Antithrombotic therapy can prevent recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE). It is, however, associated with an increased risk for major bleeding. This meta-analysis systematically reviewed the evidence regarding the duration of antithrombotic therapy to assess benefits and harms. We systematically searched for randomized controlled trials (RCTs) that compared shorter (3-6 months) with longer (>6 months) courses of anticoagulation for the primary treatment of venous thromboembolism (VTE) or that compared discontinued with indefinite antithrombotic therapy for the secondary prevention of VTE. Pairs of reviewers screened the eligible trials and collected data. This study included 22 RCTs (11 617 participants). Pooled estimates showed that, for the primary treatment of unprovoked VTE, VTE provoked by chronic risk factors or transient risk factors, treating patients with a longer course (>6 months) of anticoagulation, as opposed to a shorter course (3-6 months), probably reduced recurrent PE (risk ratio [RR], 0.66; 95% confidence interval [CI], 0.42-1.02) and DVT (RR, 0.85; 95% CI, 0.63-1.14), but it was associated with increased mortality (RR, 1.43; 95% CI, 0.85-2.41) (moderate certainty) and a higher risk for major bleeding (RR, 2.02; 95% CI, 1.02-3.98; high certainty). For the secondary prevention of unprovoked VTE and VTE provoked by chronic risk factors, when compared with discontinuing treatment, indefinite anticoagulation therapy was associated with decreased mortality (RR, 0.54; 95% CI, 0.36-0.81), a reduction in recurrent PE (RR, 0.25; 95% CI, 0.16-0.41) and DVT (RR, 0.15; 95% CI, 0.10-0.21), and an increase in the risk for bleeding (RR, 1.98; 95% CI, 1.18-3.30), all supported by high certainty. Indefinite antiplatelet therapy may be associated with decreased mortality (RR, 0.95; 95% CI: 0.53-1.68; low certainty), probably a reduction in recurrent PE (RR, 0.65; 95% CI, 0.41-1.03) and DVT (RR, 0.44; 95% CI, 0.17-1.13) (moderate certainty), and may increase the risk for bleeding (RR, 1.28; 95% CI, 0.48-3.41; low certainty). In summary, for the primary treatment of all types of VTE, shorter (3-6 months) duration of anticoagulation is more beneficial. For the secondary prevention of unprovoked VTE or VTE provoked by chronic risk factors, indefinite antithrombotic treatment is more beneficial.
Introduction
Deep vein thrombosis (DVT) and a pulmonary embolism (PE) are 2 manifestations of a venous thromboembolism (VTE), which may cause considerable morbidity and mortality.1,2 It occurs in 1 to 2 individuals per 1000 each year, which translates to 300 000 to 600 000 events in the United States annually.3 Following a primary VTE event, recurrent events may occur in patients with an unprovoked VTE, a VTE associated with transient risk factors, or a VTE associated with chronic risk factors.4-9
Anticoagulation remains the cornerstone of treatment for patients with a VTE based on proven efficacy in reducing the risk for recurrent events.10 However, it is also associated with an increased risk for bleeding. Therefore, when determining the duration of anticoagulation therapy, it is crucial to balance the benefits (namely, the prevention of recurrent VTE) against the harms, primarily major bleeding and all-cause mortality. The American Society of Hematology (ASH) 2020 guidelines for the management of VTE11 describe the treatment decisions for VTE in the following 3 phases (Figure 1): (1) initial management, which occurs from the time of diagnosis through the first 3 weeks of therapy; (2) primary treatment, which is a time-limited phase that typically runs for a minimum of 3 months; and (3) secondary prevention, which begins after completion of the primary treatment phase and extends for a prolonged, usually indefinite, period of time.
