Key Points
The quadruplet regimen Dara-CTD was active and safe as frontline therapy in ASCT-eligible NDMM.
Dara-CTD may be an alternative to potentially more toxic and costly quadruplet regimens containing PIs and lenalidomide.
Visual Abstract
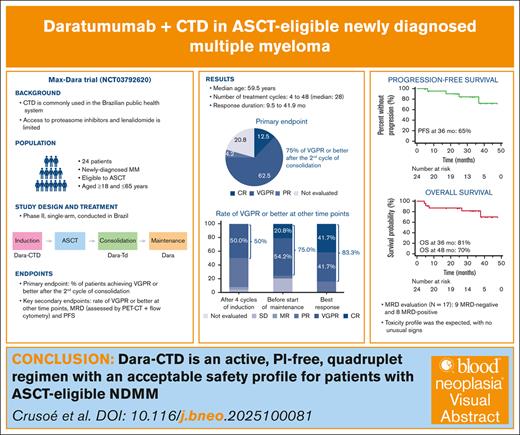
Anti-CD38 monoclonal antibodies have been successfully combined with immunomodulatory agents, proteasome inhibitors (PIs), alkylators, and corticosteroids in newly diagnosed multiple myeloma (NDMM). We assessed a regimen of daratumumab, cyclophosphamide, thalidomide, and dexamethasone (Dara-CTD) for patients with NDMM eligible to autologous stem cell transplantation (ASCT). Patients received 4 28-day cycles of induction therapy with Dara-CTD followed by ASCT, 4 cycles of consolidation Dara-TD, and single-agent daratumumab maintenance until progression or limiting toxicity. The primary end point was the percentage of patients achieving at least a very good partial response (VGPR) after the second cycle of consolidation. A key secondary end point was progression-free survival (PFS). We enrolled 24 patients, with a median age of 59.5 years, 62.5% of whom were female. Patients received a median of 28 treatment cycles. The rate of VGPR or better was 75.0% (95% confidence interval, 68.3-98.7); of 18 responding patients, 3 had a complete and 15 had a VGPR. The response duration varied between 9.5 and 41.9 months (median not reached). The estimated PFS rate at 36 months was 65%, and the overall survival rate at 48 months was 70%. The most frequent toxicity was constipation, febrile neutropenia, lymphopenia, neutropenia, peripheral neuropathy, and stomatitis. There were 7 documented cases of coronavirus disease 2019, 2 of which fatal. Two patients had permanent treatment discontinuation due to toxicity, 1 case each attributed to cyclophosphamide and daratumumab. Dara-CTD is an active, PI-free, quadruplet regimen with an acceptable safety profile for patients with ASCT-eligible NDMM. This trial was registered at www.ClinicalTrials.gov as #NCT03792620 and in the Plataforma Brasil at https://plataformabrasil.saude.gov.br as #CAAE96591818.0.0000.0048.
Introduction
Multiple myeloma (MM) accounted for ∼14% of hematologic malignancies worldwide in 2020.1 Over the past few decades, considerable improvements in overall survival (OS) have been achieved in MM, in part through the use of autologous stem cell transplantation (ASCT) in patients who are eligible for this procedure as part of frontline therapy.2 Moreover, the introduction of proteasome inhibitors and immunomodulatory drugs has contributed to improvements in long-term outcomes, as these agents have been incorporated into frontline and salvage therapy.2,3 Although various combinations of novel agents and ASCT have been tested, the best induction regimen for ASCT-eligible patients has not been established. In settings in which this is feasible, triplet induction regimens including a proteasome inhibitor, an immunomodulatory drug and dexamethasone have become standard of care for ASCT-eligible patients.4,5 In recent phase 3 trials of triplet therapy used in patients who are newly diagnosed and eligible to ASCT, the OS at 5 years ranged between ∼70% and 80%.6-9 This is a notable improvement from the median OS of ∼50% when ASCT was first found to improve patient prognosis.10
The anti-CD38 monoclonal antibody daratumumab has considerably affected the management of patients with relapsed/refractory MM and those with newly diagnosed disease ineligible for ASCT.11-14 Thus, there is increasing interest in quadruplet regimens containing an anti-CD38 antibody for the treatment of ASCT-eligible patients with newly diagnosed MM.4,5 CD38 is highly expressed on the surface of myeloma cells and has limited expression on healthy lymphoid and myeloid cells.15,16 Moreover, the favorable toxicity profile and ability to spare stem cell collection enable combinations between