Key Points
Oligocentric CD is an intermediate phenotype distinctly characterized from unicentric and iMCD.
Oligocentric CD cases demonstrate a pattern of oligocentric or regional lymphadenopathy and few inflammatory symptoms.
Visual Abstract
Castleman disease (CD) describes a group of rare lymphoproliferative disorders that exhibit a wide range of symptomatology and degree of lymphadenopathy, particularly across the 2 forms of CD with unknown etiology, unicentric CD (UCD), and human herpesvirus-8–negative/idiopathic multicentric CD (iMCD). Whereas UCD cases typically present with localized lymphadenopathy and mild symptoms, iMCD involves multicentric lymphadenopathy and cytokine storm–driven symptoms with 3 recognized clinical phenotypes. Increasingly, there are anecdotal reports of cases that do not fit into this framework, but these cases have not been systematically described. Herein, we use the ACCELERATE natural history registry to characterize the spectrum of CD based on disease features, symptomatology, and severity. Our results characterize a cohort of 179 CD cases, which were reviewed and confirmed by an expert panel of clinicians and hematopathologists. We show that patients with CD present on a continuous spectrum of clinical phenotypes, and we describe oligocentric CD (OligoCD), an intermediate phenotype that does not fit the criteria for UCD or iMCD. These cases tend to have “oligocentric” lymphadenopathy (median [interquartile range] regions of lymphadenopathy, 3.0 [2.0-4.0]) in a regional pattern and exhibit a mild clinical phenotype that is more similar to UCD than iMCD. We also show that patients with OligoCD are inconsistently categorized as UCD vs iMCD, highlighting the need for this characterization. Future data collected through ACCELERATE may further elucidate the natural history and risk profile of these patients.
Introduction
Castleman disease (CD) comprises a group of lymphoproliferative disorders with a spectrum of shared lymph node (LN) histopathological features and highly variable clinical symptomatology. CD was first reported in 1956 by Benjamin Castleman, who characterized hyaline vascular histopathologic findings in 2 localized cases, subsequently termed unicentric CD (UCD).1 The histopathologic definition of CD has since broadened to include cases with characteristic plasmacytic and mixed histopathologic findings and, in addition to UCD, now includes a set of disorders with multicentric lymphadenopathy, termed multicentric CD (MCD). A subset of MCD cases is caused by uncontrolled human herpesvirus-8 (HHV8) infection (HHV8-associated MCD), most often seen among individuals infected with HIV or who are otherwise immunocompromised.2 Among the MCD cases negative for HHV8, a small proportion are associated with polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS-associated MCD).2 The remaining HHV8-negative MCD cases have an unknown etiology and are termed idiopathic MCD (iMCD).2
Diagnosis of iMCD requires ≥2 enlarged LNs (≥1 centimeter in short-axis diameter), LN histopathological features consistent with CD, and at least 2 of 11 minor diagnostic criteria, with at least 1 being a laboratory abnormality.3 Histopathologic features consistent with CD are observed across a spectrum and include regressed or hypertrophic germinal centers, follicular dendritic cell prominence, vascularity, and plasmacytosis. Clinicopathologic findings are nonspecific to iMCD, and without a definitive diagnostic biomarker, iMCD remains a diagnosis of exclusion. This results in a substantial risk of misdiagnosing patients with closely overlapping disorders and heightens the need for clinicopathological recognition.
Among patients meeting iMCD criteria, several distinct clinical phenotypes have been reported and recognized. The most severe phenotype of iMCD includes patients who have thrombocytopenia, anasarca, fever/inflammation, renal failure/reticulin fibrosis of bone marrow, and organomegaly (TAFRO).4,5 These patients tend to experience the most intense periods of active disease, characterized by prolonged hospitalizations and invasive health care interventions due to multiorgan failure, separated by periods of disease remission after effective treatment.6 A second phenotype of patients present with thrombocytosis and hypergammaglobulinemia. These patients were described as early as 1980 and have been labeled as having the idiopathic plasmacytic lymphadenopathy (IPL) subtype.7-9 Patients with iMCD who do not meet the criteria of either of these phenotypes have a phenotype that is not otherwise specified (NOS).10,11 Patients with IPL and NOS subtypes tend to have fewer hospitalizations and interventions than those with TAFRO, but often experience a longer lower-grade disease flare.6
The etiology of iMCD is currently unknown, and it is not clear whether etiology differs between the 3 clinical phenotypes. Several causes for iMCD have been proposed, including autoimmune/autoinflammatory, infectious, and neoplastic origins.11-13 Current research suggests the cause is unlikely to be infectious and ongoing investigations into alternative hypotheses are underway; however, no single cause has yet been proven.13,14 Regardless of the trigger, the systemic symptoms, generalized lymphadenopathy, and multiorgan dysfunction of iMCD are driven by a cytokine storm that often includes interleukin 6 (IL-6). Indeed inhibition of IL-6 with the monoclonal antibody siltuximab is the only approved therapy for iMCD.15-17 All patients with iMCD are recommended to receive siltuximab, or, when siltuximab is unavailable, tocilizumab, which targets the IL-6 receptor.18 Severity of disease and response to anti–IL-6 therapy dictate subsequent treatment recommendations.18
As with iMCD, the etiology of UCD is not well understood, although a clonal expansion of LN nonhematopoietic stromal cells has been implicated.2,19 Diagnosis requires a solitary enlarged LN region with CD histopathology and exclusion of overlapping disorders; no laboratory or clinical abnormalities are required to diagnose UCD. Surgical resection is recommended and highly effective when feasible.20 For cases for which resection is not feasible, treatment recommendations are based on the site of involvement, the degree of compression of adjacent structures, and the presence of systemic inflammation. Patients with UCD typically experience fewer and less severe symptoms than iMCD. However, some patients with UCD can have an inflammatory syndrome. A recent report highlighted that a subset of patients with UCD experience inflammatory symptoms at presentation, and ongoing symptoms can persist in some patients, even after complete LN excision.21,22
CD has historically been thought of as either occurring with a single region of lymphadenopathy and mild symptoms (UCD) or many regions of lymphadenopathy and severe symptoms (iMCD). However, anecdotally, some patients do not fit into this framework and rather show CD histopathology and a clinical phenotype that falls between that of UCD and iMCD.20 Case reports and anecdotal accounts describe these patients as having >1 region of lymphadenopathy, which is often adjacent or regionally limited and milder systemic symptoms than patients with iMCD.20,23-26 Given the differences in natural history, recommended treatment approaches for iMCD may not be appropriate for these patients. As such, an international consensus group defining diagnostic and treatment guidelines for UCD provided similar recommendations for treating this intermediate phenotype with surgical debulking and removal, when possible, over systemic therapies such as IL-6–blocking antibodies or cytotoxic chemotherapies.20 These intermediate CD cases have not been characterized comprehensively and are currently not included as a recognized CD subtype, which may lead to misdiagnosis and patients who feel unrecognized.
Previous studies of CD have described the bifurcation of CD into UCD and MCD and have subtyped MCD based on presence or absence of HHV8 and POEMS syndrome.2,7,10,27,28 These classifications have helped to better inform treatment decisions, especially for clear cases of UCD, iMCD, HHV8-associated MCD, and POEMS-associated MCD. However, there remain large knowledge gaps among borderline cases of CD subtypes with unknown etiology, and filling these gaps remains crucial to improving treatment and outcomes. Herein, we draw on the ACCELERATE natural history registry of CD29 to report the phenotypic continuum of CD among cases with no clearly identifiable etiology, namely UCD, iMCD, and undefined CD cases. We describe cases with an undefined phenotype as “oligocentric” CD (OligoCD) and provide data on disease characteristics and treatment patterns.
Methods
Patients
Patients of all ages from the United States and globally and who have ever received a pathologic diagnosis of CD were invited to self-enroll into the ACCELERATE natural history registry online. All patients consented to the research and provided Health Insurance Portability and Accountability Act waivers for collection of complete medical data. This research protocol was approved by the University of Pennsylvania institutional review board.