Time frame for making decisions regarding anticoagulant treatment. Time frame of the decisions. Initial management (yellow box) spans the first 5 to 21 days following a diagnosis of a new VTE and includes issues concerning whether the patient can be treated at home or it they require admission to the hospital, the use of thrombolytic therapy, whether an inferior vena cava filter needs to be placed, and the initial anticoagulant therapy. During primary treatment, anticoagulant therapy is continued for 3 to 6 months total and represents the minimal duration of treatment for the VTE. After completion of the primary treatment, the next decision concerns whether anticoagulant therapy will be discontinued or if it will be continued for secondary prevention of recurrent VTE. Typically, secondary prevention is continued indefinitely, although patients should be reevaluated on a regular basis to review the benefits and risks of continued anticoagulant therapy. Our choice of terminology reflects the distinct clinical intentions of the different phases of VTE management, linking them to important clinical decisions addressed in the guidelines rather than using terms that reflect the relative duration of therapy. Adapted from the ASH 2020 guidelines for the management of VTE.11
Time frame for making decisions regarding anticoagulant treatment. Time frame of the decisions. Initial management (yellow box) spans the first 5 to 21 days following a diagnosis of a new VTE and includes issues concerning whether the patient can be treated at home or it they require admission to the hospital, the use of thrombolytic therapy, whether an inferior vena cava filter needs to be placed, and the initial anticoagulant therapy. During primary treatment, anticoagulant therapy is continued for 3 to 6 months total and represents the minimal duration of treatment for the VTE. After completion of the primary treatment, the next decision concerns whether anticoagulant therapy will be discontinued or if it will be continued for secondary prevention of recurrent VTE. Typically, secondary prevention is continued indefinitely, although patients should be reevaluated on a regular basis to review the benefits and risks of continued anticoagulant therapy. Our choice of terminology reflects the distinct clinical intentions of the different phases of VTE management, linking them to important clinical decisions addressed in the guidelines rather than using terms that reflect the relative duration of therapy. Adapted from the ASH 2020 guidelines for the management of VTE.11
Because of the continuing risk for bleeding and the uncertainty regarding the risk for recurrent VTE, it is important to review the evidence to guide decision-making in each of these phases. In the context of developing the recommendations for treatment of VTEs for the ASH guidelines,11 we systematically reviewed the evidence using standardized inclusion criteria, pooled analysis methods, and assessment of the evidence methods to evaluate decisions for 2 of the 3 therapeutic management phases. Specifically, this review provided the evidence base and updates based on new evidence to support 5 key recommendations in the 2020 ASH VTE treatment guidelines (recommendations 12, 13, 14, 18, and 19; see details in supplemental Table 1). For the primary treatment of VTE, the ASH guideline panel suggests a shorter anticoagulation course (3-6 months) instead of a longer course (>12 months), regardless of whether the VTE is provoked by a transient or chronic risk factor or if it was unprovoked; for secondary prevention, after completing primary treatment for unprovoked VTE or VTE caused by a chronic risk factor, the panel suggests indefinite antithrombotic therapy over stopping anticoagulation.
Methods
This systematic review was performed as evidence base for the ASH guidelines on the treatment of VTE, developed in partnership with the McMaster University’s Grading of Recommendations Assessment, Development and Evaluation (GRADE) Centre. The evidence synthesis team consisted of methodologists and clinical experts in hematology. The review and meta-analysis methodology followed the Cochrane Handbook,12 and reporting was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.13
Eligibility criteria
Studies were eligible if they included adult patients (≥18 years of age) diagnosed with primary or recurrent VTE that was unprovoked, provoked by transient risk factors, or provoked by chronic risk factors. Studies were included if they evaluated the duration of anticoagulation (direct oral anticoagulants [DOAC] or vitamin K antagonist [VKA]/low molecular weight heparin [LMWH]) for the primary treatment period (3-6 months vs >6 months duration) or the duration of antithrombotic treatment (DOAC, VKA/LMWH, or antiplatelet) during the secondary prevention period (discontinued vs indefinite duration). Only randomized controlled trials (RCTs) were eligible. We excluded studies that included patients with cancer with VTE.
To evaluate the effect of duration of the primary treatment for unprovoked VTE, VTE provoked by chronic risk factors, and VTE provoked by transient risk factors, we included studies that compared shorter duration (3-6 months) with longer duration (>6 months) anticoagulant treatment after the initial management phase. Studies were also eligible if they included patients who had completed treatment with anticoagulants for 3 to 6 months (ie, everyone completed the short duration) and who were then randomized to receive either a placebo or to continue additional treatment. Studies were included if the outcomes were assessed in both groups at the end of the follow-up period rather than at the end of treatment. We only included RCTs that measured the outcomes of both groups 3 months or longer after the end of longer anticoagulation treatment.
To evaluate whether to continue secondary prevention indefinitely for unprovoked VTE and VTE provoked by chronic risk factors, we included studies that compared discontinuing treatment after 3 to 12 months with treatment for indefinite duration that lasted longer than 12 months. Studies were included if the outcomes were assessed in both groups immediately at the end of the longer duration of antithrombotic therapy.
