TO THE EDITOR:
Teclistamab is a T-cell–redirecting bispecific antibody against B-cell maturation antigen approved for patients with relapsed or refractory multiple myeloma (MM).1 Infections and hypogammaglobulinemia manifest consistently over the entire treatment period,2 prompting the use of IV immunoglobulin (IVIG) and antimicrobial prophylaxis.3-6 Although rare in immunocompetent individuals, cytomegalovirus (CMV) DNAemia can occur in patients with MM after autologous stem cell transplant7,8 or chimeric antigen receptor (CAR) T-cellular therapy.9 Recent studies have detected CMV DNAemia in 5% to 8% of the patients treated with anti–B-cell maturation antigen T-cell engagers10; however, data remain limited regarding the actual prevalence and clinical significance of CMV DNAemia in these patients.11
We studied 23 consecutive patients who received teclistamab for relapsed or refractory MM between January 2023 and May 2024 at our institution and were evaluated for CMV reactivation. Median follow-up was 5.8 months (range, 0.2-15.9). The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by The Ohio State University Institutional Review Board committee.
Patients included in the analysis (supplemental Table 1) had a median of 6 prior lines of therapy (range, 4-17), with 13 of 23 patients (56.5%) having received a previous autologous stem cell transplant and 1 patient having received CAR T-cellular therapy. At baseline, the median absolute lymphocyte count (ALC) values were 690/μL (range, 320-3200), with 7 of 23 (30.4%) patients having at least grade 3 baseline lymphopenia. Similarly, baseline hypogammaglobulinemia was present in 20 of 23 patients (87%). After starting treatment, ALC levels rapidly decreased within the first 3 doses, with a median lowest ALC of 195/μL (range, 0-590) but improved during treatment within the first month (median day 28 ALC, 1170/μL; range, 280-4360) (supplemental Figure 1). Total IgG levels remained low, requiring IVIG supplementation in 13 of 23 (73.9%) patients.
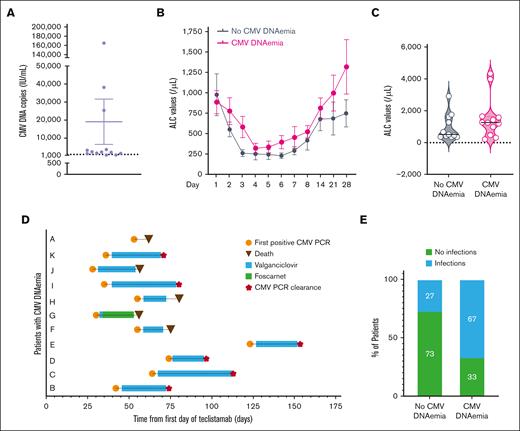
CMV DNAemia (>500 copies IU/mL) was detected in 12 of 23 (52.2%) patients (median, 2295.5; range, 653-165 000 copies IU/mL) (Figure 1A). The median time to CMV DNAemia was 54 days (range, 28-123). The median lowest ALC value at any point was 195/μL (range, 50-590), whereas it was 1280/μL (range, 220-4370) at the time of reactivation, with only 3 patients having an ALC <500/μL. Symptoms, including fatigue, hepatic enzyme abnormalities, fever, worsening leukopenia, and thrombocytopenia, were present in 9 of 12 (75%) patients. CMV syndrome, which is defined as the presence of CMV DNAemia plus at least 2 of the following criteria (fever for at least 2 days, new or increased fatigue, leukopenia or neutropenia on 2 separate measurements, thrombocytopenia, or elevation of hepatic aminotransferases)12 occurred in 5 of 12 patients (42%). One patient had possible CMV colitis with diarrhea and bowel wall thickening on imaging without pathological confirmation.
CMV DNAemia in patients treated with teclistamab. (A) Plot of the highest CMV DNA values by quantitative polymerase chain reaction in patients treated with teclistamab. CMV reactivation was defined as >500 IU/mL copies of CMV DNA. n = 12 patients. The mean ± standard error of the mean is shown. (B) ALC values (microliter) in patients with or without CMV DNAemia. The mean ± standard error of the mean is shown. ALC counts were obtained on days 1, 2, 3, 4, 5, 6, 7, 8, 14, 21, and 28 of cycle 1 of teclistamab. Analysis of variance, P < .0001. (C) Violin plots of ALC values (microliter) in 12 patients without CMV DNAemia and 11 patients with CMV DNAemia. The median ALC on the day of the first detectable CMV DNAemia or day 54 of teclistamab (median day of CMV reactivation) is highlighted with a solid black line. P = .21, not significant. (D) Swimmer plot of 11 patients who experienced CMV DNAemia with their treatment, time of CMV DNAemia clearance, and survival status. (E) Rate of infections other than CMV DNAemia in patients with and without CMV DNAemia. Fisher exact test, P = .003. PCR, polymerase chain reaction.
