TO THE EDITOR:
Bispecific CD20×CD3 antibodies have shown promising results in the treatment of relapsed or refractory B-cell lymphomas and may have a less toxic adverse effect profile compared with chimeric antigen receptor (CAR) T-cell therapy with comparable efficacy; however, direct comparisons are lacking.1-7 Loss of antibody target (ie, CD19) has been found to negatively affect treatment outcomes in CD19 CAR T-cell therapy in diffuse large B-cell lymphoma.8,9
Nevertheless, the impact of CD20 tumor expression level, before treatment with CD20×CD3 bispecific antibodies, is unclear. No studies have, to our knowledge, evaluated correlation of CD20 expression level and survival. One study (in a relatively small number of phase 1 trial patients treated with highly variable doses of CD20×CD3 bispecific antibody) found no association between CD20 expression level and initial response rate.10 The aim of this study was to evaluate whether CD20 tumor expression level influences response and survival after treatment with CD20×CD3 antibodies in a larger cohort of patients with B-cell lymphoma.
In this retrospective study, we included patients from 3 Danish centers who received CD20×CD3 bispecific antibodies between 2017 and 2024 in phase 1/2 trials.1-7
The patients’ electronic health records and all pathology reports were reviewed. Immunohistochemical analyses from 148 pretreatment and 60 posttreatment tumor samples were included. New biopsies after bispecific CD20×CD3 antibody therapy were only performed in case of clinical or radiographic relapse. A team of four experienced hematopathologists consisting members from all three centers reassessed biopsy results that in the original pathology report had revealed either a negative or a reduced immunohistochemical expression of CD20 antigen to confirm this finding.
Immunohistochemical CD20 expression was divided into the following three groups: “normal,” “reduced,” and “negative.” Negative was defined as true CD20 negativity. Reduced was defined as weak, heterogeneous, or partial expression of CD20. Anything seeming reduced in comparison to positive controls was deemed reduced. Examples of each category are depicted in supplemental Figures 1-3.
Refractory disease was defined as a best response of progression or stable disease to the most recent treatment before CD20×CD3 antibody. Progression-free survival (PFS) was defined as the time from first initiation of CD20×CD3 antibody treatment to the earliest date of either disease progression or death from any cause. In addition, progression events were defined according to the Lugano classification.11
Kaplan-Meier estimates were calculated for all time-to-event end points. Hazard ratios (HRs) with 2-sided 95% confidence intervals (CIs) were calculated from a Cox proportional hazards model. We calculated log-rank P values (2 sided) for time-to-event end points. Adjustment for multiple testing was done using false discovery rate correction.
Of the 167 patients in the cohort treated with a bispecific CD20×CD3 antibody, pretreatment immunohistochemical assessments were available in 149 patients. These were distributed across 100 patients with diffuse large B-cell lymphoma, 36 with follicular lymphoma, and 13 with other lymphoid neoplasms. Of these 149 patients, pretreatment biopsy results revealed strong CD20 expression in 128 patients, reduced CD20 expression in 13 patients, but no CD20 expression in 5 patients (Table 1). Median follow-up from first treatment with bispecific CD20×CD3 antibody was 22.2 months. Of the 149 patients, 131 received CD20×CD3 bispecific antibody as monotherapy and 18 received combination therapies. These patients received a combination of CD20×CD3 bispecific antibody with polatuzumab, rituximab, and lenalidomide or R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone). Separate baseline characteristics for patients with aggressive and indolent lymphomas can be found in supplemental Tables 1 and 2, respectively. Previous treatments and their order are available in supplemental Tables 3 and 4 and supplemental Figures 5 and 6.