Procedures and definitions
Patients enrolled into ACCELERATE between October 2016 and April 2023. After enrollment, complete medical records were collected and abstracted by trained research analysts; hematoxylin and eosin–stained LN slides were also collected and made available for review. A panel of 4 clinicians and 3 hematopathologists with expertise in the CD field reviewed and adjudicated each case for the likelihood of accurate CD diagnosis. At the time of analysis, 343 cases had been enrolled, extracted into the study database, and were available for inclusion in analysis. Cases of confirmed HHV8-associated MCD (n = 12) and POEMS-associated MCD (n = 10) were excluded from the analysis. Seventeen cases that were missing diagnostic radiologic data were also excluded.
To describe the spectrum of CD, we categorized all cases as either probable UCD, probable iMCD, or probable CD of undefined subtype before panel review. Probable UCD was defined as patients with a solitary enlarged LN region and a pathology report compatible with CD.20 Probable iMCD was defined as patients with ≥2 enlarged LN regions, 2 of 11 minor diagnostic criteria with at least 1 abnormal laboratory parameter, and a pathology report compatible with CD.3 Probable CD of undefined subtype (CD-undefined) was defined as all patients with a pathology report compatible with CD and neither met the probable UCD nor or probable iMCD categories. Panel review confirmed or rejected diagnosis with CD and identified the respective histopathological subtype. For 9 cases, a hematoxylin and eosin–stained slide was not available for review, but the panel was able to confirm a diagnosis by review of the initial diagnostic pathology report, and histopathologic subtype was imputed from the diagnostic pathology report. Patients with iMCD were also subcategorized according to phenotypic subtypes TAFRO, IPL, and NOS. Criteria and categorizations for CD subtypes and phenotypes can be found in supplemental Table 1, and the number of cases that met criteria for TAFRO and IPL can be found in supplemental Table 2.
Inflammatory syndrome was defined as at least 2 of 3 occurring simultaneously within at least 90 days of diagnosis: anemia; hypoalbuminemia; and inflammation, defined as hemoglobin of <11.5 g/dL (males) or <10.5 g/dL (females), albumin of <3.5 g/dL; and either C-reactive protein (CRP) of >20 mg/dL or erythrocyte sedimentation rate of >30 mm/h, respectively. Regimens were defined according to treatment start dates. Response assessment was determined according to the change in the proportion of symptoms present before and after a given regimen was initiated. A durable response was recorded if there was at least 50% improvement in the proportion of symptoms present after regimen initiation and that response lasted at least 1 year. For a patient who received a given regimen more than once, the best response ever achieved was documented. Lymph node response was defined as at least 50% decrease in the short axis measurement of the enlarged LN or 50% reduction in the number of enlarged LNs.
A blinded radiological review was undertaken to investigate differences in the distribution and size of enlarged LNs between patients who were panel confirmed and patients who were not panel confirmed. Among the ACCELERATE cohort, there were 108 patients with available radiological images (including computed tomography or positron emission tomography with or without contrast) for review by a blinded radiologist. For each image, the radiologist reviewed and extracted the number of enlarged LNs (short axis of >1 cm) per location (according to the Ann Arbor LN staging system30) and recorded the size of the largest LN. From these data, we compared those patients who were probable iMCD and panel confirmed (n = 53) and those who were probable iMCD and not panel confirmed (n = 22).
Quantification and statistical analysis
Data are primarily presented descriptively. Comparisons between groups were performed by the χ2 or Fisher exact test for categorical data and by Wilcoxon rank-sum test for quantitative data. Bonferroni adjustment was made for multiple comparisons. Three-way group testing was done by Kruskal-Wallis with a post hoc Dunn test. Data analysis was performed by S.K.P. using R version 4.0.5.
Results
Richly characterized cohort of patients with CD reveals a continuous spectrum of clinical phenotypes
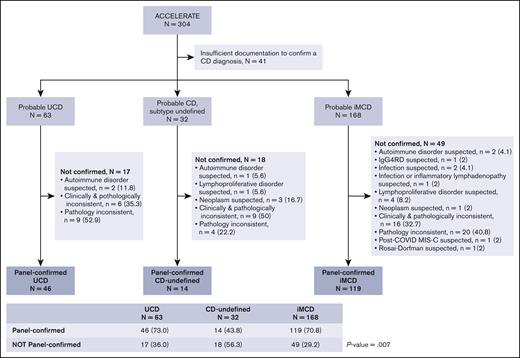
To assemble a cohort of clinically annotated CD cases, we accessed the ACCELERATE natural history registry of CD,29 in which patients with a pathology report suggestive of CD self-enrolled into ACCELERATE. At the time of analysis, 304 cases met criteria and underwent study-team preliminary review and assessment of radiological and medical data for categorization by probable subtype (as defined in “Methods”) based on meeting diagnostic criteria for UCD, iMCD, or neither (Figure 1). Forty-one cases had insufficient documentation to determine a probable subtype, 63 cases were probable UCD, 168 probable iMCD, and 32 probable CD-undefined (Figure 1). Forty-six of the 63 probable UCD cases (73%), 119 of the 168 probable iMCD cases (70.8%), and 14 of the 32 probable CD-undefined (43.8%) were confirmed by the expert panel. Cases whose CD diagnosis was panel confirmed were significantly more likely to be classified as UCD or iMCD than as CD-undefined (P = .007). Of note, all 14 of these CD-undefined cases have received care at an academic-affiliated hospital at least once in their follow-up.
A large and richly annotated cohort of patients with CD reveals a small subset of cases with a form of CD that does not meet UCD or iMCD criteria. A total of 304 cases with sufficient diagnostic radiologic data underwent preliminary review for categorization by probable subtype. Forty-one cases had insufficient documentation to undergo panel review. The remaining cases were categorized probable UCD (n = 63), probable iMCD (n = 168), and probable CD with an undefined subtype (n = 32). After expert panel review for diagnosis adjudication, 46 UCD, 119 iMCD, and 14 CD-undefined cases were panel confirmed. Cases whose CD diagnosis was panel confirmed were significantly more likely to be classified as UCD or iMCD than as CD-undefined (P = .007). IgG4RD, IgG4 related disease; MIS-C, multisystem inflammatory syndrome in children.
A large and richly annotated cohort of patients with CD reveals a small subset of cases with a form of CD that does not meet UCD or iMCD criteria. A total of 304 cases with sufficient diagnostic radiologic data underwent preliminary review for categorization by probable subtype. Forty-one cases had insufficient documentation to undergo panel review. The remaining cases were categorized probable UCD (n = 63), probable iMCD (n = 168), and probable CD with an undefined subtype (n = 32). After expert panel review for diagnosis adjudication, 46 UCD, 119 iMCD, and 14 CD-undefined cases were panel confirmed. Cases whose CD diagnosis was panel confirmed were significantly more likely to be classified as UCD or iMCD than as CD-undefined (P = .007). IgG4RD, IgG4 related disease; MIS-C, multisystem inflammatory syndrome in children.
For both probable UCD and probable iMCD, the most frequent reason for not meeting panel confirmation was pathologic inconsistency (52.9% and 40.8%, respectively). However, for probable CD of undefined subtype, the most frequent reason for not meeting panel confirmation was lack of overall consistency with CD (defined as being clinically and pathologically inconsistent with CD) in 50%. When feasible, the panel identified suspected alternative diagnoses for patients not confirmed (Figure 1). In-depth radiological review performed on a subset of patients with probable iMCD for whom imaging was available (n = 75) failed to reveal any clear differences in the distribution and size of enlarged LNs between 53 patients with iMCD that were panel confirmed and 22 that were not confirmed (supplemental Figure 1). Demographic and diagnostic characteristics of the unconfirmed patients can be found in supplemental Table 3, and treatment patterns can be found in supplemental Table 4. Interestingly, the unconfirmed iMCD group was significantly older than the confirmed iMCD group (mean, 47.7 vs 36.0 years; P = 8.9 × 10−5) and had a significantly shorter follow-up time (median, 1.2 vs 2.9 years; P = .007). Three deaths occurred, all of which were in the unconfirmed iMCD group (3/49; 6.1%). Among unconfirmed iMCD cases, 11 had been suspected TAFRO, 14 suspected IPL, and 24 suspected NOS. Of note, 5 of 7 (71.4%) patients with unconfirmed iMCD who were suspected to have IPL responded to siltuximab (supplemental Table 4).
