Dysregulated JAK/STAT signaling underlies the pathogenesis of myelofibrosis, a myeloproliferative neoplasm characterized by cytopenias, splenomegaly, and constitutional symptoms. JAK inhibitors, such as fedratinib, are the primary therapeutic option for patients with high-risk or symptomatic myelofibrosis. Fedratinib has characteristics that distinguish it from other commercially available JAK inhibitors, such as its preferential inhibition of JAK2 and its inhibitory effects on kinases such as Fms-like tyrosine kinase 3 and BRD4. Fedratinib is most often used in the second-line setting after intolerance or resistance to other JAK inhibitors, but there is substantial evidence that it is an effective first-line option in the appropriate patient population. Prevention and early treatment of fedratinib-related gastrointestinal toxicity is key to maintaining adequate drug exposure, and clinicians must remain vigilant for Wernicke encephalopathy during treatment. Fedratinib’s JAK2 selectivity and kinome profile make it an appealing agent for alternative indications, such as myelodysplastic/myeloproliferative neoplasms and maintenance after bone marrow transplantation, which are under active investigation.
Introduction
Significant progress has been made following the identification of JAK2 V617F in the pathogenesis of myeloproliferative neoplasms (MPNs).1 In the following decade, recognition of CALR2 and MPL3 mutations solidified the role of aberrant JAK/STAT signaling in MPN development. More recently, dysregulated signaling pathways have been identified that cooperate and contribute to the development of MPNs, specifically myelofibrosis (MF). These disease relevant pathways and regulators of cell fate, such as bromodomain and extraterminal domain (BET), Bcl-xL/Bcl-2, and PI3 kinase/AKT, have been identified as potential therapeutic targets for MF.4
Currently, JAK inhibition remains the primary therapeutic option for patients with MF who require treatment. Fedratinib was the second JAK inhibitor (JAKi) approved by the US Food and Drug Administration (FDA; in 2019) and was designed for selectivity to JAK2, compared with the equipotent JAK1/2 inhibitor ruxolitinib, which was approved 8 years earlier (in 2011).5 Since the approval of fedratinib, the FDA has approved 2 additional JAKis, pacritinib and momelotinib, each with distinct kinome profiles, toxicities, and applications. Fedratinib, similar to its counterparts, has a unique therapeutic and toxicity profile that determines its place in the MF treatment paradigm. Although fedratinib is underused in practice due to a myriad of reasons, including toxicity concerns and a previous FDA hold, it remains a potent JAKi and offers an important option for the treatment of MF.
Preclinical and early-phase data
Fedratinib was synthesized using structure-based drug design to selectively inhibit JAK2 over related kinases (ie, JAK1, JAK3, and TYK2).5 In vitro and pharmacokinetic studies confirmed a half maximal inhibitory concentration (IC50) >300× for JAK3, ∼35× for JAK1, and ∼135× for TYK2, compared with JAK2 (IC50 ∼ 3 nM). Additionally, fedratinib was found to have an IC50 of 15 nM for Fms-like tyrosine kinase 3 (FLT3) and 48 nM for Ret. Later, fedratinib was noted to inhibit the BET family of proteins, notably BRD4 (IC50 ∼ 130 nM), as well as BRD2, BRD3, and BRDT.6,7
The phase 1 dose-escalation trial (NCT00631462) of fedratinib enrolled adult patients (aged ≥18 years) with intermediate- or high-risk MF, as defined by the Mayo Prognostic Scoring System.8 Fifty-nine JAKi-naïve patients were treated with doses ranging from 30 to 800 mg daily in the dose-escalation phase, and 43 patients (73%) continued fedratinib past 24 weeks in the extension phase. The maximum tolerated dose (MTD) was 680 mg daily, and the median treatment dose during the extension phase was 440 mg daily.
Grade 1/2 treatment-emergent adverse events (TEAEs) in the MTD cohort included nausea (77.5%), diarrhea (62.5%), vomiting (67.5%), and increased aspartate transaminase (32.5%)/alanine aminotransferase (22.5%). Grade 3/4 anemia and thrombocytopenia occurred in 54.2% and 27.5% of patients at the MTD, respectively, most of whom entered the study with baseline grade 1/2 cytopenias. After 6 cycles, a sustained (≥8 week) palpable spleen reduction ≥50% was observed in 45% of patients in the MTD cohort.
A phase 2, dose-finding study randomized 31 JAKi-naïve patients with intermediate-2 or high-risk MF (per International Working Group (IWG)–Myeloproliferative Neoplasms Research and Treatment9 criteria) to fedratinib 300 mg (n = 10), 400 mg (n = 10), or 500 mg (n = 11) once daily.10 At 24 weeks, the proportion of patients with a ≥35% reduction in splenomegaly (spleen volume reduction [SVR35W24]) was 30% (300 mg), 60% (400 mg), and 55% (500 mg). At 24 weeks, the proportion of patients with a ≥50% reduction in total symptom score (TSS50W24), as assessed by the MPN Symptom Assessment Form (MPN-SAF), was 33% (300 mg), 60% (400 mg), and 38% (500 mg).