Baseline characteristics of all 149 patients with indolent and aggressive lymphoma stratified by immunohistochemical expression of CD20 antigen
| . | Strong . | Reduced . | Negative . | Total . |
|---|---|---|---|---|
| (n = 131) . | (n = 13) . | (n = 5) . | (N = 149) . | |
| Sex, n (%) | ||||
| Female | 49 (37.4) | 8 (61.5) | 1 (20.0) | 58 (38.9) |
| Male | 82 (62.6) | 5 (38.5) | 4 (80.0) | 91 (61.1) |
| Age, y | ||||
| Median (range) | 71.1 (21.5-87.0) | 71.8 (52.0-79.3) | 72.1 (58.9-84.0) | 71.1 (21.5-87.0) |
| Treatment diagnosis, n (%) | ||||
| DLBCL | 84 (64.1) | 11 (84.6) | 5 (100) | 100 (67.1) |
| FL | 34 (26.0) | 2 (15.4) | 0 (0) | 36 (24.2) |
| Other | 13 (9.9) | 0 (0) | 0 (0) | 13 (8.7) |
| No. of previous lines of therapy, median (range) | 3.00 (0-9.00) | 2.00 (0-6.00) | 3.00 (1.00-4.00) | 2.00 (0-9.00) |
| ECOG performance status, median (range) | 0 (0-2.00) | 0 (0-1.00) | 1.00 (0-1.00) | 0 (0-2.00) |
| Ann Arbor stage, median (range) | 4.00 (1.00-4.00) | 3.00 (2.00-4.00) | 3.00 (3.00-4.00) | 4.00 (1.00-4.00) |
| Baseline lactate dehydrogenase, median (range), U/L | 233 (64.0-2500) | 312 (183-1390) | 210 (182-1340) | 245 (64.0-2500) |
| Previous autologous stem cell transplant, n (%) | 19 (14.5) | 4 (30.8) | 0 (0) | 23 (15.4) |
| . | Strong . | Reduced . | Negative . | Total . |
|---|---|---|---|---|
| (n = 131) . | (n = 13) . | (n = 5) . | (N = 149) . | |
| Sex, n (%) | ||||
| Female | 49 (37.4) | 8 (61.5) | 1 (20.0) | 58 (38.9) |
| Male | 82 (62.6) | 5 (38.5) | 4 (80.0) | 91 (61.1) |
| Age, y | ||||
| Median (range) | 71.1 (21.5-87.0) | 71.8 (52.0-79.3) | 72.1 (58.9-84.0) | 71.1 (21.5-87.0) |
| Treatment diagnosis, n (%) | ||||
| DLBCL | 84 (64.1) | 11 (84.6) | 5 (100) | 100 (67.1) |
| FL | 34 (26.0) | 2 (15.4) | 0 (0) | 36 (24.2) |
| Other | 13 (9.9) | 0 (0) | 0 (0) | 13 (8.7) |
| No. of previous lines of therapy, median (range) | 3.00 (0-9.00) | 2.00 (0-6.00) | 3.00 (1.00-4.00) | 2.00 (0-9.00) |
| ECOG performance status, median (range) | 0 (0-2.00) | 0 (0-1.00) | 1.00 (0-1.00) | 0 (0-2.00) |
| Ann Arbor stage, median (range) | 4.00 (1.00-4.00) | 3.00 (2.00-4.00) | 3.00 (3.00-4.00) | 4.00 (1.00-4.00) |
| Baseline lactate dehydrogenase, median (range), U/L | 233 (64.0-2500) | 312 (183-1390) | 210 (182-1340) | 245 (64.0-2500) |
| Previous autologous stem cell transplant, n (%) | 19 (14.5) | 4 (30.8) | 0 (0) | 23 (15.4) |
‘Other’ diagnoses includes mantle cell lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, and chronic lymphoid leukemia.
DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; IQR, interquartile range.
