. H.H. reports grants from Takeda and Janssen. N.K. reports research funding through Amgen, Janssen, Epizyme, and AbbVie; consults for CCOPharma, OncLive, and Intellisphere Remedy Health; and participated in an advisory board for Janssen and MedImmune. K.M. reports grant support from American Society of Hematology (ASH), Multiple Myeloma Research Foundation, and International Myeloma Foundation. S.M. reports research funding from Janssen Oncology, BMS, Allogene Therapeutics, Fate Therapeutics, Caribou Therapeutics, and Takeda Oncology; received consulting fees from Evicore, Optum, BioAscend, Janssen Oncology, BMS, AbbVie, HMP Education, and Legend Biotech; and honoraria from OncLive, Physician Education Resource, MJH Life Sciences, and Plexus Communications. U.A.S. reports research funding from the Memorial Sloan Kettering Paul Calabresi Career Development Award for Clinical Oncology K12CA184746, Paula and Rodger Riney Foundation, Parker Institute for Cancer Immunotherapy at MSK, HealthTree Foundation, International Myeloma Society, ASH, and Allen Foundation Inc, as well as nonfinancial support from the ASH Clinical Research Training Institute and Transdisciplinary Research on Energetis and Cancer Training Workshop R25CA203650 (PI: Melinda Irwin). U.A.S. also reports other research support from Celgene/BMS and Janssen; personal fees from Association of Cancer Care Centers, MashUpMD, Janssen Biotech, Sanofi, BMS, MJH LifeSciences, Intellisphere, Phillips Gilmore Oncology Communications, i3 Health, and RedMedEd. C.R.T. reports research funding from Janssen and Takeda; personal fees from Physician Educations Resource and MJH Life Sciences; and has participated in advisory boards for Janssen and Sanofi, outside of the submitted work. D.J.C. receives research funding from Genentech. O.B.L. reports serving on the advisory board for MorphoSys, Kite, and Daiichi Sankyo; and has served as a paid consultant for Incyte. H.J.L. has served as a paid consultant for Takeda, Genzyme, Janssen, Karyopharm, Pfizer, Celgene, and Caelum Biosciences; and has received research support from Takeda. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc, and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc, Omeros Corporation, and Amgen, Inc; served on ad hoc advisory boards for Kite, a Gilead company; and received honoraria from i3 Health, Medscape, and CancerNetwork for Continuous Medical Education-related activity. G.L.S. reports research funding from Janssen, Amgen, BMS, Beyond Spring; and serves on the data safety monitoring board for ArcellX. S.A.G. reports personal fees and advisory role (scientific advisory board) from Actinium, Celgene, BMS, Sanofi, Amgen, Pfizer, GSK, Jazz Pharmaceuticals, Janssen, Omeros, Takeda, and Kite. T.S. reports honoraria from Roche-Genentech. A.M.L. reports grants from Novartis during the conduct of the study; grants from BMS; personal fees from Trillium Therapeutics; grants, personal fees, and nonfinancial support from Pfizer; and grants and personal fees from Janssen outside the submitted work. A.M.L. also has a patent US20150037346A1 with royalties paid. S.Z.U. reports grants and personal fees from AbbVie, Amgen, BMS, Celgene, GSK, Janssen, Merck, MundiPharma, Oncopeptides, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, and Takeda. D.J.C. receives research funding from Genentech. The remaining authors declare no competing financial interests.
Correspondence: Malin Hultcrantz, Myeloma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: hultcram@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Malin Hultcrantz (hultcram@mskcc.org).