Concern for WE
Fedratinib’s tortuous path to FDA approval is described in detail elsewhere,11 but the signal of Wernicke encephalopathy (WE) is important for review. WE results from vitamin B1 (thiamine) deficiency and is classically characterized by confusion, oculomotor abnormalities, and ataxia. Although WE deficits are generally reversible, a proportion of patients progress to the more permanent Korsakoff syndrome.12
The FDA hold on fedratinib started in November 2013, and at that time WE was suspected in 8 of 670 patients treated across several clinical trials.13 In vitro studies posited potential mechanisms for WE, including inhibition of human thiamine transporter-2 (THTR2) in the gut.14 Later, in vitro studies that closely replicated in vivo conditions identified an IC50 >30 μM against THTR2, well above the concentrations achieved with therapeutic doses of fedratinib.15-17
Independent analysis of the suspected WE cases determined that thiamine levels or magnetic resonance imaging findings were not supportive of WE in 3 patients and were inconclusive in 2 others.12 The remaining patients had significant baseline malnutrition and experienced gastrointestinal (GI) toxicity, implicating impaired oral intake in fedratinib-related WE. The FDA hold on fedratinib was lifted in 2018, but subsequent approval of fedratinib came with a black box warning regarding potentially fatal encephalopathy.18
Use in 2024
Currently, 4 JAKis are approved for MF, and treatment decisions require consideration of patient- and disease-associated factors. Fedratinib has data supporting its use in the second-line setting after intolerance or resistance to first-line JAKi, as well as randomized phase 3 data in the frontline setting for JAKi-naïve patients. Key studies of fedratinib can be found in Table 1.
Selected clinical studies of fedratinib
| Frontline studies . | ||||
|---|---|---|---|---|
| Study . | Patient population . | Treatment . | Outcomes . | Notes . |
| TED12037; NCT00631462 (phase 1) | Mayo PSS intermediate- or high-risk, Plt ≥50 × 109/L | Fedratinib 30-800 mg per day (dose-escalation phase, n = 28) Fedratinib 680 mg per day (dose-confirmation phase, n = 40) | DLT in 2/6 patients at 800 mg per day: G3/4 hyperamylasemia ≥50% decrease in palpable spleen size for at least 8 weeks at W24 in 39% of patients | 28/40 patients (70%) treated with 680 mg per day required dose reduction Median dose during extension phase was 440 mg per day |
| ARD11936; NCT01420770 (phase 2) | IWG-MRT int-2 or high-risk, splenomegaly (palpable ≥5 cm below LCM), Plt ≥50 × 109/L | Fedratinib 300, 400, or 500 mg per day (1:1:1 randomization, n = 31) | SVR35W24∗: 30%/50%/64% in 300/400/500 mg per day TSS50W24†: 33%/60%/38% in 300/400/500 mg per day | Mean hemoglobin levels are numerically lowest in the 500 mg group G3/4 TEAEs: 80% in 300 and 400 mg per day, and 100% in the 500 mg per day |
| JAKARTA; NCT01437787 (phase 3) | IWG-MRT int-2 or high-risk, splenomegaly (palpable ≥ 5 cm below LCM), Plt ≥50 × 109/L | Fedratinib 400 mg per day, 500 mg per day, or placebo (1:1:1 randomization, n = 289) | SVR35W24 (confirmed 4 weeks later): 36%/40%/1% in 400 mg per day/500 mg per day/placebo (P < .001) TSS50W24: 36%/34%/7% in 400 mg per day/500 mg per day/placebo (P < .001) | Discontinued fedratinib due to AEs during the first 24 weeks: 14% (400 mg per day), 25% (500 mg per day), and 8% (PBO) SVR35W24 without 4-week confirmation, 47% |
| Second-line studies | ||||
| Study | Patient population | Treatment | Outcomes | |
| JAKARTA2; NCT01523171 (phase 2) | DIPSS int-1 (with constitutional symptoms) or ≥int-2, prior ruxolitinib treatment ≥14 days or discontinued for intolerance, splenomegaly (palpable ≥5 cm below LCM), Plt ≥50 × 109/L | Fedratinib 400 mg per day (n = 97) | SVR35W24: 55%, TSS50W24: 26% Stringent criteria ( | In subgroup analyses, SVR rates not significantly influenced by reason for prior ruxolitinib discontinuation (relapsed/refractory or intolerant) |