Heterogeneous characteristics observed between CD subtypes and among iMCD phenotypes
After identifying a cohort of panel-confirmed cases for each subtype, we investigated characteristics and relationships between the confirmed subtypes. Mean (standard deviation) age at diagnosis of patients with UCD was 41.4 (12.5) years, 34.0 (16.1) years for CD-undefined, and 36.0 (15.8) years for iMCD cases (Table 1). Continuous distribution in age between iMCD, UCD, and CD-undefined was not different (P = .084). Exploratory post hoc pairwise testing was performed given the small sample and showed no difference between iMCD and CD-undefined (P = .46) or between UCD and CD-undefined (P = .09), but there was a difference between iMCD and UCD (P = .01) (supplemental Figure 2A). Nine (7.6%) patients with iMCD had died at the time of analysis (5 occurring in the first year after diagnosis), compared with 1 (2.2%) UCD and 0 CD-undefined patients. Median survival time could not be determined because of the small number of deaths. iMCD cases were evenly distributed between males (n = 59; 49.6%) and females (n = 60; 50.4%), whereas both CD-undefined and UCD cases were approximately two-thirds female (71.4% and 67.4%, respectively). Hypervascular/hyaline vascular histopathology predominated among all 3 subtypes. Mixed (n = 31; 26.1%) and plasmacytic (n = 8; 6.7%) were more prevalent in iMCD compared with the other categories, but still relatively uncommon. According to our definition and among those with sufficient data, inflammatory syndrome was noted among a minority of patients with UCD (n = 7; 25.9% among those with sufficient data) and not noted among patients with CD-undefined (n = 0; 0%). Eighteen (17.6%) patients with iMCD and sufficient data did not meet these criteria for an inflammatory syndrome, which is a more stringent definition than the iMCD minor diagnostic criteria; 17 did not have sufficient information to confirm inflammation.
Demographics and baseline characteristics of confirmed CD cases
| . | iMCD n = 119 . | CD-undefined n = 14 . | UCD n = 46 . |
|---|---|---|---|
| Age, mean (SD), y | 36.0 (15.8) | 34.0 (16.1) | 41.4 (12.5) |
| <18, n (%) | 21 (18.1) | 2 (14.3) | 2 (4.3) |
| Deceased, n (%) | 9 (7.6) | 0 | 1 (2.2) |
| Sex, n (%) | |||
| Female | 60 (50.4) | 10 (71.4) | 31 (67.4) |
| Male | 59 (49.6) | 4 (28.6) | 15 (32.6) |
| Race, n (%) | |||
| American Indian/Alaska Native | 1 (0.8) | 0 | 0 |
| Asian | 14 (11.8) | 0 | 2 (4.3) |
| Black | 12 (10.1) | 0 | 1 (2.2) |
| Native Hawaiian/Pacific Islander | 1 (0.8) | 0 | 0 |
| White | 80 (67.2) | 14 (100) | 42 (91.3) |
| Not provided | 11 (9.2) | 0 | 1 (2.2) |
| Histopathological subtype, n (%) | |||
| Hyaline vascular/hypervascular | 80 (67.2) | 11 (78.6) | 40 (87) |
| Mixed | 31 (26.1) | 3 (21.4) | 6 (13) |
| Plasmacytic | 8 (6.7) | 0 | 0 |
| Assessed number of minor criteria, median (IQR) | 11 (10-11) | 7 (6.3-10) | 9 (8-10) |
| Abnormal number of minor criteria, median (IQR) | 7 (5-8) | 1 (0-1) | 2 (1-3) |
| Inflammatory syndrome,∗n (%) | |||
| Present | 84 (82.3) | 0 | 7 (25.9) |
| Absent | 18 (17.6) | 7 (100) | 20 (74.1) |
| Insufficient informatoin | 17 | 7 | 19 |
| No. of enlarged node stations, median (IQR) | 8.0 (5.0-10.5) | 3.0 (2.0-4.0) | 1.0 (1.0-1.0) |
| Follow-up time, median (IQR), y | 2.9 (1.2-5.2) | 2.7 (1.1-5.7) | 1.3 (0.4-4.3) |
| . | iMCD n = 119 . | CD-undefined n = 14 . | UCD n = 46 . |
|---|---|---|---|
| Age, mean (SD), y | 36.0 (15.8) | 34.0 (16.1) | 41.4 (12.5) |
| <18, n (%) | 21 (18.1) | 2 (14.3) | 2 (4.3) |
| Deceased, n (%) | 9 (7.6) | 0 | 1 (2.2) |
| Sex, n (%) | |||
| Female | 60 (50.4) | 10 (71.4) | 31 (67.4) |
| Male | 59 (49.6) | 4 (28.6) | 15 (32.6) |
| Race, n (%) | |||
| American Indian/Alaska Native | 1 (0.8) | 0 | 0 |
| Asian | 14 (11.8) | 0 | 2 (4.3) |
| Black | 12 (10.1) | 0 | 1 (2.2) |
| Native Hawaiian/Pacific Islander | 1 (0.8) | 0 | 0 |
| White | 80 (67.2) | 14 (100) | 42 (91.3) |
| Not provided | 11 (9.2) | 0 | 1 (2.2) |
| Histopathological subtype, n (%) | |||
| Hyaline vascular/hypervascular | 80 (67.2) | 11 (78.6) | 40 (87) |
| Mixed | 31 (26.1) | 3 (21.4) | 6 (13) |
| Plasmacytic | 8 (6.7) | 0 | 0 |
| Assessed number of minor criteria, median (IQR) | 11 (10-11) | 7 (6.3-10) | 9 (8-10) |
| Abnormal number of minor criteria, median (IQR) | 7 (5-8) | 1 (0-1) | 2 (1-3) |
| Inflammatory syndrome,∗n (%) | |||
| Present | 84 (82.3) | 0 | 7 (25.9) |
| Absent | 18 (17.6) | 7 (100) | 20 (74.1) |
| Insufficient informatoin | 17 | 7 | 19 |
| No. of enlarged node stations, median (IQR) | 8.0 (5.0-10.5) | 3.0 (2.0-4.0) | 1.0 (1.0-1.0) |
| Follow-up time, median (IQR), y | 2.9 (1.2-5.2) | 2.7 (1.1-5.7) | 1.3 (0.4-4.3) |
ESR, erythrocyte sedimentation rate; SD, standard deviation.
Inflammatory syndrome was defined as at least 2 of 3 parameters occurring simultaneously within at least 90 days of diagnosis: anemia, hypoalbuminemia, and inflammation, defined as hemoglobin of <11.5 g/dL (males) or <10.5 g/dL (females), albumin of <3.5 g/dL, and either CRP of >20 mg/dL or ESR of >30 mm/h, respectively. Insufficient information reflects cases for which hemoglobin, albumin, and CRP/ESR were not measured or were not measured concurrently and therefore there was not sufficient information to assign inflammatory syndrome.