daratumumab and several other agents, leading to its use as a component of quadruplet regimens.17 Among patients with newly diagnosed MM who are ASCT eligible, the CASSIOPEIA trial showed that the addition of daratumumab to a triplet regimen led to higher objective response rates, more frequent minimal residual disease (MRD) negativity, and prolonged progression-free survival (PFS), when compared with the triplet regimen alone.18 Moreover, maintenance therapy with daratumumab improved PFS in this same patient population, when compared with observation.19 Finally, the addition of subcutaneous daratumumab to a triplet regimen was associated with improved objective response rates and PFS, when compared with the triplet regimen alone, in transplantation-eligible patients.20
Triplet regimens can be very expensive depending on the agents used, and in countries facing more severe resource limitation and reduced access to novel agents, alternative induction regimens are of great interest.21,22 One such regimen comprises oral cyclophosphamide, thalidomide, and dexamethasone (CTD); this regimen can be safely combined with ASCT and provides a more affordable alternative to conventional triple therapy for newly diagnosed patients.7,22,23 CTD is commonly used at public institutions in Brazil, in which access to novel agents is limited.24 Although daratumumab is not widely available in public institutions in Brazil, we have postulated that the addition of this antibody to CTD could improve the efficacy of the triplet regimen alone as frontline treatment for ASCT-eligible patients with newly diagnosed MM, at the same time representing a tolerable regimen. Moreover, the cost of this resulting quadruplet regimen could compare favorably with those containing a proteasome inhibitor and lenalidomide in settings for which a quadruple regimen would be preferable to triple regimens, potentially also informing regulatory decisions on reimbursement in such settings. The current single-arm, phase 2 trial represented the first step toward testing this hypothesis.
Methods
Trial design and oversight
The trial (Max Dara) was designed by the authors and registered in Plataforma Brasil https://plataformabrasil.saude.gov.br, the Brazilian registry of clinical trials identifier CAAE96591818.0.0000.0048, as well as in www.ClinicalTrials.gov (identifier NCT03792620). The trial was conducted under the auspices of the Brazilian Multiple Myeloma study group (GBRAM). All participating patients provided written informed consent after protocol approval by the ethics committee overseeing the participating institution.
Patient eligibility
Eligible patients were aged ≥18 and ≤65 years, had newly diagnosed MM (according to the International Myeloma Working Group [IMWG] 2014 criteria25), no previous treatment for the disease (except for the use of corticosteroids [≤40 mg of dexamethasone or equivalent] within the last 30 days), adequate bone marrow, renal, and hepatic functions, and an Eastern Cooperative Oncology Group performance status score between 0 and 2. Moreover, fertile patients should practice an effective contraceptive method, and those with asthma or chronic obstructive pulmonary disease required proof of adequate lung function. Patients were excluded if they had any neoplasms within the last 5 years (except skin carcinomas, in situ carcinoma of the cervix, or other neoplasms with minimal risk of recurrence); recent use (≤14 days) of radiotherapy; other clinical conditions (echocardiogram with left ventricular ejection fraction of <45%, heart block, untreated arrhythmia, clinically significant abnormalities on the electrocardiogram, HIV seropositivity, active hepatitis B or C, Chagas disease with cardiac involvement, or a psychiatric illness that compromised participation, including drug addiction or alcoholism); allergies or hypersensitivities to study drugs; or use of investigational drugs, including vaccines.