| FREEDOM; NCT03755518 (phase 3b) | DIPSS ≥ int-1, splenomegaly ≥ 450 cm3 or palpable ≥5 cm below LCM, ruxolitinib intolerance/resistance (stringent criteria), Plt ≥ 50 × 109/L | Fedratinib 400 mg per day (n = 38) | SVR35W24: 26%, TSS50W24: 44% | SVR35W24 of 37% when including missing W24 spleen measurements by using LOCF analysis |
| FREEDOM2; NCT03952039 (phase 3) | DIPSS ≥ int-2, splenomegaly ≥ 450 cm3, ruxolitinib intolerance/resistance (stringent criteria), Plt ≥50 × 109/L | Fedratinib 400 mg per day or BAT (2:1 randomization, n = 201) BAT (n = 67): ruxolitinib (78%), RBC transfusions (28%), hydroxyurea (19%) | SVR35W24: 36% in fedratinib vs 6% in BAT (P < .0001) TSS50W24: 34% in fedratinib vs 17% in BAT (P = .0033) | SVR35W24 benefit numerically higher in ruxolitinib intolerant vs ruxolitinib resistant |
| Frontline studies . | ||||
|---|---|---|---|---|
| Study . | Patient population . | Treatment . | Outcomes . | Notes . |
| TED12037; NCT00631462 (phase 1) | Mayo PSS intermediate- or high-risk, Plt ≥50 × 109/L | Fedratinib 30-800 mg per day (dose-escalation phase, n = 28) Fedratinib 680 mg per day (dose-confirmation phase, n = 40) | DLT in 2/6 patients at 800 mg per day: G3/4 hyperamylasemia ≥50% decrease in palpable spleen size for at least 8 weeks at W24 in 39% of patients | 28/40 patients (70%) treated with 680 mg per day required dose reduction Median dose during extension phase was 440 mg per day |
| ARD11936; NCT01420770 (phase 2) | IWG-MRT int-2 or high-risk, splenomegaly (palpable ≥5 cm below LCM), Plt ≥50 × 109/L | Fedratinib 300, 400, or 500 mg per day (1:1:1 randomization, n = 31) | SVR35W24∗: 30%/50%/64% in 300/400/500 mg per day TSS50W24†: 33%/60%/38% in 300/400/500 mg per day | Mean hemoglobin levels are numerically lowest in the 500 mg group G3/4 TEAEs: 80% in 300 and 400 mg per day, and 100% in the 500 mg per day |
| JAKARTA; NCT01437787 (phase 3) | IWG-MRT int-2 or high-risk, splenomegaly (palpable ≥ 5 cm below LCM), Plt ≥50 × 109/L | Fedratinib 400 mg per day, 500 mg per day, or placebo (1:1:1 randomization, n = 289) | SVR35W24 (confirmed 4 weeks later): 36%/40%/1% in 400 mg per day/500 mg per day/placebo (P < .001) TSS50W24: 36%/34%/7% in 400 mg per day/500 mg per day/placebo (P < .001) | Discontinued fedratinib due to AEs during the first 24 weeks: 14% (400 mg per day), 25% (500 mg per day), and 8% (PBO) SVR35W24 without 4-week confirmation, 47% |
| Second-line studies | ||||
| Study | Patient population | Treatment | Outcomes | |
| JAKARTA2; NCT01523171 (phase 2) | DIPSS int-1 (with constitutional symptoms) or ≥int-2, prior ruxolitinib treatment ≥14 days or discontinued for intolerance, splenomegaly (palpable ≥5 cm below LCM), Plt ≥50 × 109/L | Fedratinib 400 mg per day (n = 97) | SVR35W24: 55%, TSS50W24: 26% Stringent criteria ( | In subgroup analyses, SVR rates not significantly influenced by reason for prior ruxolitinib discontinuation (relapsed/refractory or intolerant) |
| FREEDOM; NCT03755518 (phase 3b) | DIPSS ≥ int-1, splenomegaly ≥ 450 cm3 or palpable ≥5 cm below LCM, ruxolitinib intolerance/resistance (stringent criteria), Plt ≥ 50 × 109/L | Fedratinib 400 mg per day (n = 38) | SVR35W24: 26%, TSS50W24: 44% | SVR35W24 of 37% when including missing W24 spleen measurements by using LOCF analysis |
| FREEDOM2; NCT03952039 (phase 3) | DIPSS ≥ int-2, splenomegaly ≥ 450 cm3, ruxolitinib intolerance/resistance (stringent criteria), Plt ≥50 × 109/L | Fedratinib 400 mg per day or BAT (2:1 randomization, n = 201) BAT (n = 67): ruxolitinib (78%), RBC transfusions (28%), hydroxyurea (19%) | SVR35W24: 36% in fedratinib vs 6% in BAT (P < .0001) TSS50W24: 34% in fedratinib vs 17% in BAT (P = .0033) | SVR35W24 benefit numerically higher in ruxolitinib intolerant vs ruxolitinib resistant |
DLT, Dose limiting toxicity; G3/4, grade 3/4; int-1, intermediate-1; int-2, intermediate-2; IWG-MRT, IWG–Myeloproliferative Neoplasms Research and Treatment; LCM, left costal margin; PBO, placebo; Plt, platelet; PSS, prognostic scoring system.
