Key Points
Dose level 3 was identified as the maximum tolerated dose in this phase 1 study of venetoclax and CLAG-M for patients with adverse-risk AML.
Overall, 65% of patients achieved CR or CR with incomplete hematologic recovery, and 28-day mortality was 5%.
Visual Abstract
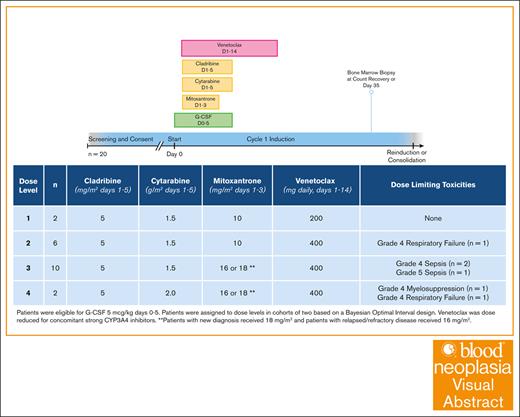
Intensifying induction by combining venetoclax with a high-dose cytarabine regimen may improve outcomes for high-risk populations such as adult patients with adverse-risk newly diagnosed or relapsed acute myeloid leukemia. In a phase 1 trial testing the novel combination of venetoclax and CLAG-M (cladribine, high-dose cytarabine, granulocyte colony-stimulating factor [G-CSF], and mitoxantrone), the maximum tolerated dose was venetoclax 400 mg on days 1 through 14, combined with cladribine 5 mg/m2 on days 1 through 5, cytarabine 1.5 g/m2 on days 1 through 5, G-CSF 5 μg/kg on days 0 through 5, and mitoxantrone 16 or 18 mg/m2 on days 1 through 3 (for relapsed/refractory and newly diagnosed adverse-risk patients, respectively). The 28-day mortality rate was 5%. Composite complete remission (CR) rate (CR + CR with incomplete hematologic recovery) was 65%. These findings support further phase 2 study of venetoclax in combination with CLAG-M. This trial was registered at www.ClinicalTrials.gov as #NCT04797767.
Introduction
Acute myeloid leukemia (AML) is an aggressive cancer of the blood and bone marrow that is typically treated with intensive induction chemotherapy in fit patients. A “7+3” regimen has been the historical standard of care; however, some centers have adopted regimens with higher doses of cytarabine delivered as an IV bolus rather than a continuous infusion. One such induction regimen is CLAG-M (cladribine, high-dose cytarabine, granulocyte colony-stimulating factor [G-CSF], and mitoxantrone). In a single-arm phase 1/2 trial examining CLAG-M, 71% of newly diagnosed (ND) patients achieved complete remission (CR) without measurable residual disease (MRD).1 Although randomized data are not available, the MRD-negative CR rate was higher than matched historical controls induced with 7+3 (53%, P = .01). The composite CR rate (CR or CR with incomplete hematologic recovery [CRi]) for those with ND adverse-risk disease was 76% and 60% for relapsed/refractory (R/R) patients.1,2 By comparison, the CR/CRi rate for favorable- and intermediate-risk patients with ND disease was 96% and 93%, respectively. Considering these suboptimal outcomes, there is a need for improved induction regimens for fit patients who fall into high-risk categories.
Venetoclax is an oral BCL-2 inhibitor that was approved in 2018 by the US Food and Drug Administration in combination with low-dose cytarabine or hypomethylating agents to treat patients with ND AML who are unfit for intensive induction therapy.3,4 More recently, venetoclax has been safely and effectively combined with other intensive induction regimens, including standard cytarabine and anthracycline (7+3); FLAG-ida (fludarabine, cytarabine, G-CSF, and idarubicin); and CLIA (cladribine, idarubicin, and cytarabine).5-7
We hypothesized that the addition of venetoclax to CLAG-M would provide enhanced activity in treating patients with AML. Thus, we conducted a phase 1 dose-finding study to estimate the maximum tolerated dose (MTD) of venetoclax in combination with CLAG-M. Given the poor outcomes with the current standards of care, we included patients with ND adverse-risk AML as well as R/R AML and high-grade myeloid neoplasms.