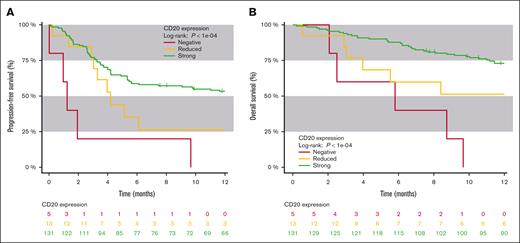
Patients with a reduced CD20 expression had a 26% PFS rate after 12 months (95% CI, 1-51) compared with 53% in patients with strong CD20 expression (95% CI, 44-62; log-rank P = .05; supplemental Figure 2A). Overall survival (OS) rate after 12 months in patients with a reduced CD20 expression was 51% (95% CI, 23-80) compared with 73% in patients with a strong CD20 expression (95% CI, 65-80; log-rank P = .03; supplemental Figure 2B). Negative CD20 expression was associated with inferior OS and PFS to bispecific CD20×CD3 therapy (Figure 1). Isolating aggressive lymphomas, patients with a reduced CD20 expression had a 31% PFS rate after 12 months (95% CI, 26-61) compared with 44% in patients with strong CD20 expression (95% CI, 33-54; log-rank P = .0009; supplemental Figure 3A). Isolating indolent lymphomas, patients with a reduced CD20 expression had a 0% PFS rate after 12 months (95% CI, 0-100) compared with 70.5% in patients with strong CD20 expression (95% CI, 57-84; log-rank P = .02; supplemental Figure 4A,).
Survival in strong, reduced, and negative immunohistochemical expression of CD20. (A) PFS of patients with either strong, reduced, or negative immunohistochemical expression of CD20 antigen. (B) OS of patients with either strong, reduced, or negative immunohistochemical expression of CD20 antigen.
Survival in strong, reduced, and negative immunohistochemical expression of CD20. (A) PFS of patients with either strong, reduced, or negative immunohistochemical expression of CD20 antigen. (B) OS of patients with either strong, reduced, or negative immunohistochemical expression of CD20 antigen.
Evaluating OS rates in patients with aggressive lymphoma, those with a reduced CD20 expression had a 51% OS rate after 12 months (95% CI, 20-82) compared with 62% in those with strong CD20 expression (95% CI, 52-73; log-rank P = .0007; supplemental Figure 3B). Isolating indolent lymphomas, patients with a reduced CD20 expression had a 50% OS rate after 12 months (95% CI, 0-100) compared with 91% in patients with strong CD20 expression (95% CI, 82-100; log-rank P = .2; supplemental Figure 4B).
In a univariate Cox regression (supplemental Table 3), reduced CD20 expression was significantly associated with inferior OS with a 2.23 HR (95% CI, 1.06-4.70; P = .035). Each percentage change of Ki67 was associated with inferior OS in indolent but not aggressive lymphoma with HR of 1.03 (per percentage change in Ki67, 1.00-1.05; P = .027) and 1.00 (per percentage change in Ki67, 0.99-1.02; P = .4), respectively. Worse Eastern Cooperative Oncology Group (ECOG) performance status and a higher number of previous lines of therapy were significantly associated with inferior OS (HR = 1.69 [P = .006] and HR = 1.27 [P = .001], respectively). BCL6 rearrangement was significantly associated with superior OS (HR = 0.52; P = .02). All factors remained either significant or borderline significant after adjustment for multiple testing.
Reduced CD20 expression was borderline significantly associated with inferior PFS (HR = 1.93; 95% CI, 1.00-3.75; P = .054) in a univariate Cox regression (supplemental Table 2).
In a multivariate Cox regression analysis with adjustment for age, sex, and ECOG performance status, reduced CD20 expression trended toward worse PFS (HR = 1.77; 95% CI 0.90-3.47; P = .097) but remained significantly associated with inferior OS (HR = 2.25; 95% CI 1.06-4.44; P = .035; supplemental Figure 1A-B).
Of the 60 patients who had biopsy-confirmed relapse after treatment with CD20×CD3 antibody, 40 had altered CD20 expression (35 with a negative expression and 5 with a reduced expression). Of these 40 patients, 33 had a strong pretreatment immunohistochemical CD20 expression. Of the 13 patients with reduced pretreatment immunohistochemical CD20 expression who had a biopsy-confirmed relapse (n = 3), all had a negative CD20 expression after the treatment.