Spleen volume reduction ≥35% at week 24.
Total symptom score reduction ≥35% at week 24.
Second line after JAKi
JAKARTA2 was a single-arm, nonrandomized phase 2 study that evaluated fedratinib in ruxolitinib-exposed patients.19 Participants had palpable splenomegaly, platelet count ≥50 × 109/L, and intermediate-1 (with symptoms) or higher risk disease by the Dynamic International Prognostic Scoring System (DIPSS). Patients with ruxolitinib resistance had to have received ruxolitinib for ≥14 days, whereas those with intolerance could have received it for <14 days. Resistance was defined by the investigator as lack of response, stable disease, progression of disease, or loss of response.
Ninety-seven patients received fedratinib 400 mg daily. The trial was terminated ∼2.5 months after the completion of accrual (due to the FDA hold), and the median follow-up was 6.0 months. The median ruxolitinib exposure was 10.3 months, and 66% of patients were resistant to ruxolitinib. The primary end point, SVR35W24, was achieved by 55% of 83 evaluable patients. Outcome reporting used a last-observation-carried-forward (LOCF) analysis, which allowed for the last spleen assessment before the end of cycle 6 to be used for SVR35W24 response. TSS50W24, assessed using a modified MF Symptom Assessment Form (MFSAF), was achieved in 26% of evaluable patients. Grade 1/2 GI TEAEs were common (diarrhea, 58%; nausea, 56%); and grade 3/4 anemia and thrombocytopenia occurred in 38% and 22% of patients, respectively.
A reanalysis of JAKARTA2 using more stringent criteria for ruxolitinib resistance or intolerance defined resistance as ≥3 months of treatment with spleen regrowth (to <10% SVR) or <30% decrease in baseline spleen size; and intolerance as ≥28 days of treatment complicated by grade 3 anemia/thrombocytopenia, red blood cell transfusion dependence, or bleeding.20 Results showed an SVR35W24 of 31% without using LOCF analysis and an SVR35W24 of 30% in the stringent criteria cohort. TSS50W24 was reported in 27% of patients with available postbaseline assessments.
FREEDOM was a single-arm study of fedratinib in ruxolitinib-treated patients with DIPSS intermediate- or high-risk MF, conducted after the FDA hold was lifted.21 Stringent criteria were used to define ruxolitinib resistance. FREEDOM opened in March 2019 and closed in October 2020 due to competing factors negatively affecting accrual, such as the COVID-19 pandemic and commercial drug availability.
Thirty-eight patients were enrolled in FREEDOM, and 27 received ≥6 cycles of fedratinib. The primary end point, SVR35W24, was achieved by 9 of 35 patients (25.7%) in the evaluable population and by 13 of 35 (37.1%) using LOCF analysis. TSS50W24 (measured by the MFSAF) was achieved in 44.4% of patients. Grade 1/2 GI TEAEs were common (diarrhea, 39.4%; nausea, 39.5%), although these were decreased compared with JAKARTA-2. Grade 1/2 thiamine decreases occurred in 6 of 38 patients (15.8%), although all resolved after supplementation.
FREEDOM2 randomized patients outside North America with prior ruxolitinib exposure in a 2:1 manner to fedratinib or best available therapy (BAT).22 Eligibility criteria were similar to FREEDOM, with a notable difference of excluding DIPSS intermediate-1 risk MF. A preemptive strategy to reduce GI TEAEs was used, and an amendment mandated prophylactic thiamine supplementation. BAT largely consisted of ruxolitinib (78%) and hydroxyurea (19%).
In total, 201 patients were enrolled; 134 received fedratinib, and 67 received BAT. SVR35W24 was achieved more often with fedratinib than BAT (36% vs 6%; P < .0001). Spleen responses with fedratinib were durable, with a median duration of 86.3 weeks by imaging and 118.3 weeks by palpation. TSS50W24 (assessed by MFSAF) was more common with fedratinib than BAT (34% vs 17%; P = .003). Despite prophylaxis, GI TEAEs (60% vs 9%) and grade 3 GI adverse events (4% vs 0%) were more common with fedratinib. Five patients treated with fedratinib developed thiamine deficiency (vs 1 treated with BAT).