Methods
Eligible patients were adults aged ≥18 years with a diagnosis of AML or high-grade myeloid neoplasm (≥10% myeloid blasts in the bone marrow or peripheral blood) and either (1) ND adverse-risk disease by European LeukemiaNet (ELN) 2017 criteria, or (2) R/R disease.8,9 Relevant inclusion criteria were adequate organ function (aspartate transaminase and alanine transaminase a ≤3 times upper limit of normal, bilirubin at ≤1.5 times upper limit of normal, creatinine clearance of ≥30 mL/min, and left ventricular ejection fraction of ≥45%), Eastern Cooperative Oncology Group performance status score of ≤2, and treatment-related mortality score of <13.1.10 To mitigate risk of tumor lysis syndrome (TLS), white blood cell (WBC) count in the peripheral blood had to be <25 × 103/μL before initiation of study therapy. Cytoreduction with hydroxyurea and/or cytarabine (up to 2 doses of ≤500 mg/m2 each) was allowed before starting study therapy. Exclusion criteria included known active AML in the central nervous system, concomitant illness associated with a likely survival of <1 year, active systemic infection, and active or clinically significant cardiac disease. The protocol (ClinicalTrials.gov identifier: NCT04797767) was approved by the Fred Hutchinson Cancer Center institutional review board, and patients were treated in accordance with the Declaration of Helsinki.
The primary objective was to identify the recommended phase 2 dose (RP2D), for which the MTD was defined as the minimum dose associated with a true dose-limiting toxicity (DLT) rate of >30%. A sample size of 20 provided reasonable operating characteristics under a variety of assumed-true dose-DLT scenarios (supplemental Table 1). A DLT was defined as (1) any grade ≥4 nonhematologic toxicity; (2) any grade ≥3 nonheme toxicity lasting >48 hours and resulting in a treatment delay beyond day 56; or (3) neutrophil count of <500 cells per μL or platelet count of <50 × 103/μL for >49 days after initiation of therapy, in conjunction with bone marrow showing no evidence of disease, because of the expected myelosuppressive effects of venetoclax.11 Grade ≥3 nonhematologic adverse effects were collected and graded according to the common terminology criteria for adverse events, version 5.0. Two patients were treated at dose level (DL) 2 (Table 1) and a Bayesian optimal interval (BOIN) design was used to recommend subsequent DLs in cohorts of 2, with a true targeted DLT rate of 30%.12
Prespecified levels for dose escalation of CLAG-M and venetoclax
| Level . | Cladribine . | Cytarabine . | Mitoxantrone . | Venetoclax . |
|---|---|---|---|---|
| 1 | 5 mg/m2 days 1-5 | 1.5 g/m2 days 1-5 | 10 mg/m2 days 1-3 | 200 mg daily days 1-14 |
| 2 | 5 mg/m2 days 1-5 | 1.5 g/m2 days 1-5 | 10 mg/m2 days 1-3 | 400 mg daily days 1-14 |
| 3 | 5 mg/m2 days 1-5 | 1.5 g/m2 days 1-5 | 16 or 18 mg/m2 days 1-3∗ | 400 mg daily days 1-14 |
| 4 | 5 mg/m2 days 1-5 | 2 g/m2 days 1-5 | 16 or 18 mg/m2 days 1-3∗ | 400 mg daily days 1-14 |
| Level . | Cladribine . | Cytarabine . | Mitoxantrone . | Venetoclax . |
|---|---|---|---|---|
| 1 | 5 mg/m2 days 1-5 | 1.5 g/m2 days 1-5 | 10 mg/m2 days 1-3 | 200 mg daily days 1-14 |
| 2 | 5 mg/m2 days 1-5 | 1.5 g/m2 days 1-5 | 10 mg/m2 days 1-3 | 400 mg daily days 1-14 |
| 3 | 5 mg/m2 days 1-5 | 1.5 g/m2 days 1-5 | 16 or 18 mg/m2 days 1-3∗ | 400 mg daily days 1-14 |
| 4 | 5 mg/m2 days 1-5 | 2 g/m2 days 1-5 | 16 or 18 mg/m2 days 1-3∗ | 400 mg daily days 1-14 |
Patients with new diagnosis received a dose of 18 mg/m2 and patients with R/R disease received a dose of 16 mg/m2.