The following outcomes were prioritized as critical for clinical decision-making by the ASH guideline panel: all-cause mortality; nonfatal PE, which was considered present if documented objectively; DVT or recurrent DVT, which was considered present if documented objectively (either a venous segment of thrombus on ultrasonography or a new intraluminal filling defect on contrast venography); and major bleeding during the treatment or follow-up period after the initial DVT or PE diagnosis, defined using the International Society on Thrombosis and Haemostasis criteria.14
Information sources and search
We searched MEDLINE (1996 to 31 December 2018, and updated the search on 21 December 2024), Embase (1974 to 31 December 2018, and updated the search on 12 December 2024), and the Cochrane Central Register of Controlled Trials (until 31 December 2018) (supplemental Table 2). The search strategy consisted of predefined key words specific to each database. The searches were restricted to RCTs of human subjects but not restricted by language. In addition, the reference lists of relevant studies and systematic reviews were scanned, and clinical experts in the field of anticoagulation management were consulted for additional references. We used Epistemonikos to identify relevant published systematic reviews and screened the references.
Study selection
Reviewers worked in pairs, independently screened titles, abstracts, and the full text of relevant articles based on prespecified inclusion and exclusion criteria. Disagreements were resolved by consensus and by a third reviewer when needed (Y.-Q.Z.).
Data abstraction
Pairs of reviewers independently extracted data and discussed the data to reach consensus, and a third reviewer resolved discrepancies when needed (Y.-Q.Z.). The data collected included patient characteristics (mean age and proportion of females), length of treatment and number of patients in each study arm, characteristics of the initial VTE (percentage first VTE episode, percentage DVT, and percentage unprovoked), and time of outcome assessment (counted from randomization).
Quality assessment and analysis
Information on the risk of bias was collected and assessed for each outcome in each included study using the Cochrane risk of bias tool for RCTs.12 The risk ratios (RRs) and 95% confidence intervals (CIs) were calculated by pooling the results from the RCTs using the Mantel-Haenszel method and the random effects model. Heterogeneity was assessed using the I2 index and was deemed as moderate to high at an I2 > 50%.12
Because the relative effect was consistent across the population, we combined and analyzed patients with unprovoked VTE, VTE provoked by transient risk factors (eg, following trauma or surgery), and VTE provoked by chronic risk factors (eg, chronic immobility) or a mixed type of VTE. Because this review informs the ASH clinical practice guidelines and to enable evaluation of the evidence for each of the 3 types of VTE, we also calculated the absolute risk reduction (ARR) using baseline risks from large observational studies or meta-analyses of unprovoked VTE, VTE provoked by transient risk factors, and VTE provoked by chronic risk factors.15-18 When no observational study or meta-analysis was available, we took the median of the control group from eligible RCTs as the baseline risk.
Data were analyzed using ReviewManager (RevMan, version 5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, 2012, Copenhagen, Denmark). Pairs of reviewers evaluated the certainty of the evidence for each outcome using the GRADE approach, and a third adjudicator (Y.-Q.Z.) was involved if the pair could not reach an agreement. We made judgments regarding the risk of bias, precision, consistency, directness, and likelihood of publication bias. The certainty of the evidence was assessed as high, moderate, low, or very low and summarized in a GRADE Evidence Profile.19 Because anticoagulants may have different effects than antiplatelet agents, we stratified the pooled effect estimate by type of antithrombotic therapy. In addition, if there was substantial heterogeneity among anticoagulants, we further performed subgroup analyses by anticoagulant type (DOAC or VKA/LMWH).
Ethics and patient consent statement
This study is based on the existing trials and data in the databases, thus not subject to ethical review and patient consent.
Results
Search results
After excluding duplicates, a total of 12 210 titles and abstracts were screened for eligibility. Of these, we excluded 12 143, leaving 67 for full-text screening. A total of 45 were excluded (see supplemental Figure 1 for Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram), leaving 22 eligible studies that were included in this systematic review and meta-analysis (Table 1). Of the 22 eligible studies, 6 assessed the outcomes at some point after treatment was stopped (evaluated decision on length of primary treatment only), 4 assessed outcomes at some point after treatment was stopped and immediately after treatment was stopped (evaluated decisions on length of primary treatment and secondary prevention), and 12 assessed outcomes immediately after treatment was stopped without any long-term follow-up (evaluated decision on length of secondary prevention only). The results are presented separately for studies that evaluated primary treatment and secondary prevention.