Real-world studies
A retrospective study from the Oncology Provider Extended Network aimed to elucidate the role of fedratinib in the post-ruxolitinib setting.23 Patients (n = 150) had discontinued first-line ruxolitinib and were mostly treated at community practices (91.7%) by general oncologists. Ruxolitinib was most often discontinued for progressive disease (70%) or suboptimal response (24%). In patients with ≥6 months of follow-up, significant improvements in splenomegaly were observed (mean, 13.6 cm vs 7.2 cm; P = .01). Spleen reduction ≥50% at 6 months was achieved by 26.8% in those with baseline palpable splenomegaly.
Gangat et al described fedratinib use in 28 patients with MF after first-line ruxolitinib at the Mayo Clinic.24 Three patients (13%) had a spleen response by imaging, and 32% of patients achieved TSS50 by the MPN-SAF. Prior treatment with ruxolitinib ≥40 mg daily correlated with decreased fedratinib response, whereas pretreatment spleen size and fedratinib dose did not. Notably, 25% of patients experienced accelerated- or blast-phase transformation during follow-up, indicative of a poor-risk cohort.
An investigation regarding the impact of fedratinib on survival using the Flatiron Health Database compared outcomes of 229 patients with MF who discontinued ruxolitinib and either subsequently received fedratinib (n = 70) or did not (n = 159).25 Notable imbalances in baseline characteristics included a higher proportion of older patients and Eastern Cooperative Oncology Group ≥2 in the non-fedratinib subgroup. Survival at 1 year after ruxolitinib was 71.6% in the fedratinib group vs 47.9% in the non-fedratinib group.
Frontline, JAKi naive
JAKARTA was a phase 3 trial that randomized patients with IWG–Myeloproliferative Neoplasms Research and Treatment intermediate-2 or high-risk MF in a 1:1:1 fashion to fedratinib 400 mg daily, 500 mg daily, or placebo.26 Patients were JAKi naïve and had palpable splenomegaly and a platelet count ≥50 × 109/L. Crossover to fedratinib was permitted after 24 weeks or earlier if progressive disease was documented (increased spleen volume ≥25% from baseline, leukemic transformation, or an increase in peripheral blast percentage ≥20% for ≥1 week).
JAKARTA enrolled 289 patients: 96 to fedratinib 400 mg daily, 97 to fedratinib 500 mg daily, and 96 to placebo. SVR35W24 with confirmation 4 weeks later (primary end point) was achieved more often with fedratinib (400 mg, 36%; 500 mg, 40%) than placebo (1%). In the 400 mg group, SVR35W24 was achieved in 53% and 38% of patients with intermediate-2 and high-risk disease, respectively, and in 33% and 55% of patients with wild-type JAK2 and mutant JAK2 status, respectively. TSS50W24 (per modified MFSAF) was more common with fedratinib (400 mg, 36%; 500 mg, 34%) than placebo (7%).
Outcomes of patients treated with fedratinib 400 mg daily were later reanalyzed ahead of US regulatory approval.27 SVR35W24 improved to 47% without requiring the 4-week confirmatory scan, which compared favorably with similar studies that did not require 4-week confirmation such as COMFORT-I, which reported an SVR35W24 of 41.9% with ruxolitinib in a similar patient population.28
The clinical hold on fedratinib led to early termination of both JAKARTA and JAKARTA-2, which limited longitudinal follow-up. The longest follow-up of JAKi-naïve patients treated with fedratinib comes from the open-extension phase of the phase 1 dose-escalation study in 59 patients.8 After a median of 30 cycles (median dose, 440 mg daily), reported durable spleen response with SVR50 by palpation was 55% at 24 months and 61% at 30 months.10
Prospective data regarding fedratinib vs other JAKi in the JAKi-naïve setting are lacking. A systematic review that compared the 4 FDA-approved JAKis, ruxolitinib, fedratinib, pacritinib, and momelotinib, found no significant difference in spleen response between ruxolitinib, fedratinib, and momelotinib in the JAKi-naïve population.29 The same study found no difference in TSS50W24 between ruxolitinib (COMFORT-I) and fedratinib (JAKARTA).