All patients received standard antiviral, antibiotic, and antifungal prophylaxis. Venetoclax was dose-adjusted for concomitant use of voriconazole (supplemental Table 2). Patients were admitted to the hospital for chemotherapy administration and venetoclax ramp-up, but were then eligible for early hospital discharge in which supportive care during their count nadir was provided in the outpatient setting.13 Bone marrow evaluation was performed at the time of count recovery or by day 35 after initiation of chemotherapy if peripheral counts had not yet recovered. MRD was measured using flow cytometry and cytogenetics (karyotype and fluorescence in situ hybridization). A second cycle of induction with CLAG-M and venetoclax was allowed for persistent disease (morphologic or MRD) after the first cycle. Participants with CR or CRi without MRD by ELN 2017 criteria were eligible to receive up to 4 consolidation cycles of venetoclax plus cladribine and cytarabine at the same DL as induction.14
Secondary objectives included response and survival, as measured by CR and CRi rate; overall survival (OS); and event-free survival (EFS), with events defined as death, relapse, or persistent disease on postinduction evaluation. Median time to count recovery was calculated using a cumulative incidence function, with death or initiation of a subsequent line of treatment treated as competing events. Statistical analysis was performed using Stata statistical software, release 15.1 (StataCorp LP, College Station, TX).
Results
Patient characteristics
Enrollment occurred between January 2022 and April 2024 in cohorts of 2 for a total of 20 participants. Patient characteristics are summarized in Table 2. Only 1 patient with high-grade myelodysplastic syndrome was enrolled; all other patients had AML. Median age was 61 (range, 30-74) years. Six patients had ND adverse-risk disease, and 14 had R/R disease. Among R/R patients, ELN 2017 risk was favorable in 4 (29%), intermediate in 4 (29%), and adverse in 6 (43%). Relapsed patients had a median of 1 prior line of therapy (range, 1-3), and 4 had a prior transplant. Three patients had been previously treated with venetoclax. In total, 2 patients were enrolled at DL 1, 6 at DL 2, 10 at DL 3, and 2 at DL 4, as per the BOIN design.
Patient demographics
| Patient characteristics . | N = 20 . |
|---|---|
| Median age, y (range) | 61 (30-74) |
| Male sex | 12 (60) |
| Race | |
| Black or African American | 1 (5) |
| Asian | 4 (20) |
| White | 15 (75) |
| Ethnicity | |
| Non-Hispanic or non-Latino | 17 (85) |
| Hispanic or Latino | 3 (15) |
| ECOG PS score | |
| 0 | 3 (15) |
| 1 | 16 (80) |
| 2 | 1 (5) |
| Diagnosis | |
| AML | 19 (95) |
| MDS/AML with mutated TP53 | 1 (5) |
| Disease status at enrollment | |
| ND | 6 (30) |
| Relapsed | 14 (70) |
| Median prior lines of treatment for relapsed patients (range) | 1 (1-3) |
| No. of patients relapsed after HCT | 4 (29) |
| Secondary AML | 6 (30) |
| Treatment-related AML | 3 (15) |
| ELN 2017 risk category | |
| Favorable | 4 (20) |
| Intermediate | 4 (20) |
| Adverse | 12 (60) |
| Patient characteristics . | N = 20 . |
|---|---|
| Median age, y (range) | 61 (30-74) |
| Male sex | 12 (60) |
| Race | |
| Black or African American | 1 (5) |
| Asian | 4 (20) |
| White | 15 (75) |
| Ethnicity | |
| Non-Hispanic or non-Latino | 17 (85) |
| Hispanic or Latino | 3 (15) |
| ECOG PS score | |
| 0 | 3 (15) |
| 1 | 16 (80) |
| 2 | 1 (5) |
| Diagnosis | |
| AML | 19 (95) |
| MDS/AML with mutated TP53 | 1 (5) |
| Disease status at enrollment | |
| ND | 6 (30) |
| Relapsed | 14 (70) |
| Median prior lines of treatment for relapsed patients (range) | 1 (1-3) |
| No. of patients relapsed after HCT | 4 (29) |
| Secondary AML | 6 (30) |
| Treatment-related AML | 3 (15) |
| ELN 2017 risk category | |
| Favorable | 4 (20) |
| Intermediate | 4 (20) |
| Adverse | 12 (60) |
Data are presented as number (%), unless otherwise stated.
ECOG PS, Eastern Cooperative Oncology Group performance status; MDS, myelodysplastic syndrome.