As part of our investigation into the CD spectrum, we further characterized the 3 clinical phenotypes of patients meeting iMCD criteria: TAFRO, IPL, and NOS (Table 2; supplemental Table 5). Sixty-five (54.6%) patients met TAFRO and 12 (10.1%) met IPL criteria, with 42 (35.3%) defined as NOS. Interestingly, the mean age of patients with IPL was 44 years, compared with 33.5 years for TAFRO and 37.6 years for NOS, and most patients with iMCD diagnosed at age <18 years had the TAFRO subtype (17/21, 81%). The age distribution (supplemental Figure 2B) between TAFRO, IPL, and NOS was not significantly different (P = .051). However, because evidence for a difference was weak but marginally nonsignificant, we performed a post hoc pairwise comparison to examine possible differences between groups. There was no difference between IPL and NOS (P = .10) or between TAFRO and NOS (P = .07), but comparison between TAFRO and IPL showed a difference (P = .01). These results demonstrate a trend toward TAFRO being diagnosed in younger patients; a larger sample may have achieved significance. Of the 9 patients with iMCD who died, 6 (66.7%) had TAFRO and 3 (33.3%) had NOS disease. No patients with IPL in this cohort had died at the time of analysis. Patients with TAFRO were predominantly male (n = 40; 61.5%), whereas those with IPL and NOS were predominantly female (n = 8; 66.7% and n = 27; 64.3% female, respectively). All histopathologic variants were found among patients with IPL, but the plasmacytic variant was the most common (n = 8; 66.7%). Although evaluation of the IPL group was limited by small numbers, patients with TAFRO and IPL tended to demonstrate more laboratory abnormalities and more clinical symptomatology. As expected, there were differences in the proportion of patients within each subgroup demonstrating abnormal clinical and laboratory tests (supplemental Table 5), such as thrombocytopenia (P = 1.6 × 10−14), thrombocytosis (P = 1.1 × 10−5), elevated CRP (P = .002), low hemoglobin (P = 2.1 × 10−6), elevated creatinine (P = 3.1 × 10−5), low estimated glomerular filtration rate (P = .001), elevated γ-globulin (P = 5.4 × 10−4), and elevated immunoglobulin G (P = 9.0 × 10−7). Notably, a higher proportion of patients with TAFRO and IPL exhibited elevated CRP and low hemoglobin compared with those with NOS disease, whereas patients with TAFRO had higher creatinine and lower estimated glomerular filtration rate values than both NOS and IPL, and patients with IPL showed elevated γ-globulins compared with both patients with NOS and TAFRO.
Demographic, clinical, and laboratory features of iMCD phenotypes
| . | TAFRO n = 65 . | NOS n = 42 . | IPL n = 12 . |
|---|---|---|---|
| Age, mean (SD), y | 33.5 (17.8) | 37.6 (12.8) | 44.0 (10.4) |
| <18, n (%) | 17 (26.2) | 4 (9.5) | 0 (0) |
| Deceased, n (%) | 6 (9.2) | 3 (7.1) | 0 (0) |
| Sex, n (%) | |||
| Female | 25 (38.5) | 27 (64.3) | 8 (66.7) |
| Male | 40 (61.5) | 15 (35.7) | 4 (33.3) |
| Race, n (%) | |||
| American Indian/Alaska Native | 0 (0) | 0 (0) | 1 (8.3) |
| Asian | 7 (10.8) | 1 (2.4) | 6 (50) |
| Black | 6 (9.2) | 6 (14.3) | 0 (0) |
| Native Hawaiian/Pacific Islander | 1 (1.5) | 0 (0) | 0 (0) |
| White | 47 (72.3) | 29 (69) | 4 (33.3) |
| Not provided | 4 (6.2) | 6 (14.3) | 1 (8.3) |
| Histopathological subtype, n (%) | |||
| Hyaline vascular/hypervascular | 46 (70.8) | 33 (78.6) | 1 (8.3) |
| Mixed | 19 (29.2) | 9 (21.4) | 3 (25) |
| Plasmacytic | 0 (0) | 0 (0) | 8 (66.7) |
| Inflammatory disease, n (%) | |||
| Present | 63 (100) | 13 (44.8) | 8 (80) |
| Absent | 0 (0) | 16 (55.1) | 2 (20) |
| Insufficient information | 2 | 13 | 2 |
| Follow-up time, median (IQR), y | 3.3 (1.3-5.0) | 2.0 (1.0-4.6) | 5.7 (1.9-13.6) |
| Clinical symptoms, n/assessed (%) | |||
| Constitutional symptoms | 63/65 (96.9) | 37/41 (90.2) | 11/12 (91.7) |
| Organomegaly | 59/65 (90.8) | 16/40 (40) | 5/12 (41.7) |
| Cherry hemangioma/violaceous papules | 4/56 (7.1) | 0 (0) | 0 (0) |
| Lymphocytic interstitial pneumonitis | 0 (0) | 0 (0) | 0 (0) |
| Fluid retention | 64/65 (98.5) | 23/40 (57.5) | 6/11 (54.5) |
| Platelets, median (IQR), ×103/μL | 83 (51-136) | 283 (219-348) | 451 (373-489) |
| CRP, median (IQR), mg/L | 80.5 (22.7-185.0) | 14.0 (5.0-55.0) | 114.0 (73.0-165.1) |
| ESR, median (IQR), mm/h | 78 (47.5-109.3) | 43 (22-72.0) | 73 (32.5-113.5) |
| Hemoglobin, median (IQR), g/dL | 9.2 (8.0-10.9) | 11.9 (10.8-13.4) | 9.9 (8.0-11.3) |
| Albumin, median (IQR), g/dL | 2.5 (2.1-2.9) | 3.9 (3.3-4.3) | 2.8 (2.5-3.6) |
| Creatinine, median (IQR), mg/dL | 1.3 (1.0-1.8) | 0.8 (0.6-1.0) | 0.8 (0.7-1.0) |
| eGFR, mL/min per 1.73 m2 | |||
| ≤60, n (%) | 32 (68.1) | 18 (56.3) | 5 (62.5) |
| >60, n (%) | 15 (31.9) | 14 (43.8) | 3 (37.5) |
| Not documented, n | 18 | 10 | 4 |
| IgG, median (IQR), mg/dL | 985 (736-1528) | 1137 (988-1264) | 4270 (3190-6178) |
| γ-Globulin, median (IQR), g/dL | 1.1 (0.8-1.7) | 1.2 (0.9-1.5) | 4.4 (4.1-5.9) |
| . | TAFRO n = 65 . | NOS n = 42 . | IPL n = 12 . |
|---|---|---|---|
| Age, mean (SD), y | 33.5 (17.8) | 37.6 (12.8) | 44.0 (10.4) |
| <18, n (%) | 17 (26.2) | 4 (9.5) | 0 (0) |
| Deceased, n (%) | 6 (9.2) | 3 (7.1) | 0 (0) |
| Sex, n (%) | |||
| Female | 25 (38.5) | 27 (64.3) | 8 (66.7) |
| Male | 40 (61.5) | 15 (35.7) | 4 (33.3) |
| Race, n (%) | |||
| American Indian/Alaska Native | 0 (0) | 0 (0) | 1 (8.3) |
| Asian | 7 (10.8) | 1 (2.4) | 6 (50) |
| Black | 6 (9.2) | 6 (14.3) | 0 (0) |
| Native Hawaiian/Pacific Islander | 1 (1.5) | 0 (0) | 0 (0) |
| White | 47 (72.3) | 29 (69) | 4 (33.3) |
| Not provided | 4 (6.2) | 6 (14.3) | 1 (8.3) |
| Histopathological subtype, n (%) | |||
| Hyaline vascular/hypervascular | 46 (70.8) | 33 (78.6) | 1 (8.3) |
| Mixed | 19 (29.2) | 9 (21.4) | 3 (25) |
| Plasmacytic | 0 (0) | 0 (0) | 8 (66.7) |
| Inflammatory disease, n (%) | |||
| Present | 63 (100) | 13 (44.8) | 8 (80) |
| Absent | 0 (0) | 16 (55.1) | 2 (20) |
| Insufficient information | 2 | 13 | 2 |
| Follow-up time, median (IQR), y | 3.3 (1.3-5.0) | 2.0 (1.0-4.6) | 5.7 (1.9-13.6) |
| Clinical symptoms, n/assessed (%) | |||
| Constitutional symptoms | 63/65 (96.9) | 37/41 (90.2) | 11/12 (91.7) |
| Organomegaly | 59/65 (90.8) | 16/40 (40) | 5/12 (41.7) |
| Cherry hemangioma/violaceous papules | 4/56 (7.1) | 0 (0) | 0 (0) |
| Lymphocytic interstitial pneumonitis | 0 (0) | 0 (0) | 0 (0) |
| Fluid retention | 64/65 (98.5) | 23/40 (57.5) | 6/11 (54.5) |
| Platelets, median (IQR), ×103/μL | 83 (51-136) | 283 (219-348) | 451 (373-489) |
| CRP, median (IQR), mg/L | 80.5 (22.7-185.0) | 14.0 (5.0-55.0) | 114.0 (73.0-165.1) |
| ESR, median (IQR), mm/h | 78 (47.5-109.3) | 43 (22-72.0) | 73 (32.5-113.5) |
| Hemoglobin, median (IQR), g/dL | 9.2 (8.0-10.9) | 11.9 (10.8-13.4) | 9.9 (8.0-11.3) |
| Albumin, median (IQR), g/dL | 2.5 (2.1-2.9) | 3.9 (3.3-4.3) | 2.8 (2.5-3.6) |
| Creatinine, median (IQR), mg/dL | 1.3 (1.0-1.8) | 0.8 (0.6-1.0) | 0.8 (0.7-1.0) |
| eGFR, mL/min per 1.73 m2 | |||
| ≤60, n (%) | 32 (68.1) | 18 (56.3) | 5 (62.5) |
| >60, n (%) | 15 (31.9) | 14 (43.8) | 3 (37.5) |
| Not documented, n | 18 | 10 | 4 |
| IgG, median (IQR), mg/dL | 985 (736-1528) | 1137 (988-1264) | 4270 (3190-6178) |
| γ-Globulin, median (IQR), g/dL | 1.1 (0.8-1.7) | 1.2 (0.9-1.5) | 4.4 (4.1-5.9) |
ESR, erythrocyte sedimentation rate; eGFR, estimated glomerular filtration rate; IgG, immunoglobulin G; SD, standard deviation.