Treatment
The chosen backbone regimen was CTD, which is commonly used at public institutions in Brazil, in which access to proteasome inhibitors and lenalidomide is limited. Patients were treated during 4 phases (induction, transplantation, consolidation, and maintenance). Treatment and follow-up were carried out at Centro de Hematologia e Oncologia da Bahia, with ASCT performed on an inpatient basis at Hospital Universitário Professor Edgar Santos, Federal University of Bahia; both institutions are in Salvador, Brazil. Induction consisted of 4, 4-week cycles of oral cyclophosphamide, 500 mg/d on days 1, 8, and 15 of each cycle; thalidomide, 100 mg/d (which could be increased to 200 mg/d after 15 days if well tolerated) from day 1 to day 28 of each cycle; oral dexamethasone, 40 mg/wk of each cycle; and intravenous daratumumab, 16 mg/kg weekly in cycles 1 and 2, and every other week in cycles 3 and 4 (total of 12 doses). Stem cell mobilization started between day −9 and day −8 with the use of granulocyte-colony stimulating factor at the maximum dose of 15 μg/kg per day. The stem cell collection was planned for when the peripheral CD34 cell count exceeded 10 cells per μL. Stem cell collection was performed using Cobe Spectra, with processing of 4 to 6 volemias. For stem cell infusion, the patient should have a harvest of at least 2.5 × 106 CD34 cells per kg; otherwise, plerixafor (1 vial) could be added to granulocyte-colony stimulating factor for 1 or 2 days. Transplantation consisted of high-dose melphalan followed by stem cell infusion. IV melphalan was administered at the dose of 140 to 200 mg/m2 (depending on creatinine clearance) on day −2 and day −1 up to 30 days after the fourth induction cycle. Consolidation consisted of 4, 4-week cycles of thalidomide, 100 mg/d from day 1 to day 28 of each cycle and oral dexamethasone, 40 mg/wk of each cycle, plus IV daratumumab, 16 mg/kg on day +30 after ASCT every other week for 4 cycles (total of 8 doses). Maintenance consisted of daratumumab, 16 mg/kg every 4 weeks until progression or limiting toxicity. Throughout treatment, no dose reductions were allowed for daratumumab, whereas they were allowed for the components of the CTD regimen. Thalidomide could be reduced up to 100 mg every other day, cyclophosphamide to the lowest possible dose of 1000 mg/mo, and dexamethasone could be managed following the schedule described by Weber et al.26 Because of the coronavirus disease 2019 (COVID-19) pandemic, the risk of performing ASCT was deemed too high, which led to a modification of the treatment schedule. In patients who had not yet completed the induction phase, as well as in new patients, the 2 original consolidation cycles were transferred to the induction phase, which now had 6 cycles (instead of 4), and consolidation was now carried out with 2 cycles (instead of 4).
Statistical analysis
The sample size for the trial was based on the Fleming 1-stage design, considering a historical response rate of 74% after treatment with CTD and ASCT23 and an expected absolute increase in the response rate of 20% from the addition of daratumumab to the CTD regimen.17 Thus, the initially planned sample size was 23 patients, which gave the trial a statistical power of 93% to detect the increase in the rate of very good partial response (VGPR) or better, with a 1-sided α error of 9%. The primary end point was the VGPR or better assessed after the second cycle of consolidation, which corresponded to a window of 75 to 90 days after ASCT. Secondary end points were the VGPR or better at other time points, time to response, response duration, PFS, OS, MRD negativity, and safety. The other time points for assessment of VGPR or better were after 4 cycles of induction and before the start of maintenance therapy. In addition to these time points, the best response at any time point was also evaluated. Response assessment followed the IMWG 2014 criteria.25 During the disruption brought on by the pandemic, response assessments were performed at the following time points: (1) after 4 cycles of treatment; (2) approximately on day +30 after ASCT (which would be between day +75 and day +90 of the original plan); and (3) after 2 cycles of consolidation. Therefore, the change in treatment schedule brought on by the pandemic (moving 2 consolidation cycles to the induction period) had little or no impact on the timing of assessment of the primary end point, in relation to treatment initiation, between patients outside or inside the period affected by the pandemic. Time-to-event end points were assessed using the Kaplan-Meier method, censoring patients who were free from the corresponding events on last follow-up. Median follow-up for PFS and OS was calculated using the “reverse Kaplan-Meier” method.27 MRD negativity was assessed using both positron emission tomography with computed tomography (PET-CT) and 8-color flow cytometry with a sensitivity of 10−5. PET-CT was performed at baseline and after consolidation (or earlier if a patient had a complete response [CR]). Flow cytometry for MRD evaluation was performed after 4 cycles of induction, after 2 and 4 cycles of consolidation, and 1 year after start of maintenance therapy; negativity was defined as <1 cell per 100 000 in the bone marrow. Safety was assessed by recording clinical and laboratory toxicity elicited during patient visits and hospital stays, grading the worst occurrence of such toxicity using the corresponding adverse events listed in the common terminology criteria for adverse events, version 4.0. An exploratory analysis was conducted of CD34 counts in the apheresis product after mobilization. Statistical analysis was carried out using MedCalc (Mariakerke, Belgium, version 11.3.3.0).