DLTs and adverse events
Six DLTs occurred: grade 4 respiratory failure at DL 2, grade 4 sepsis at DL 3 (n = 2), grade 4 respiratory failure at DL 4, myelosuppression >49 days at DL 4, and grade 5 sepsis at DL 3, resulting in a 5% 28-day mortality (1/20). DL 3 was identified as the RP2D. Nine patients recovered absolute neutrophil count of ≥500 cells per μL and platelet counts of ≥50 × 103/μL by day 42. Notably, the patient who died of sepsis had relapsed after haploidentical allogeneic hematopoietic cell transplant (HCT); before study enrollment, this patient had significant venetoclax exposure with enrollment on another venetoclax-based clinical trial followed by administration of azacitidine with venetoclax. His course after CLAG-M and venetoclax induction on this clinical trial was complicated by Rothia mucilaginosa bacteremia and subsequent multiorgan failure.
We prespecified TLS as an adverse event of special interest, given the known side effect profile of venetoclax. Only 1 patient developed TLS on day 2 of therapy. Before treatment initiation, his WBC count was 5.17 × 103/μL. He had grade 3 TLS by Cairo Bishop definition (creatinine peaked at 1.37 mg/dL, and uric acid at 9.5 mg/dL), which improved with intravenous fluids and allopurinol. This patient had a history of ELN 2017 favorable-risk AML (biallelic CEBPA mutation) that relapsed after initial intensive chemotherapy. No patients received rasburicase. Other grade ≥3 adverse events are enumerated in Table 3. The most common adverse events were grade 3 febrile neutropenia (15/20, 75%) and grade 3 sepsis (5/20, 25%).
Grade 3 and higher adverse events
| Adverse event term . | Grade . | n . |
|---|---|---|
| Infectious | ||
| Febrile neutropenia | 3 | 15 |
| Skin infection | 3 | 1 |
| Sepsis | 3 | 5 |
| Sepsis | 4 | 2 |
| Sepsis | 5 | 1 |
| Gastrointestinal | ||
| Blood bilirubin increased | 3 | 1 |
| Anorexia | 3 | 1 |
| Diarrhea | 3 | 1 |
| Enterocolitis | 3 | 1 |
| Mucositis oral | 3 | 3 |
| Respiratory | ||
| Dyspnea | 3 | 3 |
| Hypoxia | 3 | 1 |
| Hypoxia | 4 | 1 |
| Respiratory failure | 4 | 2 |
| Cardiac | ||
| Myocardial infarction | 3 | 1 |
| Pericarditis | 3 | 1 |
| Other | ||
| Delirium | 3 | 1 |
| Encephalopathy | 3 | 1 |
| Hyperglycemia | 3 | 1 |
| Hypokalemia | 3 | 1 |
| Rash (maculo-papular) | 3 | 1 |
| Syncope | 3 | 1 |
| TLS | 3 | 1 |
| Total | 48 | |
| Adverse event term . | Grade . | n . |
|---|---|---|
| Infectious | ||
| Febrile neutropenia | 3 | 15 |
| Skin infection | 3 | 1 |
| Sepsis | 3 | 5 |
| Sepsis | 4 | 2 |
| Sepsis | 5 | 1 |
| Gastrointestinal | ||
| Blood bilirubin increased | 3 | 1 |
| Anorexia | 3 | 1 |
| Diarrhea | 3 | 1 |
| Enterocolitis | 3 | 1 |
| Mucositis oral | 3 | 3 |
| Respiratory | ||
| Dyspnea | 3 | 3 |
| Hypoxia | 3 | 1 |
| Hypoxia | 4 | 1 |
| Respiratory failure | 4 | 2 |
| Cardiac | ||
| Myocardial infarction | 3 | 1 |
| Pericarditis | 3 | 1 |
| Other | ||
| Delirium | 3 | 1 |
| Encephalopathy | 3 | 1 |
| Hyperglycemia | 3 | 1 |
| Hypokalemia | 3 | 1 |
| Rash (maculo-papular) | 3 | 1 |
| Syncope | 3 | 1 |
| TLS | 3 | 1 |
| Total | 48 | |
Response and survival assessments
Sixteen patients received 1 induction cycle on protocol and 4 patients received 2 cycles (3 for MRD, and 1 for refractory disease). Counting best response after 1 to 2 cycles of induction, composite CR rate (CR + CRi) was 65% (13/20 patients, 95% confidence interval [CI], 41-85; Table 4). Of 6 ND patients, 2 patients achieved CR without MRD, 1 CR with MRD, 2 CRi with MRD, and 1 had refractory disease. In the cohort of R/R patients, 3 patients achieved CR without MRD, 2 CRi without MRD, 3 CRi with MRD, 3 morphologic leukemia-free state without MRD, 2 had persistent disease, and 1 died before evaluation. Median time from starting the first cycle of induction to recovering neutrophils of >500 cells per μL was 37 days (ND, 30; R/R, 40) and platelet count of >50 × 103/μL was 40 days (ND, 29; R/R, 51). Of those treated at RP2D, median time to neutrophils of >500 cells per μL was 37 days, and platelet count of >50 × 103/μL was 38 days. Five patients went on to receive at least 1 cycle of consolidation on study.