Special populations/scenarios
Thrombocytopenia
Moderate thrombocytopenia (platelet count 50 × 109/L to 100 × 109/L) is observed in ∼25% of patients with MF,30,31 and severe thrombocytopenia (platelet count <50 × 109/L) is observed in 15% to 20% of patients with MF at diagnosis.30,32 Thrombocytopenia is associated with poor outcomes in MF, including increased symptoms, leukemic transformation, and death.32,33
Harrison et al assessed the efficacy of fedratinib in patients with moderate thrombocytopenia (“low-platelet” cohort) enrolled in the JAKARTA and JAKARTA2 studies, as well as a subset of the phase 2 dose-finding study.34 The low-platelet cohort included 14 of 96 patients (15%) randomized to fedratinib 400 mg in JAKARTA and 33 of 97 patients (34%) in JAKARTA2, with median baseline platelet counts of 65 × 109/L and 76 × 109/L, respectively. SVR35W24 was 36% in both JAKARTA (vs 0% in placebo) and JAKARTA2, which was similar to that of patients with platelet counts >100 × 109/L (49% and 28% in JAKARTA and JAKARTA2, respectively). TSS50W24 was 37% in the low-platelet cohorts and was not significantly different within each trial between low- and normal-platelet groups.
Similar proportions of patients in the low-platelet (17%) and normal-platelet cohorts (15%) discontinued fedratinib for TEAEs. Bleeding events occurred in 15 of 48 patients (31%) in the low-platelet cohort vs 34 of 155 (22%) in the normal-platelet cohort. New or worsening thrombocytopenia occurred in 21 of 48 (44%) in the low-platelet cohort vs 14 of 155 (9%) in the normal-platelet cohort.
In FREEDOM, SVR35W24 was achieved by 1 patient of 9 (11.1%) with moderate thrombocytopenia vs 8 of 25 (32.0%) with mild or no thrombocytopenia.21 Notably, 10 of 134 patients (7%) treated with fedratinib and 4 of 67 patients (6%) treated with BAT in FREEDOM2 had severe thrombocytopenia at baseline (response data unavailable for this specific cohort).22 In patients with moderate thrombocytopenia in FREEDOM2, SVR35W24 was achieved by 16 of 34 patients (47.1%) treated with fedratinib vs 0 of 21 patients treated with BAT.
Anemia
Anemia is associated with poor outcomes in MF and can be worsened with fedratinib.35,36 Baseline hemoglobin in patients treated with fedratinib 400 mg daily in JAKARTA was 10.7 g/dL. Grade 3/4 anemia occurred in 41 of 96 patients (43%) treated with fedratinib vs 24 of 95 patients (25%) treated with placebo. Hemoglobin nadired ∼12 to 16 weeks into treatment and subsequently increased but did not quite reach baseline levels in both cohorts. Among baseline transfusion-dependent patients, 7 of 8 (87.5%) achieved transfusion independence with fedratinib vs 3 of 6 (50%) with placebo. Treatment-emergent grade 3/4 anemia occurred in 38% to 60% of patients across the various fedratinib trials.37
Ongoing investigations are testing anemia-mitigating interventions in JAKi-treated patients. Luspatercept, a transforming growth factor β trap, demonstrated anemia benefit in combination with ruxolitinib for patients with MF in the ACE-536-MF-001 trial.38 Transfusion-dependent patients on a stable dose of ruxolitinib treated with luspatercept exhibited a transfusion-independence rate (defined as 12 weeks without receipt of an red blood cell transfusion, per Gale criteria) of 31.6%, and 52.6% had a ≥50% reduction in transfusion burden. Luspatercept has efficacy in combination with fedratinib39 and is being studied in the phase 3 randomized INDEPENDENCE trial (vs placebo) as an add-on therapy for patients with transfusion-dependent anemia treated with JAKis (NCT04717414).40
Renal/hepatic impairment
Two studies were conducted to determine the pharmacokinetics of fedratinib in patients with renal (“the renal study”) and hepatic impairments (“the hepatic study”).41 The studies enrolled patients with chronic renal or hepatic disease, as well as healthy controls, and administered a single dose of fedratinib 300 mg.
The renal study enrolled 36 patients with mild to end-stage renal disease (per Cockcroft-Gault formula) and 17 healthy controls. The hepatic study enrolled 8 patients with mild hepatic disease (Child-Pugh score 5-6) and 9 healthy controls. Fedratinib plasma concentrations were higher in those with renal impairment; maximum fedratinib plasma concentration increased ∼1.5-fold with mild impairment and twofold with moderate-to-severe impairment. Pharmacokinetics were not significantly different between patients with mild hepatic impairment and healthy controls. Investigators concluded that mild renal or hepatic impairment should not affect fedratinib dosing, whereas moderate renal impairment requires careful dosing, and patients with severe renal impairment should receive a lower dose of fedratinib (200 mg daily).
Transplant
Hematopoietic stem cell transplantation (HSCT) is the only curative treatment for patients with MF.42 A retrospective study examined the impact of pre-HSCT fedratinib after prior ruxolitinib in 18 patients with MF.43 The median treatment duration with fedratinib was 6.9 months, and spleen size by palpation was significantly reduced 3 months after fedratinib initiation compared with baseline (15.1 cm vs 9.0 cm; P = .021). Four deaths (22.2%) occurred in patients who started fedratinib as a bridge to HSCT, at a median of 14.5 months after fedratinib initiation.