Patients with CD-undefined tend to demonstrate oligocentric lymphadenopathy
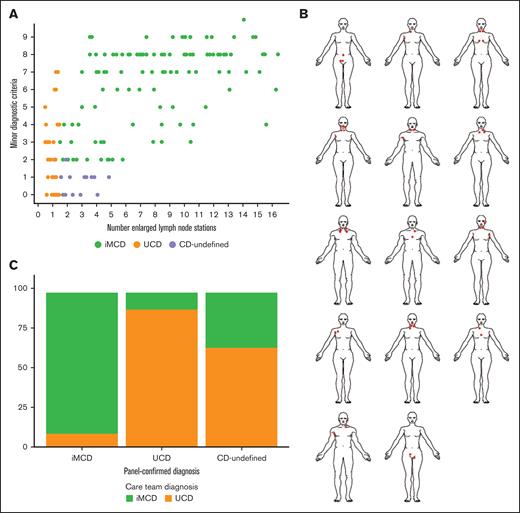
To characterize the range of lymphadenopathy and clinical symptoms across CD, we plotted the number of documented minor diagnostic criteria against the number of enlarged LN stations and visualized patterns by subtype (Figure 2A). As per definition, patients with UCD had 1 station of enlarged LNs, and the number of minor diagnostic criteria ranged from 0 to 7. Patients with iMCD demonstrated a range between 2 and 16 enlarged LN stations (median [interquartile range (IQR)]: 8.0 [5.0-10.5]) and between 2 and 10 minor diagnostic criteria. The 14 CD-undefined cases demonstrated between 2 and 5 enlarged LN stations (median [IQR]: 3.0 [2.0-4.0]) and 13 had only 1 minor diagnostic criterion. The apparent difference between CD-undefined and UCD is expected given the cohort definitions, but the apparent difference between CD-undefined and iMCD is noteworthy. One CD-undefined case had 2 minor clinical criteria but did not meet the iMCD criteria because the patient did not have at least 1 laboratory abnormality.
Investigation of involved LN stations reveals that patients with an undefined subtype demonstrate oligocentricity. (A) The relationship between the number of enlarged LN stations plotted against the number of minor diagnostic criteria. Patients with CD-undefined can be visualized separately from both UCD and iMCD. (B) Most of CD-undefined cases had ≤4 enlarged LNs in the same general region. These patients demonstrate oligocentric lymphadenopathy and henceforth will be referred to as OligoCD. (C) Cases that were panel-confirmed iMCD were typically diagnosed iMCD by their treating physician (n = 109; 91.6%), and cases that were panel-confirmed UCD were typically diagnosed UCD by their treating physician (n = 41; 89.1%). Patients with CD-undefined were diagnosed as UCD in 64.3% of cases (n = 9), and iMCD in the remaining 35.7% (n = 5).
Investigation of involved LN stations reveals that patients with an undefined subtype demonstrate oligocentricity. (A) The relationship between the number of enlarged LN stations plotted against the number of minor diagnostic criteria. Patients with CD-undefined can be visualized separately from both UCD and iMCD. (B) Most of CD-undefined cases had ≤4 enlarged LNs in the same general region. These patients demonstrate oligocentric lymphadenopathy and henceforth will be referred to as OligoCD. (C) Cases that were panel-confirmed iMCD were typically diagnosed iMCD by their treating physician (n = 109; 91.6%), and cases that were panel-confirmed UCD were typically diagnosed UCD by their treating physician (n = 41; 89.1%). Patients with CD-undefined were diagnosed as UCD in 64.3% of cases (n = 9), and iMCD in the remaining 35.7% (n = 5).
The “oligocentric” pattern with a few enlarged LNs observed in these CD-undefined cases was consistent with anecdotal clinical descriptions.23-25 These cases have also been described as “regional” because they tend to have adjacent lymphadenopathy. When we examined the location of enlarged LNs for each of the 14 CD-undefined cases (Figure 2B), we found the enlarged nodes tended to fall within adjacent or nearby LN stations. Notably, in every case, the enlarged LN stations occurred on the same side of the diaphragm. Twelve cases involved enlarged LNs that were located superiorly to the diaphragm, and 2 cases involved enlarged LNs that were located inferiorly to the diaphragm.
We also examined which subtype had been assigned to the patients with CD-undefined by their treating physicians according to documentation in patient medical records. Among the 119 patients with panel-confirmed iMCD, 91.6% (n = 109) had been diagnosed with iMCD by their treating physician and 8.4% (n = 10) diagnosed with UCD. Among the 46 patients with panel-confirmed UCD, 89.1% (n = 41) had been diagnosed with UCD by their treating physician and 10.9% (n = 5) with iMCD. Among the patients with 14 CD-undefined, 64.3% (n = 9) had been diagnosed with UCD by their treating physician and 35.7% (n = 5) diagnosed with iMCD. These data suggest greater discordance among patients with an undefined subtype (Figure 2C) and occasional ambiguity among UCD and iMCD despite diagnostic criteria. Given the oligocentric lymphadenopathy observed, we suggest the term “oligocentric CD” to describe this undefined subtype that does not meet UCD or iMCD criteria, and we will henceforth refer to the previously undefined cases as having OligoCD.
OligoCD shares clinical resemblance to UCD
Next, we sought to determine whether OligoCD shares a greater clinical resemblance to UCD or iMCD. We compared diagnostic clinical, histopathological, and laboratory features between iMCD, UCD, and OligoCD cases and found OligoCD cases exhibited a clinical phenotype more similar to UCD than to iMCD (Figure 3A-C). iMCD and OligoCD had significantly different albumin, creatinine, CRP, and hemoglobin values (P <.05), but UCD and OligoCD showed no significant differences with respect to these laboratory values (Figure 3B). We investigated whether patients with OligoCD had elevated IL-6 levels, but only 2 patients had IL-6 measured outside of a siltuximab treatment interval. One showed IL-6 within normal range, and 1 showed IL-6 at a level exceeding the reference interval of 5 pg/mL by nearly 8 times. Patients with OligoCD demonstrated relatively few clinical abnormalities, which was more similar to UCD. Conclusions from histopathology are difficult given that all 3 subtypes were predominantly hypervascular/hyaline vascular. Altogether, we note comparable laboratory and clinical findings between UCD and OligoCD, suggesting that OligoCD belongs on a spectrum of CD that includes UCD, OligoCD, and the 3 iMCD clinical phenotypes: TAFRO, IPL, and NOS (Figure 4).
Disease features of OligoCD more closely resemble UCD than iMCD. OligoCD demonstrates features that are more similar to UCD than to iMCD. (A) Clinical, (B) laboratory, and (C) histopathologic features of UCD, iMCD, and OligoCD cases demonstrate stronger similarities between UCD and OligoCD. Significance: ns, P > .05; ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗P ≤ .0001. HV, hyaline vascular; hyperV, hypervascular; ns, not significant.