Association between cytogenetic and molecular data at study enrollment with response assessment after treatment
| Disease status at enrollment . | ELN 2017 . | Cytogenetic data . | Molecular mutations . | Best response on study . |
|---|---|---|---|---|
| ND | Adverse | Complex karyotype | TP53 | CR with MRD |
| Adverse | ASXL1 | CRi with MRD | ||
| Adverse | Complex, monosomal karyotype | Germline TP53 (Li Fraumeni) | CR without MRD | |
| Adverse | Del7q and del11q | CRi with MRD | ||
| Adverse | Monosomy 7 and rearrangement of MECOM | Persistent disease/refractory | ||
| Adverse | Complex karyotype | CR without MRD | ||
| R/R | Favorable | Biallelic CEBPA | CR without MRD | |
| Favorable | FLT3-TKD, NPM1 | Persistent disease/refractory | ||
| Favorable | Biallelic CEBPA | MLFS without MRD | ||
| Favorable | MECOM rearrangement at relapse | NPM1 at diagnosis | MLFS without MRD | |
| Intermediate | Germline DDX41 | Persistent disease/refractory | ||
| Intermediate | FLT3-TKD | CRi with MRD | ||
| Intermediate | Del7q31 at relapse | FLT3-TKD, ASXL1 at diagnosis | CRi without MRD | |
| Intermediate | CR without MRD | |||
| Adverse | ASXL1, BCOR | CRi without MRD | ||
| Adverse | ASXL1, RUNX1, SRSF2, STAG2 | MLFS without MRD | ||
| Adverse | Complex karyotype | TP53 | CRi with MRD | |
| Adverse | Del7q | SF3B1 | CR without MRD | |
| Adverse | ASXL1, RUNX1 | Death before evaluation | ||
| Adverse | Complex karyotype | TP53 | CRi with MRD |
| Disease status at enrollment . | ELN 2017 . | Cytogenetic data . | Molecular mutations . | Best response on study . |
|---|---|---|---|---|
| ND | Adverse | Complex karyotype | TP53 | CR with MRD |
| Adverse | ASXL1 | CRi with MRD | ||
| Adverse | Complex, monosomal karyotype | Germline TP53 (Li Fraumeni) | CR without MRD | |
| Adverse | Del7q and del11q | CRi with MRD | ||
| Adverse | Monosomy 7 and rearrangement of MECOM | Persistent disease/refractory | ||
| Adverse | Complex karyotype | CR without MRD | ||
| R/R | Favorable | Biallelic CEBPA | CR without MRD | |
| Favorable | FLT3-TKD, NPM1 | Persistent disease/refractory | ||
| Favorable | Biallelic CEBPA | MLFS without MRD | ||
| Favorable | MECOM rearrangement at relapse | NPM1 at diagnosis | MLFS without MRD | |
| Intermediate | Germline DDX41 | Persistent disease/refractory | ||
| Intermediate | FLT3-TKD | CRi with MRD | ||
| Intermediate | Del7q31 at relapse | FLT3-TKD, ASXL1 at diagnosis | CRi without MRD | |
| Intermediate | CR without MRD | |||
| Adverse | ASXL1, BCOR | CRi without MRD | ||
| Adverse | ASXL1, RUNX1, SRSF2, STAG2 | MLFS without MRD | ||
| Adverse | Complex karyotype | TP53 | CRi with MRD | |
| Adverse | Del7q | SF3B1 | CR without MRD | |
| Adverse | ASXL1, RUNX1 | Death before evaluation | ||
| Adverse | Complex karyotype | TP53 | CRi with MRD |
MLFS, morphologic leukemia-free state; TKD, tyrosine kinase domain.