In this study, we found that in patients with B-cell neoplasms treated with CD20×CD3 bispecific antibodies, reduced CD20 expression was associated with inferior survival. We reveal that CD20 antigen loss at relapse after bispecific CD20×CD3 antibodies is a frequent finding and presumably responsible for two-thirds of relapses in our cohort. We believe that our results regarding reduced CD20 expression extend findings by other groups who have revealed that a strictly negative pretreatment immunohistochemical CD20 antigen presentation is associated with poor survival outcomes after bispecific CD20×CD3 antibody therapy.10,12,13
Our findings have multiple implications for future studies of bispecific CD20×CD3 antibodies. An important perspective is whether patients with reduced CD20 expression but strong CD19 expression may have better outcomes with CAR T-cell therapy or novel trispecific antibodies targeting multiple tumor antigens (eg, both CD20 and CD79).14,15 Regimens combining CD20×CD3 antibodies with other drugs may also reduce or overcome the negative prognostic effect of reduced CD20 expression.2,3,5,6,10,12,13,16
We demonstrate that tumor CD20 expression levels have substantial impact on survival after treatment with bispecific T-cell engagers. Because this is a very accessible and reliable biomarker, we warrant further research to validate our findings, and ultimately investigations of whether assessment of CD20 expression before bispecific CD20×CD3 antibody treatment could improve risk-based stratification of therapy.
We obeyed the laws on handling of personal information in accordance with the Danish Scientific Ethical Committees Act (Komitéloven) section 20, part 1, no. 4. The study was approved by Datatilsynet under the Capital Region umbrella application (RH-2020-561). Concerning documentation of data security, the demands of Datatilsynet have been met.
Consent was obtained when applicable. Exemption from obtainment of informed consent from these patients was granted by the Danish ethical council through the Research Biobank and Clinical Database of patients with lymphoproliferative malignancies.
Acknowledgments: The authors thank the technical staff from the Pathology Departments of Rigshospitalet (Copenhagen, Denmark) and Odense University Hospital (Odense, Denmark) for their invaluable support.
This work has been funded by Aase and Ejnar Danielsen’s Foundation, the Capital Region Health Research Foundation, the Danish Cancer Society (R306-A18107), and the Research Foundation of Rigshospitalet.
Contribution: E.R.K. and S.H. wrote the manuscript; L.S., M.D.S., T.L.G., M.H., S.H., and E.R.K. designed the research; E.R.K. and S.H. did the statistical analyses; L.S., M.D.S., T.L.G., and M.B.M. reviewed the immunohistochemistry slides; C.R., M.R.C., T.S.L., M.H., L.S.A., T.T., and E.R.K. collected the data; and all authors contributed to the interpretation of the data and approved the final version of the manuscript.
Conflict-of-interest disclosure: K.G. received research funding from Janssen and is on the advisory board of Nanexa and GlaxoSmithKline. M.H. has consulting or advisory role at AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, Roche, and Takeda; and has received research funding from AbbVie, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, Genmab, Incyte, Janssen, Merck, Novartis, Roche, and Takeda. C.U.N. received research funding and/or consultancy fees from AstraZeneca, Janssen, AbbVie, BeiGene, Genmab, CSL Behring, Octapharma, Takeda, and Novo Nordisk. T.S.L. received consultancy fees from Roche and Gilead; and research funding from Genentech. T.T. received research support from Janssen. M.G. received consultancy fees from Amgen, AstraZeneca, and Thermo Fisher Scientific; and research support from Merck. M.R.C. reports advisory role with AbbVie, AstraZeneca, Genmab, Gilead, Incyte, Janssen, and Roche; and received travel expenses from AbbVie, AstraZeneca, Genmab, Janssen, Pfizer, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Simon Husby, Grønbæk Group, Department of Hematology, Rigshospitalet, Ole Maaløes Vej 5, building 2, 3rd floor, 2200 Copenhagen, Denmark; email: simon.husby.01@regionh.dk.
References
Author notes
Data are available on request from the corresponding author, Simon Husby (simon.husby.01@regionh.dk).
The full-text version of this article contains a data supplement.