Characteristics of included studies
| Author, year . | Characteristics of primary VTE event . | Treatment by arm∗ (n patients) . | Mean age (y) (SD) . | Percentage females . | Outcome assessment at end of treatment† . | Outcome assessment at end of follow-up† . |
|---|---|---|---|---|---|---|
| Primary treatment | ||||||
| Schulman et al, 198520 | First VTE episode: 0% (2nd DVT only) DVT: 100% proximal DVT Unprovoked: 0% | Short: 6 mo (n = 10) | 63.7 | 60 | NA | 15-27 mo |
| Long: 12 mo (n = 10) | 65.5 | 60 | ||||
| Siragusa et al, 200821 | First VTE episode: 100% DVT: 100% proximal DVT Unprovoked: 77% Included patients with RVT only | Short: 3 mo (n = 92) | 61.1 (11.5) | 48 | NA | 21 mo |
| Long: 12 mo (n = 88) | 57.1 (14.1) | 47 | ||||
| Agnelli et al, 200322 | First VTE episode: 100% DVT: 0% (PE only) Unprovoked: 56% | Short: 6 mo (n = 161) | 61 (15.5) | 58 | NA | 36 mo |
| Long: 12 mo (n = 165) | 62.9 (16.3) | 61 | ||||
| Agnelli et al, 200123 | First VTE episode: 100% DVT: 100% proximal DVT Unprovoked: 100% | Short: 3 mo (n = 133) | 67.7 (7.3) | 38 | NA | 36 mo |
| Long: 12 mo (n = 134) | 66.8 (6.7) | 46 | ||||
| Vitovec et al, 200924 | First VTE episode: 100% DVT: 100% Unprovoked: 100% | Short: 6 mo (n = 25) | Overall: 58 (12) | Overall: 54 | NA | 18 mo |
| Long: 12 mo (n = 27) | ||||||
| Van Gogh Investigators, 200725 | First VTE episode: 82% DVT: 55% Unprovoked: 60% | Short: 6 mo (n = 621) | 59.9 (15.4) | 48 | NA | 12 mo |
| Long: 12 mo (n = 594) | 60.2 (15.2) | 47 | ||||
| Both primary treatment and secondary prevention | ||||||
| Farraj, 200426 | First VTE episode: 100% DVT: 72% Unprovoked: 100% | Short (discontinued): 6 mo (n = 32) | 42 (14) | 44 | 24 mo | 36 mo |
| Long (indefinite): 24 mo (n = 32) | 41 (15) | 38 | ||||
| Couturaud et al, 201527 | First VTE episode: 100% DVT: 0% (PE only) Unprovoked: 100% | Short (discontinued): 6 mo (n = 187) | 57.3 (17.4) | 45 | 18 mo | 42 mo |
| Long (indefinite): 24 mo (n = 184) | 58.7 (17.9) | 58 | ||||
| Couturaud et al, 201928 | First VTE episode: 100% DVT: 100% proximal DVT Unprovoked: 100% | Short (discontinued): 6 mo (n = 54) | 61.5 (14.5) | 38 | 18 mo | 42 mo |
| Long (indefinite): 24 mo (n = 50) | 59 (17.2) | 28 | ||||
| Eischer et al, 200929 | First VTE episode: 100% DVT: 59% Unprovoked: NR Limited to patients with high factor VIII only | Short (discontinued): 6 mo (n = 17) | 54 (16) | 65 | 24 mo | 48 mo |
| Long (indefinite): 30 mo (n = 17) | 53 (18) | 71 | ||||
| Secondary prevention | ||||||
| Belcaro et al, 199330 | First VTE episode: 100% DVT: 100% Unprovoked: NR | Discontinued: 6 mo (n = 63) | 45.7 (12) | 52 | 30 mo | NA |
| Indefinite: 36 mo (n = 60) | 45.3 (11) | 52 | ||||
| Schulman et al, 200331 | First VTE episode: 86% DVT: 65% Unprovoked: NR | Discontinued: 6 mo (n = 611) | 58 (15) | 49 | 18 mo | NA |
| Indefinite: 24 mo (n = 612) | 56 (15) | 46 | ||||
| Agnelli et al, 201332 | First VTE episode: 100% DVT: 65% Unprovoked: 92% | Discontinued: 6-12 mo (n = 829) | 57.1 (15.2) | 35 | 12 mo | NA |
| Indefinite: Finished 6-12 mo, then continued 12 mo (n = 1653) | 56.6 (15.3) to 56.4 (15.6) | 42 to 42 | ||||
| Palareti et al, 200633 | First VTE episode: 100% DVT: 63% Unprovoked: 100% | Discontinued: 3 mo (n = 120) | 68.2 (12.5) | 58 | 18 mo | NA |
| Indefinite: 21 mo (n = 103) | 70.1 (13.7) | 47 | ||||
| Ridker et al, 200334 | First VTE episode: 62% DVT: NR Unprovoked: 100% | Discontinued: 6.5 mo median (n = 253) | 53 (47-64) median | 47 | Mean of 2.1 y and up to 4.3 y, the trial was terminated prematurely | NA |