Results
Patient characteristics and exposure to treatment
Between December 2018 and December 2020, 24 patients were enrolled and treated. Selected baseline patient characteristics are shown in Table 1. The median age was 59.5 years, and 62.5% of patients were female. Two patients with an age above the age limit (aged 66 and 67 years, respectively) were enrolled because of excellent clinical conditions. There was a predominance of patients of mixed race (Black and White ancestry), consistently with the race distribution in Salvador. Regarding staging, the most frequent categories were stage I for the International Staging System (45.8%) and stage II for the Revised International Staging System (57.1%). The predominant immunoglobulin phenotype was immunoglobulin G (58.3%), and 20.8% of patients had only light chains. Approximately a third of patients tested (n = 20) were classified as being at high cytogenetic risk (del 17p; t(4;14); t(14;16); or expanded 1q21.2). Regarding the PET-CT assessment at baseline, the result was considered positive in 20 patients (83.3%) and negative in 4.
Selected baseline patient characteristics
| Characteristic . | (%) or median (range) . |
|---|---|
| Age, y | 59.5 (37-67) |
| Sex, female | 15 (62.5) |
| Race | |
| White | 2 (8.3) |
| Mixed | 13 (54.2) |
| Black | 9 (37.5) |
| Hemoglobin, g/dL | 9.8 (6.0-13.8) |
| Platelets, ×103/μL | 210 (97-309) |
| Albumin, g/dL | 4.0 (2.2-4.7) |
| Lactic dehydrogenase, U/L | 205.5 (108-621) |
| Ionized calcium, mg/dL | 1.3 (1.2-1.5) |
| β-2-microglobulin, mg/L | 4.0 (1.8-47.0) |
| International Staging System | |
| I | 11 (45.8) |
| II | 7 (29.2) |
| III | 6 (25.0) |
| Revised International Staging System∗ | |
| I | 6 (28.6) |
| II | 12 (57.1) |
| III | 3 (14.3) |
| Monoclonal pattern | |
| IgG | 15 (62.5) |
| IgA | 4 (16.7) |
| Light-chain only | 5 (20.8) |
| Cytogenetics, high risk† | 7 (35.0)∗ |
| Characteristic . | (%) or median (range) . |
|---|---|
| Age, y | 59.5 (37-67) |
| Sex, female | 15 (62.5) |
| Race | |
| White | 2 (8.3) |
| Mixed | 13 (54.2) |
| Black | 9 (37.5) |
| Hemoglobin, g/dL | 9.8 (6.0-13.8) |
| Platelets, ×103/μL | 210 (97-309) |
| Albumin, g/dL | 4.0 (2.2-4.7) |
| Lactic dehydrogenase, U/L | 205.5 (108-621) |
| Ionized calcium, mg/dL | 1.3 (1.2-1.5) |
| β-2-microglobulin, mg/L | 4.0 (1.8-47.0) |
| International Staging System | |
| I | 11 (45.8) |
| II | 7 (29.2) |
| III | 6 (25.0) |
| Revised International Staging System∗ | |
| I | 6 (28.6) |
| II | 12 (57.1) |
| III | 3 (14.3) |
| Monoclonal pattern | |
| IgG | 15 (62.5) |
| IgA | 4 (16.7) |
| Light-chain only | 5 (20.8) |
| Cytogenetics, high risk† | 7 (35.0)∗ |
IgG/A, immunoglobulin G/A.
For the Revised International Staging System, 3 patients were not classified.
For cytogenetics, only 20 patients were tested.