Disease features of OligoCD more closely resemble UCD than iMCD. OligoCD demonstrates features that are more similar to UCD than to iMCD. (A) Clinical, (B) laboratory, and (C) histopathologic features of UCD, iMCD, and OligoCD cases demonstrate stronger similarities between UCD and OligoCD. Significance: ns, P > .05; ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗P ≤ .0001. HV, hyaline vascular; hyperV, hypervascular; ns, not significant.
A summary of common clinical, laboratory, and histopathological features on the spectrum of patients with UCD to those with OligoCD and with iMCD. Patients demonstrate differences in lymphadenopathy, histopathology, laboratory and clinical abnormalities, and degree of inflammation. The patients with the least severe disease typically have UCD, and the most severe cases are typically iMCD-TAFRO. Severity of symptoms along this spectrum should be considered when making a diagnosis. HV, hyaline vascular; hyperV, hypervascular; PC, plasmacytic.
A summary of common clinical, laboratory, and histopathological features on the spectrum of patients with UCD to those with OligoCD and with iMCD. Patients demonstrate differences in lymphadenopathy, histopathology, laboratory and clinical abnormalities, and degree of inflammation. The patients with the least severe disease typically have UCD, and the most severe cases are typically iMCD-TAFRO. Severity of symptoms along this spectrum should be considered when making a diagnosis. HV, hyaline vascular; hyperV, hypervascular; PC, plasmacytic.
Next, to compare clinical outcomes between groups we investigated the incidence of flares as well as the incidence of new nodal involvement beyond the diagnostic window (supplemental Table 6). We identified patients who experienced a flare or a resurgence of symptoms after having achieved at least a 50% reduction in symptoms from the initial diagnostic flare. Forty-eight (40.3%) patients with iMCD experienced a subsequent flare, compared with 5 (35.7%) with OligoCD and 9 (19.6%) with UCD (P = .04). Furthermore, we reviewed radiology reports to identify patients who subsequently developed emergent nodal involvement at sites not previously affected during the year after their diagnosis. Of 119 patients with iMCD, 35 (29.4%) had lymphadenopathy at newly involved nodal sites at least 1 year after diagnosis, compared with 5 (35.7%) of 14 patients with OligoCD and 5 (10.9%) of 46 patients with UCD (P = .03). These findings suggest that although patients with OligoCD are more clinically comparable with patients with UCD at presentation, they may behave more like iMCD with regard to flares of disease over time.
Data on differential treatment approaches for individual clinical phenotypes are limited
Lastly, we investigated treatment patterns of patients across the spectrum from UCD through iMCD. It is important to note that some patients may not receive treatment at any time despite waxing and waning disease, whereas others may need multiple rounds of different treatments for refractory or relapsing disease. Among UCD, OligoCD, and the 3 clinical phenotypes of iMCD (TAFRO, IPL, and NOS), we categorized best durable response (lasting at least 1 year; Table 3) and LN response (supplemental Table 7) to treatment for regimens that are either consensus recommended or commonly administered. Response is more likely to be unknown in patients with few or no clinical symptoms other than lymphadenopathy. Patients with UCD received a median (IQR) of 1 (1.0-2.0) treatment regimens, OligoCD received 2 (2.0-3.0), NOS received 3 (2.0-4.0), IPL received 4 (2.8-5.0), and TAFRO received 3 (2.0-4.0).
Treatment patterns and durable response among OligoCD and iMCD subtypes
| . | TAFRO n = 65 . | IPL n = 12 . | NOS n = 42 . | OligoCD n = 14 . | UCD n = 46 . |
|---|---|---|---|---|---|
| No. of regimens administered, median (IQR) | 3 (2-4) | 4 (2.8-5) | 3 (2-4) | 2 (2-3) | 1 (1-2) |
| LN excision (partial or complete) | |||||
| Ever received, n | 11 | 10 | 31 | 14 | 43 |
| Response evaluable, n | 10 | 9 | 25 | 6 | 19 |
| Response ratio (%) | 0/10 (0) | 0/9 (0) | 2/25 (8) | 1/6 (16.7) | 12/19 (63.2) |
| Response unknown, n | 1 | 1 | 6 | 8 | 24 |
| Steroid monotherapy | |||||
| Ever received, n | 27 | 6 | 14 | 2 | 6 |
| Response evaluable, n | 24 | 5 | 10 | 1 | 1 |
| Response ratio (%) | 0/24 (0) | 0/5 (0) | 2/10 (20) | 0/1 (0) | 0/1 (0) |
| Response unknown, n | 3 | 1 | 4 | 1 | 5 |
| Anti–IL-6 w/wo steroids∗ | |||||
| Ever received, n | 39 | 10 | 21 | 2 | 3 |
| Response evaluable, n | 36 | 10 | 16 | 2 | 3 |
| Response ratio (%) | 16/36 (44.4) | 6/10 (60) | 7/16 (43.8) | 1/2 (50) | 1/3 (33.3) |
| Response unknown, n | 3 | 0 | 5 | 0 | 0 |
| Siltuximab ± steroids | |||||
| Ever received, n | 28 | 8 | 20 | 2 | 3 |
| Response evaluable, n | 24 | 8 | 15 | 2 | 3 |
| Response ratio (%) | 11/24 (45.8) | 5/8 (62.5) | 6/15 (40) | 1/2 (50) | 1/3 (33.3) |
| Response unknown, n | 4 | 0 | 5 | 0 | 0 |
| Tocilizumab w/wo steroids | |||||
| Ever received, n | 15 | 4 | 1 | 1 | 0 |
| Response evaluable, n | 14 | 3 | 1 | 1 | 0 |
| Response ratio (%) | 5/14 (35.7) | 2/3 (66.7) | 1/1 (100) | 0/1 (0) | 0 |
| Response unknown, n | 1 | 1 | 0 | 0 | 0 |
| Rituximab w/wo steroids | |||||
| Ever received, n | 12 | 6 | 21 | 4 | 5 |
| Response evaluable, n | 11 | 6 | 12 | 3 | 4 |
| Response ratio (%) | 3/11 (27.3) | 0/6 (0) | 3/12 (25) | 0/3 (0) | 1/4 (25) |
| Response unknown, n | 1 | 0 | 9 | 1 | 1 |
| Chemotherapy w/wo other agents | |||||
| Ever received, n | 29 | 0 | 3 | 2 | 2 |
| Response evaluable, n | 23 | 0 | 2 | 2 | 2 |
| Response ratio (%) | 11/23 (47.8) | 0 | 1/2 (50) | 0/2 (0) | 0/2 (0) |
| Response unknown, n | 6 | 0 | 1 | 0 | 0 |
| Other | |||||
| Ever received, n | 37 | 4 | 10 | 2 | 7 |
| Response evaluable, n | 33 | 4 | 9 | 2 | 4 |
| Response ratio (%) | 14/33 (42.4) | 0/4 (0) | 3/9 (33.3) | 1/2 (50.0) | 2/4 (50) |
| Response unknown, n | 4 | 0 | 1 | 0 | 3 |
| . | TAFRO n = 65 . | IPL n = 12 . | NOS n = 42 . | OligoCD n = 14 . | UCD n = 46 . |
|---|---|---|---|---|---|
| No. of regimens administered, median (IQR) | 3 (2-4) | 4 (2.8-5) | 3 (2-4) | 2 (2-3) | 1 (1-2) |
| LN excision (partial or complete) | |||||
| Ever received, n | 11 | 10 | 31 | 14 | 43 |
| Response evaluable, n | 10 | 9 | 25 | 6 | 19 |
| Response ratio (%) | 0/10 (0) | 0/9 (0) | 2/25 (8) | 1/6 (16.7) | 12/19 (63.2) |
| Response unknown, n | 1 | 1 | 6 | 8 | 24 |
| Steroid monotherapy | |||||
| Ever received, n | 27 | 6 | 14 | 2 | 6 |
| Response evaluable, n | 24 | 5 | 10 | 1 | 1 |
| Response ratio (%) | 0/24 (0) | 0/5 (0) | 2/10 (20) | 0/1 (0) | 0/1 (0) |
| Response unknown, n | 3 | 1 | 4 | 1 | 5 |
| Anti–IL-6 w/wo steroids∗ | |||||
| Ever received, n | 39 | 10 | 21 | 2 | 3 |
| Response evaluable, n | 36 | 10 | 16 | 2 | 3 |
| Response ratio (%) | 16/36 (44.4) | 6/10 (60) | 7/16 (43.8) | 1/2 (50) | 1/3 (33.3) |
| Response unknown, n | 3 | 0 | 5 | 0 | 0 |
| Siltuximab ± steroids | |||||
| Ever received, n | 28 | 8 | 20 | 2 | 3 |
| Response evaluable, n | 24 | 8 | 15 | 2 | 3 |
| Response ratio (%) | 11/24 (45.8) | 5/8 (62.5) | 6/15 (40) | 1/2 (50) | 1/3 (33.3) |
| Response unknown, n | 4 | 0 | 5 | 0 | 0 |
| Tocilizumab w/wo steroids | |||||
| Ever received, n | 15 | 4 | 1 | 1 | 0 |
| Response evaluable, n | 14 | 3 | 1 | 1 | 0 |
| Response ratio (%) | 5/14 (35.7) | 2/3 (66.7) | 1/1 (100) | 0/1 (0) | 0 |
| Response unknown, n | 1 | 1 | 0 | 0 | 0 |
| Rituximab w/wo steroids | |||||
| Ever received, n | 12 | 6 | 21 | 4 | 5 |
| Response evaluable, n | 11 | 6 | 12 | 3 | 4 |
| Response ratio (%) | 3/11 (27.3) | 0/6 (0) | 3/12 (25) | 0/3 (0) | 1/4 (25) |