A phase 1/2 trial is evaluating the safety and efficacy of fedratinib maintenance after HSCT for MPNs or myelodysplastic syndrome (MDS/MPN) overlap syndromes (NCT05127174). The phase 1 portion identified fedratinib 400 mg daily as the MTD, and the phase 2 portion is currently enrolling.
JAK2 wild-type MF
Although only 50% to 60% of patients with MF harbor JAK2 V617F mutations, all have dysregulated JAK-STAT signaling that is ameliorated by currently approved JAKis, which inhibit both mutant and wild-type JAK2.
SVR35W24 rates in the JAKARTA trial were similar between fedratinib-treated JAK2 V617F patients (40% at 400 mg, 43% at 500 mg, and 0% with placebo) and JAK2 wild-type patients (30% at 400 mg, 35% at 500 mg, and 3% with placebo).26 SVR35W24 rates did not differ in JAKARTA-2 between JAK2 V617F and JAK2 wild-type patients in either the original or updated stringent analyses.20 Although “triple-negative” patients with MF who lack mutations in JAK2, CALR, or MPL have inferior responses to ruxolitinib, this has not been demonstrated specifically with fedratinib.44
Future uses
Aspects of fedratinib make it an attractive partner for additional indications. Fedratinib inhibits BRD4 and FLT3, which are implicated in MF pathogenesis and progression.45,46 Aberrant FLT3 signaling leads to the activation of downstream signaling pathways such as PI3K/AKT, MAPK, and STAT5 that propagate an inflammatory bone marrow milieu and contribute to disease progression. BRD4 and JAK inhibition synergistically inhibits NF-κB activity and subsequent inflammatory cytokine production7 and has demonstrated improved spleen response rates in MF.47
Fedratinib is being studied in patients with MDS/MPN and chronic neutrophilic leukemia on the basis of FLT3/BRD4 inhibition and cellular-Myc suppression.48 Patients with MDS/MPN or chronic neutrophilic leukemia, splenomegaly, and/or a significant disease burden as measured by MPN TSS are treated with fedratinib 400 mg daily. At the last data cutoff, 10 of 19 of evaluable patients (53%) had a response by MDS/MPN IWG criteria, and spleen volume decreased by an average of 32% in 13 patients with baseline splenomegaly who received at least 24 weeks of fedratinib. Expression of cellular-Myc (% positive cells × staining intensity) decreased in 7 of 9 patients (78%) with paired samples by an average of 30%.
Fedratinib’s selectively for JAK2 may help avoid unintended T-cell inhibition and immunosuppression. Although single-agent immune checkpoint inhibition has not demonstrated significant efficacy in MF,49,50 there is evidence that JAK2 V617F aberrant signaling promotes synthesis of programmed cell death ligand-1 in MPN cells and assists with immune escape.51 FRACTION is a phase 2 study of fedratinib and nivolumab, an anti–PD-1 antibody, in patients with MF who have failed treatment or have had suboptimal responses to prior JAKi.52 Fedratinib is also being studied in combination with decitabine for accelerated- and blast-phase MPNs (NCT04282187) and as maintenance therapy after HSCT to prevent disease relapse (NCT05127174).
Expert opinion
The lengthy clinical hold on fedratinib delayed FDA approval until almost a decade after that of ruxolitinib for a similar indication. This period when ruxolitinib was the only available JAKi for MF helped increase familiarity with the drug and establish it as the common first-line option, at least until the recent approval of less myelosuppressive JAKis. Other deterrents to the use of fedratinib include a boxed warning for WE and higher rates of GI toxicity.
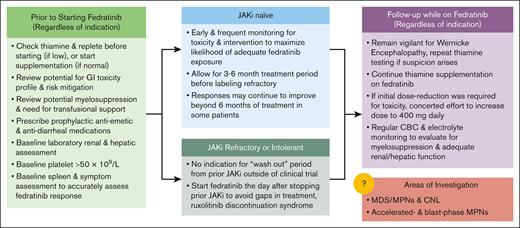
Fedratinib is a potent inhibitor of JAK2 and has good efficacy in the JAKi-naïve population, as shown in JAKARTA, with an SVR35W24 of 36% in the original analysis and 47% in a reanalysis that did not include an additional 4-week confirmatory scan. These numbers compare favorably with those seen in the registration COMFORT trials with ruxolitinib in a similar patient population. Perhaps even more compelling are the data for fedratinib in the second-line setting, particularly in patients without cytopenic MF. Large, mostly randomized studies such as JAKARTA2, FREEDOM, and FREEDOM2 have demonstrated that approximately one-third of patients will achieve SVR35W24 with second-line fedratinib. A detailed description of our approach to prescribing fedratinib can be found in Figure 1.