Patients received between 4 and 48 treatment cycles, with a median of 28. Of 24 patients, 2 did not undergo ASCT: 1 patient died during COVID-19 infection before reaching transplantation, and the second died of sepsis during induction therapy. Dose adjustments were required for thalidomide in 13 patients, and for dexamethasone in 2. Treatment discontinuation was attributed to cyclophosphamide in 15 patients (temporarily in 13, and permanent in 2), to thalidomide in 19 patients (temporarily in 15 and permanent in 4), to dexamethasone in 16 patients (temporarily in 12 and permanent in 4), and to daratumumab in 21 patients (temporarily in 11 and permanent in 10). Most temporary discontinuations were because of infectious complications, whereas permanent discontinuation was usually because of disease progression. Details are provided in supplemental Table 1 of the supplemental Materials.
Efficacy
Table 2 displays the results of response assessments after 4 cycles of induction, after the second cycle of consolidation, and before the start of maintenance therapy, alongside the best response at any time point. Assessment after the second cycle of consolidation, the primary end point, was possible in 19 cases. Five patients could not be assessed at this time point. Among these 5 patients, only 1 had a documented disease progression within the evaluation period, 2 had a subsequent assessment (with a partial response and a VGPR in 1 case each), and 2 had no further assessments. Of these patients with no further assessments, 1 died during induction, and the other within a month from ASCT. Therefore, the VGPR or better among all 24 patients was 75.0% (95% confidence interval, 68.3-98.7). The 18 patients with a response comprised 3 CRs and 15 VGPRs; moreover, 1 patient had a partial response, leading to an objective response (partial response or better) in 19 of 24 patients (Table 2). Twenty patients (10 with CR and 10 with VGPR) had a response at any time point, for a VGPR or better of 83.3%. Considering these 20 patients with at least VGPR at any time point, the median time to obtain such a response was 4 months. For these 20 patients, the duration of response varied between 9.5 and 41.9 months, and the median duration of response was not reached (see supplemental Figure 1 of the supplemental Materials).
Response assessment at different time points and best recorded response per patient
| Objective response . | n (%) . | |||
|---|---|---|---|---|
| After 4 cycles of induction . | After 2 cycles of consolidation∗ . | Before start of maintenance . | Best response . | |
| Response categories | ||||
| Stringent CR | 0 | 0 | 0 | 0 |
| CR | 0 | 3 (12.5) | 5 (20.8) | 10 (41.7) |
| VGPR | 12 (50.0) | 15 (62.5) | 13 (54.2) | 10 (41.7) |
| Partial response | 10 (41.7) | 1 (4.2) | 1 (4.2) | 4 (16.7) |
| Minimal response | 1 (4.2) | 0 | 0 | 0 |
| Stable disease | 1 (4.2) | 0 | 0 | 0 |
| Progressive disease | 0 | 0 | 0 | 0 |
| Not evaluated | 0 | 5 (20.8) | 5 (20.8) | 0 |
| Objective response rate | 95.6% | 79.2% | 79.2% | 100% |
| Objective response . | n (%) . | |||
|---|---|---|---|---|
| After 4 cycles of induction . | After 2 cycles of consolidation∗ . | Before start of maintenance . | Best response . | |
| Response categories | ||||
| Stringent CR | 0 | 0 | 0 | 0 |
| CR | 0 | 3 (12.5) | 5 (20.8) | 10 (41.7) |
| VGPR | 12 (50.0) | 15 (62.5) | 13 (54.2) | 10 (41.7) |
| Partial response | 10 (41.7) | 1 (4.2) | 1 (4.2) | 4 (16.7) |
| Minimal response | 1 (4.2) | 0 | 0 | 0 |
| Stable disease | 1 (4.2) | 0 | 0 | 0 |
| Progressive disease | 0 | 0 | 0 | 0 |
| Not evaluated | 0 | 5 (20.8) | 5 (20.8) | 0 |
| Objective response rate | 95.6% | 79.2% | 79.2% | 100% |
Primary end point.
Four patients had a documented progression during the observation period, and 5 patients died (none of these 5 patients had a documented progression before death). The median follow-up for OS was 34.3 months. Figure 1 displays the PFS, whose median had not yet been achieved; the estimated PFS rate at 36 months was 65%. Figure 2 displays the OS, whose median had also not been achieved. OS rates were estimated as 87% at 12 and 24 months, 81% at 36 months, and 70% at 48 months.