At date of data cutoff (15 November 2024), median OS from treatment start was 9.6 months (95% CI, 5.1 to not reached [NR]). ND patients had a median OS of 7.4 months (95% CI, 4.2 to NR) vs 11.2 months for R/R (95% CI, 3.9 to NR). Median follow-up of survivors was 14.7 (range, 2.8-24.6) months. Median EFS was 3.3 months (95% CI, 1.6-9.6). Median EFS for ND was 2.7 months (95% CI, 1.0 to NR) vs 3.3 months (95% CI, 1.2 to NR) for R/R. Six patients (2 ND, 4 R/R) went on to receive allogeneic HCT, at a median of 4.4 (range, 3.8-5.8) months after starting induction.
Discussion
In this trial, we examined the combination of venetoclax with an intensive induction chemotherapy regimen, CLAG-M, for patients with AML at a high risk of poor outcomes with standard therapy. We included patients with ND adverse-risk disease using ELN 2017 criteria and those with R/R disease. Using a BOIN design, we estimated the MTD of the combination of venetoclax and CLAG-M, which was DL 3: venetoclax 400 mg of days 1 through 14, combined with cladribine 5 mg/m2 on days 1 through 5, cytarabine 1.5 g/m2 on days 1 through 5, G-CSF 5 μg/kg on days 0 through 5, and mitoxantrone 16 or 18 mg/m2 on days 1 through 3 (for R/R and ND adverse-risk patients, respectively). There were several key observations from this single-center phase 1 dose escalation study. First, the combination of venetoclax and CLAG-M provides potent antileukemia effect, even in a study population consisting of notoriously difficult-to-treat patients. The composite CR rate in the entire study cohort was 65%, and 83% in the subset of ELN 2017 adverse-risk ND patients. Although this trial was initiated before the publication of the ELN 2022 risk stratification and response criteria, we reevaluated responses based on the updated criteria and found that no changes in eligibility would have been made.14 A notable number of new drugs have been approved by the US Food and Drug Administration for patients with AML in the past decade, but response rates for patients with R/R disease remain low. Furthermore, despite ongoing efforts, targeted options are not available for many patients, including those with complex karyotype or TP53 mutations. Of the 6 ND patients, 3 had complex karyotype (of whom 2 had TP53 mutation), 2 had deletions involving chromosome 7, and 1 had mutated ASXL1. The high proportion of ND patients with complex karyotype may explain the poor EFS and OS in this group despite initial responses. Four (29%) of the R/R cohort had relapsed after allogeneic HCT, another clinical scenario for which options are limited.15
Although the antileukemia effect of the combination regimen appears promising in this clinical trial, myelosuppression was also significant. During trial design, we anticipated this adverse event and designated that prolonged count recovery of >49 days would be considered a DLT. It can be difficult to determine whether myelosuppression is a toxicity of chemotherapy or the underlying disease, particularly in R/R patients who were heavily pretreated or had a history of prior allogeneic HCT. We observed 1 DLT of myelosuppression at >49 days, which occurred at DL 4. Another DLT of special interest for this venetoclax-based regimen was TLS. Only 1 patient (5%) developed laboratory TLS, which was treated conservatively with fluids and allopurinol. This low rate of TLS highlights the safety provided by the venetoclax dose ramp-up (supplemental Table 2) as well as cytoreduction before initiation of study therapy, with a requirement that the WBC count be <25 × 103/μL before study drug administration.
Based on the BOIN design implemented in this clinical trial (Table 1), DL 3 was determined to be the RP2D. This flexible design, which is now commonly incorporated into phase 1 trials, allowed for increases and decreases of the dose based on the number of observed DLTs. DL 2 was chosen as the starting dose because it uses standard-dose mitoxantrone (10 mg/m2) along with full-dose venetoclax given for 14 days, the latter being identical to the administration of venetoclax when combined with FLAG-ida.16 Half of the participants (n = 10) received DL 3, which included dose-escalated mitoxantrone at 18 mg/m2 for ND patients and 16 mg/m2 for R/R patients; dose-escalated mitoxantrone was chosen based on results from previous trials.1,2 Notably, only 2 patients were enrolled at DL 4 on this study, and both experienced DLTs: 1 with grade 4 respiratory failure, and 1 with myelosuppression at >49 days. The difference between DL 3 and DL 4 is a cytarabine dose of 1.5 vs 2 g/m2. The lower cytarabine dose was also chosen in the FLAG-ida plus venetoclax study.16 Although DL 3 is the recommended dose moving forward based on our study design, a larger cohort will be needed to determine efficacy of the regimen.