| Response unknown, n | 1 | 0 | 9 | 1 | 1 |
| Chemotherapy w/wo other agents | |||||
| Ever received, n | 29 | 0 | 3 | 2 | 2 |
| Response evaluable, n | 23 | 0 | 2 | 2 | 2 |
| Response ratio (%) | 11/23 (47.8) | 0 | 1/2 (50) | 0/2 (0) | 0/2 (0) |
| Response unknown, n | 6 | 0 | 1 | 0 | 0 |
| Other | |||||
| Ever received, n | 37 | 4 | 10 | 2 | 7 |
| Response evaluable, n | 33 | 4 | 9 | 2 | 4 |
| Response ratio (%) | 14/33 (42.4) | 0/4 (0) | 3/9 (33.3) | 1/2 (50.0) | 2/4 (50) |
| Response unknown, n | 4 | 0 | 1 | 0 | 3 |
Response assessment was determined according to the change in the proportion of symptoms present before and after a given regimen was initiated. A response was recorded if there was at least 50% improvement in the proportion of symptoms present after a regimen initiation compared with before or at the time of initiation.
w/wo, with/without.
Inclusive of patients ever treated with either siltuximab w/wo steroids and/or tocilizumab w/wo steroids. Best response among those regimens is listed.
We found a 63.2% (12/19 evaluable) durable clinical response and an 85.7% (30/35 evaluable) LN response to surgical LN excision among patients with UCD, a 16.7% (1/6 evaluable) durable clinical response and a 40% (4/10 evaluable) LN response to surgical LN excision among patients with OligoCD, and a 0% (0/10 evaluable) durable clinical response and 0% (0/4 evaluable) LN response among patients with iMCD. A closer examination into the 8 patients with OligoCD who were categorized as not having an evaluable durable clinical response to surgical excision revealed that 4 had too few clinical symptoms at the start of treatment to assess a response, 2 achieved a response but there was not enough follow-up data to determine the durability of that response, and 2 did not have enough data for a determination. These results suggest that patients with OligoCD and those with UCD may have too few clinical/laboratory abnormalities before initiating a regimen to be able to reliably assess response to therapy. In fact, we found that across all regimen types (including any reason for which a patient may have started a new treatment), patients with OligoCD (8/14, 57.1%) had a higher proportion of regimens for which a response could not be determined because of lack of symptoms at the initiation of the regimen than for UCD (17/46, 37.0%) or iMCD, (29/119, 24.4%; P = .03). Limited data on treatments and response precluded definitive statistical comparison of treatment patterns across CD subtypes and among patients with OligoCD.
Discussion
The data presented herein underscore the variability of CD and highlight that CD occurs across a spectrum rather than the previously described binary model of CD into UCD and MCD. Considering the degree of lymphadenopathy, laboratory abnormalities, symptomatology, and overall severity, we propose CD cases of unknown etiology be considered along a spectrum of symptoms and that these factors should be considered when determining treatment approaches for the various subtypes. Importantly, it is not yet known whether the etiology of the different subtypes on this spectrum is related or unrelated, and there is no evidence to suggest that patients can progress from 1 subtype to another along this spectrum of clinical subtypes. Although this study was not able to fully evaluate survival and prognostic factors because of limitations in study design and sample sizes, we report that 9 of 10 deaths in this cohort occurred in patients with iMCD, and 6 of these patients had TAFRO. A recent multicenter study in China, which included 580 iMCD cases, found 3-year overall survival across the iMCD subtypes of 65.7% for TAFRO, 87.2% for NOS, and 98.5% for IPL.10 The TAFRO subtype likely represents the most severe subtype on the multicentric end of the CD spectrum.
Characterization of the full spectrum of CD has been challenging to date because of its rarity, heterogeneity, and the fact that it remains a diagnosis of exclusion. The ACCELERATE natural history registry of CD serves as an ideal source of information because of the annotated set of data and independent diagnosis adjudication by a panel of disease experts. Diagnosis requires careful pathologic inspection to assess features of CD histopathology but high variability among pathologist interpretations can complicate diagnosis.31 In fact, depending on subtype, between one-third and two-thirds of patients in our study who received a CD diagnosis at an outside institution did not have their diagnosis confirmed when their LN tissue and medical records were reviewed by CD experts. Interestingly, we observed a 71.4% (5/7) response to siltuximab with or without steroids among patients who were probable iMCD-IPL but whose diagnosis was not confirmed by the panel. This raises the possibility that some patients who were not panel confirmed were misclassified or that siltuximab is effective for inflammatory diseases that overlap clinicopathologically with iMCD. Given the difficulty of diagnosing CD based on clinicopathologic features, the clinicopathologic spectrum of CD must be understood to facilitate its recognition and a diagnostic biomarker is needed.
We found a subset of patients who did not meet the diagnostic criteria for iMCD but who had enlarged LNs in at least 2 regions and therefore did not have UCD. We have characterized these patients with oligocentric lymphadenopathy, often in a regional pattern, as OligoCD. These patients were clinically more similar to UCD than iMCD. Although treatment data were limited, the milder clinical and laboratory abnormalities support the recommendation to approach treatment with surgical debulking and removal when feasible and to limit systemic therapies, similar to the diagnostic and treatment guidelines for UCD.20 However, extensive surgical procedures should also be avoided, and patients with inflammatory symptoms may benefit from systemic therapies. Among the small number of OligoCD cases that all exhibited mild disease severity in this study, few patients with OligoCD received drug treatment regimens, and a large proportion (8/14, 57.1%) initiated treatment with few clinical symptoms. The incidence of future lymphadenopathy and subsequent flares being more similar to iMCD suggest monitoring may be needed. Further research and expert consensus are needed to advise on appropriate treatment approaches because OligoCD may have a different natural history and risk profile than UCD and iMCD. ACCELERATE and other population-based cohorts may provide additional data on the natural course of OligoCD.10,32
Patients with OligoCD were less likely to be panel confirmed in this study, which may be because OligoCD has never been formally described by diagnostic criteria. Patients with an OligoCD-like profile may also be more likely to have an alternative disease process. Other published studies have described the presence of patients with an OligoCD phenotype,20,23,25,33 but our study provides a large and richly annotated cohort of patients with CD, allowing for characterization of cases with OligoCD along the CD spectrum.27,28,34,35 Interestingly, a larger proportion of patients in the UCD subtype had an inflammatory-like syndrome than in the OligoCD subtype, but this is, in part, driven by the definitions used. Likewise, a notable proportion of patients in the iMCD group did not have an inflammatory-like syndrome (18%). These patients with ≥2 enlarged LN stations and few minor criteria may be more similar to the OligoCD group. It remains to be known whether the underlying disease pathogenesis is the same across these subtypes.