The combined evaluation of MRD by PET-CT and flow cytometry was performed, with a negative MRD considered as cases with a negative PET-CT after treatment and negative flow cytometry at the last evaluation for each patient. Using these criteria, which could be applied to 17 patients, 9 and 8 had MRD-negative and MRD-positive results, respectively. The number of CD34+ cells was available for 22 patients and ranged from 2.54 × 106/kg to 11.08 × 106/kg (mean of 4.51 × 106/kg and standard deviation of 2.09 × 106/kg). Of 22 patients, 8 required plerixafor to achieve adequate CD34 counts. Information on time to engraftment was available for 21 patients; for neutrophils, this time ranged from 9 to 14 days, with a median of 11, whereas for platelets, the time ranged from 8 to 17 days, with a median of 12.
Safety
Tables 3 and 4 display the toxicity assessment and their grading. The toxicity profile was as expected, with no unusual signals for any of the individual components of treatment. The most frequently recorded toxicity was constipation, febrile neutropenia during ASCT, lymphopenia, neutropenia, peripheral neuropathy, stomatitis, and upper respiratory tract infection. In terms of severity, the toxicity most frequently reported as grade 3 or 4 was febrile neutropenia during ASCT, lymphopenia, and neutropenia. There was a total of 7 documented cases of COVID-19. Two patients died during an episode of COVID-19, 1 of them before and 1 after transplantation; the patient who died before transplantation also had grade 5 documented bacterial pneumonia. Of 3 additional deaths taking place at the time of analysis, 1 was because of posttransplantation febrile neutropenia, 1 was from unknown causes (the patient who was lost to follow-up), and 1 death occurred during subsequent treatment motivated by disease progression after study completion.
Assessment of hematologic toxicity and worst grade per patient
| Toxicity . | Grade . | |
|---|---|---|
| All grades . | Grades 3/4 . | |
| Lymphopenia | 22 | 11 |
| Neutropenia | 20 | 14 |
| Thrombocytopenia | 10 | 1 |
| Toxicity . | Grade . | |
|---|---|---|
| All grades . | Grades 3/4 . | |
| Lymphopenia | 22 | 11 |
| Neutropenia | 20 | 14 |
| Thrombocytopenia | 10 | 1 |
Assessment of nonhematologic toxicity and worst grade per patient
| Toxicity . | Grade . | |
|---|---|---|
| All grades . | Grades 3/4 . | |
| Bacterial pneumonia∗ | 8 | 1 |
| Constipation | 18 | 0 |
| COVID-19† | 5 | 1 |
| Cushing syndrome | 2 | 0 |
| Dizziness | 9 | 0 |
| Fatigue/asthenia | 11 | 0 |
| Febrile neutropenia during ASCT‡ | 21 | 21 |
| Herpes zoster virus | 3 | 1 |
| Hyperglycemia | 17 | 0 |
| Hypertension | 5 | 4 |
| Infusion reaction | 9 | 2 |
| Insomnia | 7 | 0 |
| Joint pain | 8 | 0 |
| Muscle pain | 9 | 0 |
| Nausea | 13 | 1 |
| Peripheral edema | 14 | 1 |
| Peripheral neuropathy | 23 | 1 |
| Pruritus | 11 | 0 |
| Skin rash | 13 | 1 |
| Somnolence | 2 | 0 |
| Stomatitis during ASCT | 21 | 6 |
| Syncope | 5 | 3 |
| Tremors | 9 | 1 |
| Upper respiratory tract infection | 19 | 0 |
| Venous thrombosis | 1 | 0 |
| Toxicity . | Grade . | |
|---|---|---|
| All grades . | Grades 3/4 . | |
| Bacterial pneumonia∗ | 8 | 1 |
| Constipation | 18 | 0 |
| COVID-19† | 5 | 1 |
| Cushing syndrome | 2 | 0 |
| Dizziness | 9 | 0 |
| Fatigue/asthenia | 11 | 0 |
| Febrile neutropenia during ASCT‡ | 21 | 21 |
| Herpes zoster virus | 3 | 1 |
| Hyperglycemia | 17 | 0 |
| Hypertension | 5 | 4 |
| Infusion reaction | 9 | 2 |
| Insomnia | 7 | 0 |
| Joint pain | 8 | 0 |
| Muscle pain | 9 | 0 |
| Nausea | 13 | 1 |
| Peripheral edema | 14 | 1 |
| Peripheral neuropathy | 23 | 1 |
| Pruritus | 11 | 0 |
| Skin rash | 13 | 1 |
| Somnolence | 2 | 0 |
| Stomatitis during ASCT | 21 | 6 |
| Syncope | 5 | 3 |
| Tremors | 9 | 1 |
| Upper respiratory tract infection | 19 | 0 |
| Venous thrombosis | 1 | 0 |
There was 1 case of grade 5 bacterial pneumonia.