CMV DNAemia in patients treated with teclistamab. (A) Plot of the highest CMV DNA values by quantitative polymerase chain reaction in patients treated with teclistamab. CMV reactivation was defined as >500 IU/mL copies of CMV DNA. n = 12 patients. The mean ± standard error of the mean is shown. (B) ALC values (microliter) in patients with or without CMV DNAemia. The mean ± standard error of the mean is shown. ALC counts were obtained on days 1, 2, 3, 4, 5, 6, 7, 8, 14, 21, and 28 of cycle 1 of teclistamab. Analysis of variance, P < .0001. (C) Violin plots of ALC values (microliter) in 12 patients without CMV DNAemia and 11 patients with CMV DNAemia. The median ALC on the day of the first detectable CMV DNAemia or day 54 of teclistamab (median day of CMV reactivation) is highlighted with a solid black line. P = .21, not significant. (D) Swimmer plot of 11 patients who experienced CMV DNAemia with their treatment, time of CMV DNAemia clearance, and survival status. (E) Rate of infections other than CMV DNAemia in patients with and without CMV DNAemia. Fisher exact test, P = .003. PCR, polymerase chain reaction.
Comparing patient and disease characteristics, no differences were noted between patients with or without CMV DNAemia (supplemental Table 2), including similar ALC levels within the first 30 days of therapy or on day 54 (median number of days for detectable CMV DNAemia; Figure 1B-C; ALC with CMV DNAemia, median 1280/μL [range, 220-4370]; ALC without CMV DNAemia, median 520/μL [range, 370-2940]). IgG CMV seropositivity was more common in patients with CMV DNAemia (91.7% vs 45.5%), whereas cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, or use of tocilizumab were not associated with increased risk of CMV DNAemia. One patient with grade 2 neurotoxicity received high-dose dexamethasone and later developed CMV DNAemia.
Within the first month of therapy, the rates of grade 3 anemia, thrombocytopenia, lymphopenia, neutropenia, and hypogammaglobulinemia were similar between the 2 groups (supplemental Table 3). The incidence of ≥2 signs or symptoms associated with CMV syndrome was comparable between the 2 groups (patients without CMV DNAemia, 5/11 [45.5%]; patients with CMV DNAemia, 5/12 [42%]). However, patients without CMV DNAemia exhibited “CMV syndrome-like” symptoms during the initial month of teclistamab treatment. In contrast, those with CMV DNAemia presented with new and more severe signs and symptoms (worsening thrombocytopenia or leukopenia, new liver enzyme abnormalities, and fatigue), coinciding with the detection of CMV DNA in the blood.
Oral valganciclovir (9 patients) or IV ganciclovir (2 patients) was started in 11 of 12 patients (Figure 1D). The patient who did not receive treatment had a single transient CMV-positive polymerase chain reaction result (654 IU/mL; not shown in Figure 1D). Four of 11 (36.4%) patients with CMV DNAemia developed at least grade 1 neutropenia, and 2 experienced severe neutropenia. Similarly, 4 of the 11 patients without CMV DNAemia had at least grade 1 neutropenia. The median time to CMV clearance was 26 days (range, 21-43 days). Four patients died due to disease progression before CMV DNAemia clearance, 1 patient received valganciclovir followed by valacyclovir, and 6 patients were treated until CMV DNAemia clearance, followed by valganciclovir maintenance therapy. Only 1 patient discontinued valganciclovir due to neutropenia. Teclistamab was continued during anti-CMV therapy in all patients except for 1. Patients with CMV DNAemia were also more prone to other types of infections (67% vs 27%, P = .003) (Figure 1E), including bacterial, viral, and fungal infections, and grade 3 or 4 infections (supplemental Figure 2; supplemental Table 4). These infections occurred after CMV clearance but while on anti-CMV maintenance therapy.