| Indefinite: Finished 6.5 mo median, followed by mean of 2.1 y and up to 4.3 y (n = 255) | 53 (46-65) median | 47 | ||||
| Kearon et al, 199935 | First VTE episode: 100% DVT: 75% Unprovoked: 100% | Discontinued: 3 mo (n = 83) | 58 (16) | 47 | 24 mo | NA |
| Indefinite: 27 mo (n = 79) | 59 (16) | 32 | ||||
| Bauersachs et al, 201036 | First VTE episode: 100% DVT: 62% Unprovoked: 74% | Discontinued: 6-12 mo (n = 594) | 58 (16) | 43 | 12 mo | NA |
| Indefinite: Finished 6-12 mo, then continued 6 or 12 mo (n = 602) | 58 (16) | 41 | ||||
| Schulman et al, 199737 | First VTE episode: 0% (2nd VTE only) DVT: 85% Unprovoked: NR | Discontinued: 6 mo (n = 111) | 65 (12.4) | 37 | 48 mo | NA |
| Indefinite: 48 mo (n = 116) | 64 (12.5) | 41 | ||||
| Schulman et al, 201338 | First VTE episode: NR DVT: 65% Unprovoked: NR | Discontinued: 6-18 mo, mean of 10 mo (n = 681) | 56.1 (15.5) | 44 | 6 mo | NA |
| Indefinite: Finished 6-18 mo, then continued 6 mo (n = 662) | 55.5 (15.1) | 45 | ||||
| Becattini et al, 201239 | First VTE episode: 100% DVT: 63% Unprovoked: 100% | Discontinued: 6-18 mo (n = 197) | 62.1 (15.1) | 38 | 24 mo | NA |
| Indefinite: Finished 6-18 mo, then continued 24 mo (n = 205) aspirin | 61.9 (15.3) | 34 | ||||
| Brighton et al, 201240 | First VTE episode: 100% DVT: 57% Unprovoked: 100% | Discontinued: 89% of 3-12 mo (n = 411) | 54 (15.8) | 46 | The median duration of follow-up was 37.2 mo | NA |
| Indefinite: Finished 1.5-24 mo, then continued 24-48 mo (n = 411) aspirin | 55 (16.0) | 45 | ||||
| Bradbury et al, 202041 | First VTE episode: 100% DVT: 51% Unprovoked: 100% | Discontinued: 3 mo (n = 140) | 63.3 (12.7) | 33 | 24 mo | NA |
| Indefinite: 27 mo (n = 141) | 62.2 (13) | 32 | ||||
| Author, year . | Characteristics of primary VTE event . | Treatment by arm∗ (n patients) . | Mean age (y) (SD) . | Percentage females . | Outcome assessment at end of treatment† . | Outcome assessment at end of follow-up† . |
|---|---|---|---|---|---|---|
| Primary treatment | ||||||
| Schulman et al, 198520 | First VTE episode: 0% (2nd DVT only) DVT: 100% proximal DVT Unprovoked: 0% | Short: 6 mo (n = 10) | 63.7 | 60 | NA | 15-27 mo |
| Long: 12 mo (n = 10) | 65.5 | 60 | ||||
| Siragusa et al, 200821 | First VTE episode: 100% DVT: 100% proximal DVT Unprovoked: 77% Included patients with RVT only | Short: 3 mo (n = 92) | 61.1 (11.5) | 48 | NA | 21 mo |
| Long: 12 mo (n = 88) | 57.1 (14.1) | 47 | ||||
| Agnelli et al, 200322 | First VTE episode: 100% DVT: 0% (PE only) Unprovoked: 56% | Short: 6 mo (n = 161) | 61 (15.5) | 58 | NA | 36 mo |
| Long: 12 mo (n = 165) | 62.9 (16.3) | 61 | ||||
| Agnelli et al, 200123 | First VTE episode: 100% DVT: 100% proximal DVT Unprovoked: 100% | Short: 3 mo (n = 133) | 67.7 (7.3) | 38 | NA | 36 mo |
| Long: 12 mo (n = 134) | 66.8 (6.7) | 46 | ||||
| Vitovec et al, 200924 | First VTE episode: 100% DVT: 100% Unprovoked: 100% | Short: 6 mo (n = 25) | Overall: 58 (12) | Overall: 54 | NA | 18 mo |
| Long: 12 mo (n = 27) | ||||||
| Van Gogh Investigators, 200725 | First VTE episode: 82% DVT: 55% Unprovoked: 60% | Short: 6 mo (n = 621) | 59.9 (15.4) | 48 | NA | 12 mo |
| Long: 12 mo (n = 594) | 60.2 (15.2) | 47 | ||||
| Both primary treatment and secondary prevention | ||||||
| Farraj, 200426 | First VTE episode: 100% DVT: 72% Unprovoked: 100% | Short (discontinued): 6 mo (n = 32) | 42 (14) | 44 | 24 mo | 36 mo |
| Long (indefinite): 24 mo (n = 32) | 41 (15) | 38 | ||||