There were 2 cases of grade 5 COVID-19.
There was 1 case of grade 5 febrile neutropenia.
Discussion
Despite the unprecedented achievements of the past few decades, MM remains incurable, and novel treatments are needed.2 Our results show that the addition of daratumumab to CTD is safe and does not seem to be associated with unexpected toxicity in newly diagnosed, ASCT-eligible patients. Moreover, the quadruplet regimen used in our trial allowed adequate stem cell mobilization in all cases, despite the need to use 6 cycles of induction because of the COVID-19 pandemic. Finally, the use of maintenance daratumumab until progression also appears feasible and beneficial.
With regard to the efficacy of this novel quadruplet regimen, the VGPR or better rate of 75%, did not meet the primary end point (an expected absolute increase in the response rate of 20% from the addition of daratumumab to the CTD regimen from 74%-94%). Nevertheless, these results are promising and potentially useful in a health care context with more severe constraints. Evidently, our results do not allow definitive conclusions before a randomized trial is conducted. Although this response rate of 75% is nominally higher than the historical rates of at least VGPR reported for CTD alone (74% in the trial by Morgan et al23 and 39% by Vasquez et al22), ours was a single-arm trial, and the lack of a control arm precludes definitive assessment of causal links between treatment and outcomes. Moreover, we observed a rate of VGPR or better that was nominally lower than that reported in for CTD in the control arm of the Myeloma XI trial (77%).7 The same reasoning applies when contrasting the VGPR or better observed in our trial and that reported by GRIFFIN investigators with daratumumab, lenalidomide, bortezomib, and dexamethasone (91% at the end of consolidation).17 Likewise, Costa et al have reported a response of at least VGPR in 95% of patients after ASCT using daratumumab, carfilzomib, lenalidomide, and dexamethasone28; whereas in the CASSIOPEIA study, a response of at least VGPR was 83% with daratumumab plus bortezomib, thalidomide, and dexamethasone.18 Notwithstanding potential differences in terms of response criteria in the trial by Morgan et al23 and Vasquez et al22 (using earlier IMWG criteria) and the other trials with quadruplet regimens, and the potential influence of differing baseline prognostic features across these trials, it is conceivable that the rate of at least VGPR reported here is lower than that seen with other backbone regimens to which daratumumab has been added (ie, containing a proteasome inhibitor and lenalidomide). In contrast, we believe that the addition of daratumumab to a backbone of CTD warrants further investigation in settings in which conventional quadruplet regimens cannot be widely used because of concerns with cost or toxicity. This includes patients with contraindications to proteasome inhibitors. Additionally, an important point in interpreting our results is an indirect comparison with a conventional frontline triple regimen, such as bortezomib, lenalidomide, and dexamethasone. In Brazil, the price for lenalidomide is nearly the same between the original and generic formulations, which makes that regimen less accessible for patients treated in public institutions in this country. A similar consideration applies to the generalizability of our results, whose relevance may depend on the specific health care context. When our trial was designed, CTD was the only backbone triple regimen to which daratumumab could be realistically added, because bortezomib was not available in public institutions in Brazil.
Of note, we have observed deep responses despite the absence of a proteasome inhibitor or lenalidomide as components of the backbone regimen. For the assessment of MRD, our combined use of flow cytometry with sensitivity of 10−5 and of PET-CT arguably allowed for a more reliable evaluation of deep responses. Given the heterogenous distribution of malignant plasma cells in the bone marrow and the potential presence of such cells in other locations, the use of flow cytometry and PET-CT is thought to provide a broader assessment of both medullary and extramedullary MRD.29
A noteworthy feature of our trial is that all but 2 patients were of mixed or Black race, usually underrepresented in trials among patients with MM.30 The extent to which treatment results in MM may depend on differing biological characteristics among races remains controversial,31 and we do not believe that inferences should be drawn from our results regarding their preferential applicability to mixed-race or Black populations.