To mimic real-world experience, we allowed for concomitant administration of azole antifungals for prophylaxis against, and treatment of, mold infections, which are a common complication in this patient population given prolonged neutropenia from underlying AML as well as chemotherapy.11,17 Because of CYP3A4 interactions between azole antifungals and venetoclax, the US prescribing information for venetoclax delineates guidelines for decreased dosage of venetoclax when azoles are coadministered. Posaconazole and voriconazole, both strong CYP3A4 inhibitors commonly prescribed to patients with AML, have different recommendations for the final dosage of venetoclax after initial ramp-up (70 mg for concomitant posaconazole and 100 mg for concomitant voriconazole, compared with a dose of 400 mg when administered without an azole). To simplify the administration of venetoclax, especially given the need for multiple different doses during the 4-day ramp-up, we settled on a requirement of voriconazole as the antifungal for patients on this clinical trial. Future studies examining plasma drug concentrations and evaluating efficacy may help determine the optimal antifungal to administer with concomitant venetoclax.
Although the composite CR rate in our clinical trial suggests that further study of the venetoclax and CLAG-M is warranted, we acknowledge some limitations in this trial. This study was a phase 1, dose-escalation trial, with a population of only 20 patients. Rare DLTs may not have been observed because of the small sample size. Because the inclusion criteria allowed ND adverse-risk patients as well as R/R patients (both populations who experience poor responses to standard therapy), the small numbers preclude further comparison of differences in DLT rates, TLS frequency, or other risks and benefits of this regimen among subgroups. Because the phase 1 design was only powered to assess toxicity, we are planning a phase 2 study to determine the efficacy of this novel combination induction regimen.
Acknowledgments
Eli Estey was indispensable in the conception and design of this study.
This study was funded, in part, by a philanthropic gift from Jihad Othman. Research reported in this publication was supported, in part, by the National Heart, Lung, and Blood Institute T32 grant (award number T32HL007093) and National Cancer Institute (NCI) Cancer Center support grant (NCI 5 P30 CA015704-49). Study drug (venetoclax) was provided by AbbVie.
Authorship
Contribution: T.A.G. and M.-E.M.P. designed the research; A.B.H., J.S.A., C.M.G, P.C.H., R.D.C., R.B.W., and M.-E.M.P. consented and treated patients; S.R., A.R., K.Q., E.G., and M.-E.M.P. collected and assembled the data; S.R., T.A.G., and M.-E.M.P. analyzed and interpreted the data; S.R. and M.-E.M.P. wrote the manuscript; and all authors reviewed and revised the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.B.H. provided consultancy for Karyopharm Therapeutics; and received research funding from Imago Biosciences, Merck, Bayer Pharmaceuticals, Gilead Sciences, Jazz Pharmaceuticals, Incyte, Karyopharm Therapeutics, Disc Medicine, Protagonist, PharmEssentia, and Sumitomo. J.S.A. received honoraria from Incyte. R.B.W. did consultancy for Wugen, Inc; and received research funding from Aptevo, Celgene/Bristol Myers Squibb (BMS), ImmunoGen, Janssen, Jazz, Kite, Kura, Pfizer, and Vor Biopharma. R.D.C. received research funding from Amgen, Kite/Gilead, Incyte, Merck, Pfizer, Servier, and Vanda Pharmaceuticals; provided consultancy for/received honoraria from Autolus, Amgen, Jazz, Kite/Gilead, and Pfizer; reports membership on a board or advisory committee for Autolus and PeproMene Bio; and reports that a spouse was employed by and owned stock in Seagen. M.-E.M.P. received research funding from AbbVie, Ascentage, Astex, Biosight, BMS/Celgene, Glycomimetics, Immunogen, Nohla Therapeutics, Oscotec, Pfizer, Telios, and VinceRx. The remaining authors declare no competing financial interests.
Correspondence: Mary-Elizabeth M. Percival, Fred Hutchinson Cancer Center, 825 Eastlake Ave E, MS LG-700, Seattle, WA 98109; email: mperciva@uw.edu.
References
Author notes
Individual participant data will not be shared. The clinical trial protocol is included in the supplemental information.
The full-text version of this article contains a data supplement.