| Couturaud et al, 201527 | First VTE episode: 100% DVT: 0% (PE only) Unprovoked: 100% | Short (discontinued): 6 mo (n = 187) | 57.3 (17.4) | 45 | 18 mo | 42 mo |
| Long (indefinite): 24 mo (n = 184) | 58.7 (17.9) | 58 | ||||
| Couturaud et al, 201928 | First VTE episode: 100% DVT: 100% proximal DVT Unprovoked: 100% | Short (discontinued): 6 mo (n = 54) | 61.5 (14.5) | 38 | 18 mo | 42 mo |
| Long (indefinite): 24 mo (n = 50) | 59 (17.2) | 28 | ||||
| Eischer et al, 200929 | First VTE episode: 100% DVT: 59% Unprovoked: NR Limited to patients with high factor VIII only | Short (discontinued): 6 mo (n = 17) | 54 (16) | 65 | 24 mo | 48 mo |
| Long (indefinite): 30 mo (n = 17) | 53 (18) | 71 | ||||
| Secondary prevention | ||||||
| Belcaro et al, 199330 | First VTE episode: 100% DVT: 100% Unprovoked: NR | Discontinued: 6 mo (n = 63) | 45.7 (12) | 52 | 30 mo | NA |
| Indefinite: 36 mo (n = 60) | 45.3 (11) | 52 | ||||
| Schulman et al, 200331 | First VTE episode: 86% DVT: 65% Unprovoked: NR | Discontinued: 6 mo (n = 611) | 58 (15) | 49 | 18 mo | NA |
| Indefinite: 24 mo (n = 612) | 56 (15) | 46 | ||||
| Agnelli et al, 201332 | First VTE episode: 100% DVT: 65% Unprovoked: 92% | Discontinued: 6-12 mo (n = 829) | 57.1 (15.2) | 35 | 12 mo | NA |
| Indefinite: Finished 6-12 mo, then continued 12 mo (n = 1653) | 56.6 (15.3) to 56.4 (15.6) | 42 to 42 | ||||
| Palareti et al, 200633 | First VTE episode: 100% DVT: 63% Unprovoked: 100% | Discontinued: 3 mo (n = 120) | 68.2 (12.5) | 58 | 18 mo | NA |
| Indefinite: 21 mo (n = 103) | 70.1 (13.7) | 47 | ||||
| Ridker et al, 200334 | First VTE episode: 62% DVT: NR Unprovoked: 100% | Discontinued: 6.5 mo median (n = 253) | 53 (47-64) median | 47 | Mean of 2.1 y and up to 4.3 y, the trial was terminated prematurely | NA |
| Indefinite: Finished 6.5 mo median, followed by mean of 2.1 y and up to 4.3 y (n = 255) | 53 (46-65) median | 47 | ||||
| Kearon et al, 199935 | First VTE episode: 100% DVT: 75% Unprovoked: 100% | Discontinued: 3 mo (n = 83) | 58 (16) | 47 | 24 mo | NA |
| Indefinite: 27 mo (n = 79) | 59 (16) | 32 | ||||
| Bauersachs et al, 201036 | First VTE episode: 100% DVT: 62% Unprovoked: 74% | Discontinued: 6-12 mo (n = 594) | 58 (16) | 43 | 12 mo | NA |
| Indefinite: Finished 6-12 mo, then continued 6 or 12 mo (n = 602) | 58 (16) | 41 | ||||
| Schulman et al, 199737 | First VTE episode: 0% (2nd VTE only) DVT: 85% Unprovoked: NR | Discontinued: 6 mo (n = 111) | 65 (12.4) | 37 | 48 mo | NA |
| Indefinite: 48 mo (n = 116) | 64 (12.5) | 41 | ||||
| Schulman et al, 201338 | First VTE episode: NR DVT: 65% Unprovoked: NR | Discontinued: 6-18 mo, mean of 10 mo (n = 681) | 56.1 (15.5) | 44 | 6 mo | NA |
| Indefinite: Finished 6-18 mo, then continued 6 mo (n = 662) | 55.5 (15.1) | 45 | ||||
| Becattini et al, 201239 | First VTE episode: 100% DVT: 63% Unprovoked: 100% | Discontinued: 6-18 mo (n = 197) | 62.1 (15.1) | 38 | 24 mo | NA |
| Indefinite: Finished 6-18 mo, then continued 24 mo (n = 205) aspirin | 61.9 (15.3) | 34 | ||||
| Brighton et al, 201240 | First VTE episode: 100% DVT: 57% Unprovoked: 100% | Discontinued: 89% of 3-12 mo (n = 411) | 54 (15.8) | 46 | The median duration of follow-up was 37.2 mo | NA |
| Indefinite: Finished 1.5-24 mo, then continued 24-48 mo (n = 411) aspirin | 55 (16.0) | 45 | ||||
| Bradbury et al, 202041 | First VTE episode: 100% DVT: 51% Unprovoked: 100% | Discontinued: 3 mo (n = 140) | 63.3 (12.7) | 33 | 24 mo | NA |
| Indefinite: 27 mo (n = 141) | 62.2 (13) | 32 | ||||
NA, not assessed; NR, not reported; RVT, residual vein thrombosis.