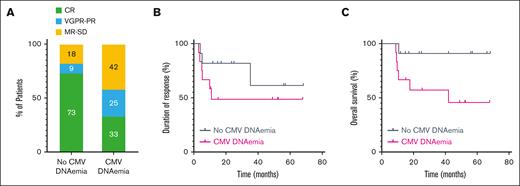
At the time of data censoring, 9 of 11 (82%) patients without CMV DNAemia were still receiving teclistamab, compared with 4 of 12 (33%) patients with CMV DNAemia. The percentage of patients achieving a partial response or better was lower in patients with CMV DNAemia (82% vs 58%; Fisher exact test, P = .0003), with complete responses occurring in 73% vs 33% of patients (Figure 2A). Patients with CMV DNAemia received less teclistamab than those without CMV DNAemia (21 vs 9 doses). However, the duration of response was not different between the 2 groups (Hazard Ratio, 0.465; 95% confidence interval, 0.116-1.863) (Figure 2B). At the time of CMV DNAemia, 7 of 12 (58.3%) patients achieved at least a partial response, 2 of 12 (16.7%) had stable disease, and 3 of 12 (25%) had progressive disease. This suggests that CMV DNAemia may develop independently of the disease status and result from broader immune dysfunction, which also contributes to a diminished depth of response and increased susceptibility to other infections. Overall survival was shorter in patients with CMV DNAemia (Hazard Ratio, 0.018-1.279; P = .08) (Figure 2C).
Outcomes of patients treated with teclistamab based on CMV DNAemia. (A) Response rates in patients with (n = 12) and without (n = 11) CMV DNAemia. (B) Duration of response to teclistamab in patients with and without CMV DNAemia. Log-rank, P = .2677. (C) Overall survival from the first day of teclistamab in patients with and without CMV DNAemia. Log-rank, P = .0461. CR, complete response; MR, minimal response; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
Outcomes of patients treated with teclistamab based on CMV DNAemia. (A) Response rates in patients with (n = 12) and without (n = 11) CMV DNAemia. (B) Duration of response to teclistamab in patients with and without CMV DNAemia. Log-rank, P = .2677. (C) Overall survival from the first day of teclistamab in patients with and without CMV DNAemia. Log-rank, P = .0461. CR, complete response; MR, minimal response; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
In summary, our study highlights that CMV DNAemia and CMV syndrome are complications of teclistamab therapy. Previous studies have reported CMV reactivation rates of 5% to 8% without routine monitoring,2,10 whereas a recent report using monthly CMV surveillance detected a significantly higher rate of 49%,13 consistent with our findings. Patients with CMV DNAemia showed lower response rates and higher infection risks even after CMV clearance, indicating broader immune dysregulation. Although a low ALC is a known risk factor,14 it does not alone predict CMV DNAemia. Instead, tumor- and teclistamab-induced T-cell exhaustion, along with chronic antigen stimulation, may impair CMV-specific immunity in certain patients.15
The limitations of this study include its single-center design, small patient number, and the lack of standardized CMV DNAemia testing protocols. Additionally, 1 patient received prior CAR T-cellular therapy, which may confer additional risks due to prolonged immunosuppression, and CD4 counts were not available for our patients.
In conclusion, although routine CMV polymerase chain reaction monitoring is not currently recommended by the guidelines, larger prospective studies are needed to determine the optimal timing for CMV testing, refine antiviral and prophylactic strategies, and assess the impact of discontinuing teclistamab therapy on restoring anti-CMV immunity.
Acknowledgments: The authors thank The Ohio State University (OSU) MM physicians and clinical research team members who consented patients to the MM registry, and all patients included in the registry.
F.C. reports grants from the International Myeloma Society and Paula and Rodger Riney Foundation Translational Research Award, The Elsa U. Pardee Foundation, OSU College of Medicine, and National Cancer Institute (1K08CA26347601A1).
Contribution: C.P. wrote the institutional review board (IRB) protocol, performed chart review, collected and analyzed patient data, and drafted the manuscript; L.B. and N.S. performed chart review and collected data; N.B., A.M.K., S.D., and E.U. consented patients on the multiple myeloma registry; D.B., A.R., and Z.E.B. provided comments and reviewed the manuscript; and F.C. wrote the IRB protocol, designed the study, supervised data collection and accuracy, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesca Cottini, Division of Hematology, Department of Internal Medicine, College of Medicine, The Ohio State University Wexner Medical Center, 385G Wiseman Hall, 400 W 12th Ave, Columbus, OH 43210-1240; email: francesca.cottini@osumc.edu.
References
Author notes
Data are available on request from the corresponding author, Francesca Cottini (francesca.cottini@osumc.edu).
The full-text version of this article contains a data supplement.