There are several limitations to this study. Patients are invited to self-enroll, which may lead to a self-selection sample bias. Our study does have a high proportion of individuals with iMCD, and particularly with TAFRO, who tend to be the most severely affected patients. It is possible that these patients are more motivated to enroll in a research study because of the severity of their disease. Our rigorous confirmation process, however, helps to exclude patients who may be less likely to have an accurate CD diagnosis and who might not be excluded in large claims database research in which clinicopathological adjudication is not performed. Furthermore, real-world data present challenges for analysis compared with clinical trials. Data are collected at nonstandard intervals. To address this, subtypes were defined using criteria collected within 90 days of diagnosis, which may not reflect a real-world diagnosis timeframe. Our finding that most cases with a probable intermediate/OligoCD subtype are not panel confirmed may reflect the lack of diagnostic criteria for OligoCD, which could bias selection against these patients. The unequal and small number of patients in some subtypes limits our statistical power to make comparisons; however, most of our findings are descriptive in nature and generally support previous anecdotal reports related to OligoCD.
Herein, we have described the full spectrum of CD among subtypes with no clear etiology, which has allowed us to characterize the OligoCD subtype. Careful consideration should be given to alternative diagnoses for patients who do not meet UCD or iMCD diagnostic criteria. Investigations into a larger cohort of patients with OligoCD will enable the development of diagnostic and treatment guidance.
Acknowledgments
The authors thank all the patients and their families for their participation in the ACCELERATE registry; the Castleman Disease Collaborative Network and the ACCELERATE registry team for their support; and Tom Uldrick, Jason Ruth, David Wu, Kojo Elenitoba-Johnson, Nikhil Munshi, Atsushi Kawakami, Peter Voorhees, Arthur Rubenstein, and Kazu Yoshizaki for their support for this research.
The ACCELERATE natural history registry has received funding from Janssen Pharmaceuticals (2016-2018); EUSA Pharma, LLC (USA), which has merged with Recordati Rare Diseases Inc (2018-2022); and the US Food and Drug Administration (R01FD007632; 2022 to present).
Authorship
Contribution: S.K.P., J.D.B., A.B., M.J.L., D.A.T., C.C., A.C., S.C., A.D., A.F., C.H., M.I., R.Z., S.M., S.N., J.-T.N., A.N., E.O., M.S., R.S.M.W., L.Z., M.S.L., G.S., F.v.R., and D.C.F. conceptualized the study; S.K.P., D.A.T., and D.C.F. contributed to the methodology; S.K.P. visualized the study, performed the formal analysis, curated the data, and wrote the original draft; S.K.P., D.A.T., M.S.B., and S.S. performed the investigation; J.D.B., D.A.T., A.B., M.J.L., D.A.T., C.C., A.C., S.C., A.D., A.F., C.H., M.I., R.Z., S.M., S.N., J.T.N., A.N., E.O., M.S.B., S.S., M.S., R.S.M.W., L.Z., M.S.L., G.S., F.v.R., and D.C.F. reviewed and edited the manuscript; J.D.B., A.B., M.J.L., D.A.T., C.C., A.C., S.C., A.D., A.F., C.H., M.I., R.Z., S.M., S.N., J.T.N., A.N., E.O., M.S., R.S.M.W., L.Z., M.S.L., G.S., F.v.R., and D.C.F. supervised the study; S.K.P. and D.C.F. contributed to project administration; and D.C.F. acquired the funding.
Conflict-of-interest disclosure: J.D.B. is supported by a Doris Duke Foundation Physician Scientist Fellowship and American Society of Transplantation and Cellular Therapy New Investigator Award; and has consulted for Recordati Rare Diseases and EUSA Pharma. D.A.T. is a cofounder of Quantitative Radiology Solutions LLC. M.J.L. reports consulting fees from Secura Bio, EUSA Pharma, and Kyowa Kirin, and travel support from Secura Bio. C.C. is a paid consultant and speaker for Recordati Rare Diseases, and receives research funding from Johnson & Johnson and Immunity Bio. A.D. has served on the advisory board and independent review committees for Janssen Pharmaceuticals; has received research funding from Alnylam, Pfizer, Takeda, and Bristol Myers Squibb; and serves on the scientific advisory board for AbbVie and HaemaLogiX. R.K. has received research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, MedImmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, TopAlliance, and the National Cancer Institute; has received consultant and/or speaker fees and/or advisory board/consultant fees from Actuate Therapeutics, AstraZeneca, Bicara Therapeutics Inc, Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, Eisai, EOM Pharmaceuticals, Iylon, LabCorp, Merck, NeoGenomics, Neomed, Pfizer, Precirix, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, and X-Biotech; has an equity interest in CureMatch Inc; serves on the board of CureMatch and CureMetrix; and is a cofounder of CureMatch. S.M. reports advisory board participation for Celgene/Acceleron, Bristol Myers Squibb, Novartis, Blueprint Medicines, Genentech, EUSA/Recordati, and AbbVie; reports honoraria from Aplastic Anemia and MDS International Foundation, Celgene (now Bristol Myers Squibb), Bristol Myers Squibb, McGraw Hill Hematology Oncology Board Review, Partnership for Health Analytic Research, LLC, and EUSA/Recordati; reports consultancy for BioPharm, Celgene, Novartis, Bristol Myers Squibb, EUSA/Recordati; and received research funding (institution funding) from Bristol Myers Squibb (formerly Celgene), Novartis, and Jazz Pharmaceuticals. S.N. reports research funding from Caribou biosciences, ONO therapeutics, Astex, Atara, LOXO/Lilly, Pharmacyclics, Genentech/Roche, and Seattle Genetics; reports advisory board membership from Genmab, ADC Therapeutics, Genentech, and Acrotech; and reports data safety monitoring board membership with Merck. J.-T.N. received research grants not related to this manuscript from Gilead and EUSA Pharma/Recordati Rare Diseases; and received honoraria not related to this manuscript from AstraZeneca, Blueprint Medicines, EUSA Pharma/Recordati Rare Diseases, Novartis, and Roche. A.N. received research funding from Pharmacyclics/AbbVie, Kite/Gilead, and Cornerstone; received consulting fees from Janssen, MorphoSys, Cornerstone, Epizyme, EUSA, TG therapeutics, ADC Therapeutics, and AstraZeneca; and received honoraria from Pharmacyclics/AbbVie. M.S. reports speakers bureau and consultancy fees from Regeneron. R.S.M.W. has received grants/research support from AbbVie, Acerta, Alexion, Amgen, Apellis, Astella, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Celgene, Daiichi-Sankyo, GSK, Janssen, Kartos, MorphoSys, MSD, Novartis, Pfizer, Regeneron, and Roche; and consultant and/or speaker fees and/or advisory board/consultant fees from Alexion, Amgen, Astella, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer-Ingelheim, Daiichi-Sankyo, GlaxoSmithKline, Novartis, Pfizer, and Roche. M.S.L. reports research funding and advisory board and speakers bureau fees from Thermo Fisher Scientific. G.S. reports speakers bureau fees from Recordati Rare Diseases. F.v.R. has received consulting fees from EUSA Pharma, GlaxoSmithKline, Janssen, Karyopharm, and Takeda; and has received research funding from Janssen Pharmaceuticals and Bristol Myers Squibb. D.C.F. receives funding from the National Heart, Lung, and Blood Institute (R01HL141408; 2018 to present); has consulted for and received research funding from Recordati Rare Diseases and EUSA Pharma; was provided study drug for a clinical trial of a Pfizer product (sirolimus); and has provisional patent applications filed by the University of Pennsylvania (provisional patent applications: 63/113,045; 62/989,437). The remaining authors declare no competing financial interests.
Correspondence: David C. Fajgenbaum, Translational Medicine and Human Genetics, Raymond and Ruth Perelman School of Medicine, University of Pennsylvania, 3535 Market St, Suite 700, Philadelphia, PA 19104; email: davidfa@pennmedicine.upenn.edu.
References
Author notes
Original data used to produce this manuscript are available on request from the corresponding author, David C. Fajgenbaum (davidfa@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.