Treatment duration includes the period that patients were on an anticoagulant or antithrombotic therapy (including the period before randomization). Short or long arms refer to studies that assessed primary treatment; these studies assessed outcomes at least 3 months after treatment was stopped in the long arm. Discontinued or indefinite arms refer to studies that assessed secondary prevention; these studies assessed outcomes immediately after treatment was stopped in the indefinite arm. Four studies assessed both primary treatment and secondary prevention and reported outcomes at 2 time points as noted in the table.
The time period was counted from randomization.
Study characteristics
Primary treatment
We identified 10 RCTs (2633 patients) that compared a duration of 3 to 6 months with >6 months for primary anticoagulation therapy. The length of treatment in the shorter duration arm (3-6 months) was 3 months in 2 studies21,23 and 6 months in the remaining studies.20,22,24-29 The length of treatment in the long duration arm was 12 months in 6 studies,20-25 30 months in 1 study,29 and 24 months in the remaining 3 studies.26-28 All studies used VKA/LMWH for VTE treatment.
The mean age ranged from 41 years to 67.7 years, and the proportion of females ranged from 28% to 71%. A total of 8 studies21-24,26-29 (80%) limited their patient population to those with a first VTE episode only, and 5 studies23,24,26-28 (50%) limited their study to unprovoked VTE only. Five studies20,21,23,24,28 included patients with DVT only, 2 studies22,27 included patients with PE only; the remaining 3 studies25,26,29 included a mix of DVTs and Pes as the primary VTE. Outcomes were assessed between 12 months and 48 months from randomization.
Secondary prevention
We identified 16 RCTs (9557 patients) that compared stopping vs continuing treatment indefinitely for the secondary prevention of VTE. Treatment in the short duration (3-6 months) or discontinued arm was stopped at 3 months in 3 studies33,35,41 and at 6 to 18 months in the remaining studies.26-32,34,36-40 Treatment in the long or indefinite arm was continued until the outcomes were assessed, which ranged from 12 months to 4.3 years. Four studies31,32,36,38 used DOAC for treatment, 2 studies39,40 used aspirin for treatment, 1 study30 used indobufen for treatment, and the remaining 9 studies26-29,33-35,37,41 used VKA/LMWH for VTE treatment.
The mean age ranged from 41 years to 70.1 years, and the proportion of females ranged from 32% to 71%. A total of 12 studies26-30,32,33,35,36,39-41 (75%) limited their patient population to those with a first VTE episode only, and 9 studies26-28,33-35,39-41 (56%) limited their study to unprovoked VTE only. Two studies28,30 included patients with DVT only, 1 study27 included patients with PE only, and the remaining 13 studies26,29,31-41 included a mix of DVTs and PEs as the primary VTE. Outcomes were assessed between 6 months and 4.3 years from randomization.
Risk of bias of the included studies
Risk of bias was assessed across the 22 included studies (supplemental Figure 2). Random sequence generation to prevent selection bias was reported in 19 (86%) studies.20-23,25-28,31-41 Allocation concealment, which also prevents selection bias, was reported in 17 (77%) studies.20-23,25-28,31-33,35,36,38-41 Performance bias was determined by reference to blinding of participants or personnel. In the included studies,11,25,27,28,31,32,34-36,38-40 (50%) reported blinding of participants and/or personnel. Blinding of outcome assessors to prevent detection bias was reported by 18 (82%) studies.21-23,25,27-29,31-41 Complete reporting of outcome data was presented in 17 (77%) studies20,22-25,27-29,31,32,34-40 to prevent attrition bias. Selective reporting was assessed by identifying outcomes assessed a priori. Eleven (50%) studies21,25,27,28,32,33,36,38-41 were free of selective reporting. Other biases included any concerns about bias not addressed in the other domains. None of the included studies reported other biases.