Recommendations for fedratinib initiation, assessment of efficacy, and toxicity monitoring. CBC, complete blood count; CNL, chronic neutrophilic leukemia.
Recommendations for fedratinib initiation, assessment of efficacy, and toxicity monitoring. CBC, complete blood count; CNL, chronic neutrophilic leukemia.
Selecting the appropriate JAKi, including fedratinib, is dependent on certain patient and disease risk factors. Fedratinib is contraindicated in patients with platelet count <50 × 109/L. Fedratinib has efficacy in patients with mild-to-moderate thrombocytopenia but should be used with caution in patients with thrombocytopenia who are at higher risk for bleeding events (eg, concurrent anticoagulation and history of prior hemorrhage). Patients with moderate-to-severe anemia may preferentially benefit from a JAKi that also inhibits ACVR1, such as momelotinib or pacritinib, especially in the setting of symptomatic anemia or significant cardiovascular comorbidities. It is important to recognize that fedratinib has demonstrated efficacy in patients with cytopenic MF and can be a reasonable option for a patient with mild cytopenias but significant splenomegaly or constitutional symptoms. Fedratinib should be used with caution in patients with severe baseline malnutrition or GI symptoms, and frequent thiamine monitoring should be used to mitigate the risk of WE.
To maximize success with fedratinib, minimizing toxicity by optimizing GI prophylaxis is essential. Although GI toxicity occurs in approximately half of treated patients, it is less common after the first 2 months of therapy and can often be managed with optimized antiemetic and antidiarrheal regimens. Managing patient expectations, especially when transiting from ruxolitinib to fedratinib, is paramount to success, given the possibility of JAKi withdrawal syndrome and new toxicities. Monitoring for and supplementation of thiamine is essential to safe administration. Thiamine levels should be assessed at baseline, and all patients should be started on thiamine supplementation before or at the time of fedratinib initiation. We do not start fedratinib in patients with low thiamine levels before correction or in patients with thiamine levels that do not normalize with supplementation. These measures resulted in no cases of irreversible encephalopathy in the 172 patients treated in the FREEDOM and FREEDOM2 trials.21,22
One should consider making the transition to fedratinib before the disease progresses fully and is refractory to any JAKi option. Therefore, when the physician first notes loss of original spleen response or after at least 3 months of maximal ruxolitinib dosing without response, a sooner rather than delayed transition in therapy should be executed.
It is also important to recognize that clinical trial evaluation of second-line therapies frequently require a “wash-out” period of ≥2 weeks to fully assess SVR. In the real-world setting with commercial fedratinib, this is both unnecessary and potentially detrimental to a smooth transition. We advise making the switch immediately from ruxolitinib to fedratinib and always administering it at the intended full dose of 400 mg once daily. In patients who are taking ruxolitinib at a dose of 10 mg twice daily or higher, tapering by 5 mg twice daily every 4 to 7 days and briefly overlapping with fedratinib may help mitigate the risk of JAKi withdrawal syndrome. We do not routinely advise the use of prophylactic steroids, but their administration and reintroduction of ruxolitinib can be considered if signs or symptoms (fever, increased splenomegaly, and hemodynamic instability) of JAKi withdrawal occur. Although in vitro studies demonstrate the potential for fedratinib discontinuation to provoke JAKi withdrawal syndrome, we do not routinely recommend a taper when stopping, given the low likelihood and lack of reported cases.53 As the trials demonstrate, the expected myelosuppression may warrant transfusional support in the first several months, and a careful monitoring of the patients through laboratory assessments is important to navigate early fedratinib treatment.
Authorship
Contribution: A.C. performed the literature search, wrote the original draft, and created the figures; J.M. provided significant edits and contributed crucial editorial oversight; and both authors conceptualized the article, critically reviewed the manuscript for intellectual content, and approved the final manuscript.
Conflict-of-interest disclosure: J.M. reports research funding paid to the institution from Incyte, Novartis, Roche, Merck, Geron, CTI BioPharma, Bristol Myers Squibb, AbbVie, Celgene, Kartos, and PharmaEssentia, and consulting fees from Incyte, CTI BioPharma, Constellation, Geron, Kartos, Bristol Myers Squibb, Celgene, Novartis, Karyopharm, Sierra Oncology, AbbVie, PharmaEssentia, and Galecto. A.C. declares no competing financial interests.
Correspondence: Alexander Coltoff, Hollings Cancer Center, Medical University of South Carolina, 39 Sabin St 7th Floor, Office 714, Charleston, SC 29425; email: coltoff@musc.edu.