Our study suffers from the usual limitation of single-arm trials, in that no definitive conclusions can be made regarding the comparative efficacy between this and other regimens. Also, like many studies conducted during the past few years, this study was considerably affected by the COVID-19 pandemic. Especially during the earlier phases of the pandemic, COVID-19 added considerable morbidity and arguably increased mortality among patients with MM.32 The extent to which our results have been affected by the pandemic situation cannot be clearly ascertained. Given the relatively small size of this phase 2 trial, the documentation of this infection in 7 patients, and the fact that 2 died from COVID-19, it is reasonable to speculate that the efficacy of the quadruplet regimen of CTD plus daratumumab estimated here is lower than the expected results in ideal conditions. A final note about potential shortcomings of our study pertains to the use of intravenous daratumumab, as opposed to the more recent practice of subcutaneous injection, and notwithstanding the similar efficacy and safety profiles of both formulations.33
In summary, we have multiple classes of agents and we still do not know exactly which, and how many, should be combined for patients with newly diagnosed disease eligible for ASCT. We have proposed the first trial with daratumumab/immunomodulatory drug/alkylator in this population. The current results suggest that the quadruplet regimen of CTD plus daratumumab has an acceptable safety profile and sufficient activity to be considered an alternative to potentially more toxic and costly quadruplet regimens containing proteasome inhibitors and lenalidomide. Although access to ASCT is possible under the Brazilian public health system, constraints exist; this constraint, coupled with the frequent lack of access to a proteasome inhibitor in frontline treatment may represent a useful approach in this setting. Moreover, this regimen is practical (daratumumab can be given subcutaneously, and other agents are oral), does not preclude adequate stem cell collection, and is relevant in settings in which a proteasome inhibitor is not available or may be omitted intentionally. Therefore, this option (daratumumab-CTD) provides 4 active drugs and spares use of a proteasome inhibitor, leaving the option for proteasome inhibitor–based regimens at relapse. This is of particular importance in low- to middle-income countries, in which progress in the treatment of MM has lagged in comparison with high-income countries.
Acknowledgments
The authors thank Debora Sacramento and Amanda Lessa for administrative support and Luciano Costa for comments and suggestions. Editorial support was provided by Dendrix, São Paulo, Brazil, with no influence from the financial sponsor.
This study was financially supported by Janssen-Cilag, São Paulo, Brazil.
The financial sponsor had no role in data collection, study conduct, or analysis.
Authorship
Contribution: E.d.Q.C designed, developed, and performed research, analyzed the data, and wrote and approved the final manuscript; V.H. developed and performed research, analyzed the data, and wrote and approved the final manuscript; and all authors contributed to the development of, and performed, research, and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.d.Q.C. has received honoraria from Janssen, Amgen, Bristol Myers Squibb (BMS), Takeda, GlaxoSmithKline (GSK), and Sanofi; and has received a research grant from Janssen. V.H. has received honoraria from Janssen, Amgen, BMS, Takeda, and GSK. M.A.S. has received honoraria from Janssen, Amgen, AbbVie, Takeda, and Kite Pharmaceuticals. J.d.A.S. and L.F.L have received honoraria from Janssen. The remaining authors declare no competing financial interests.
A complete list of members of the Brazilian multiple myeloma study group GBRAM appears in the supplemental Appendix.
Correspondence: Edvan de Queiroz Crusoe, Rede D’or Oncologia and Hospital Universitário Professor Edgar Santos, Federal University of Bahia, Ave Araújo Pinho, 439, Salvador 40110-150, Brazil; email: edvancrusoe@gmail.com.
References
Author notes
∗Member of Brazilian Multiple Myeloma Group, GBRAM.
The protocol and data set are available on request from the corresponding author, Edvan de Queiroz Crusoé (edvancrusoe@gmail.com).
The full-text version of this article contains a data supplement.