Synthesis of results
Primary treatment
ALL-CAUSE MORTALITY
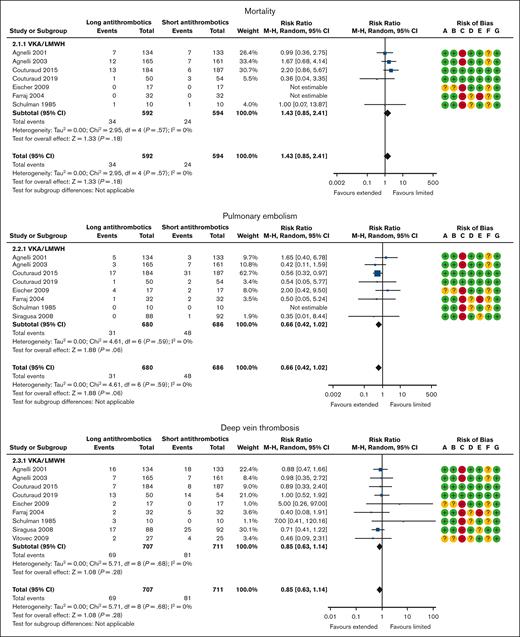
Mortality was reported in 7 VKA trials20,22,23,26-29 with 1186 patients and a mean follow-up of 37 months. Overall, 24 of the 594 patients (4.0%) assigned to the shorter duration (3-6 months) arm and 34 of 592 patients (5.7%) assigned to the longer duration arm died. When compared with the shorter duration (3-6 months) therapy, a longer duration of anticoagulation therapy(>6 months) was probably associated with increased all-cause mortality (RR, 1.43; 95% CI, 0.85-2.41; Figure 2; ARR, 6 more per 1000 [95% CI, 2 fewer to 20 more] for patients with unprovoked VTE, VTE provoked by chronic risk factors, or transient risk factors; moderate certainty; Tables 2, 3, and 4).
Primary treatment: shorter duration (3-6 months) vs longer duration (>6 months) of anticoagulation. Risk of bias legend: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias.
Primary treatment: shorter duration (3-6 months) vs longer duration (>6 months) of anticoagulation. Risk of bias legend: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias.
Summary of the effects of shorter (3-6 months) vs longer (>6 months) duration of anticoagulation treatment for primary treatment in patients with unprovoked VTE
| Certainty of evidence assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With short-term anticoagulation (3 to 6 mo) . | With long-term anticoagulation (>6 mo) . | Risk with short-term anticoagulation (3 to 6 mo) . | Risk difference with long-term anticoagulation (>6 mo) . | ||||||||
| Mortality (follow-up: mean 37 mo) | |||||||||||
| 1186 (7 RCTs)20,22,23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 24/594 (4.0) | 34/592 (5.7) | RR 1.43 (0.85-2.41) | Study population | |
| 40 per 1000 | 17 more per 1000 (from 6 fewer to 56 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000† | 6 more per 1000 (from 2 fewer to 20 more) | ||||||||||
| Nonfatal PE (follow-up: mean 35 mo) | |||||||||||
| 1366 (8 RCTs)20-23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 48/686 (7.0) | 31/680 (4.6) | RR 0.66 (0.42-1.02) | Study population | |
| 70 per 1000 | 24 fewer per 1000 (from 41 fewer to 1 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 36 per 1000 (16, 17)‡ | 12 fewer per 1000 (from 21 fewer to 1 more) | ||||||||||
| DVT (follow-up: mean 33 mo) | |||||||||||
| 1418 (9 RCTs)20-24,26-29 | Not serious | Not serious | Not serious | Serious∗ | none | ⨁⨁⨁◯ MODERATE | 81/711 (11.4) | 69/707 (9.8) | RR 0.85 (0.63-1.14) | Study population | |
| 114 per 1000 | 17 fewer per 1000 (from 42 fewer to 16 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 44 per 1000 (16, 17)‡ | 7 fewer per 1000 (from 16 fewer to 6 more) | ||||||||||
| Major bleeding (follow-up: mean 33 mo) | |||||||||||
| 2581 (9 RCTs)20-23,25-29 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 11/1307 (0.8) | 36/1274 (2.8) | RR 2.02 (1.02-3.98) | Study population | |
| 8 per 1000 | 8 more per 1000 (from 0 fewer to 24 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 21 per 1000 (15)§ | 21 more per 1000 (from 0 more to 63 more) | ||||||||||
| Certainty of evidence assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With short-term anticoagulation (3 to 6 mo) . | With long-term anticoagulation (>6 mo) . | Risk with short-term anticoagulation (3 to 6 mo) . | Risk difference with long-term anticoagulation (>6 mo) . | ||||||||
| Mortality (follow-up: mean 37 mo) | |||||||||||
| 1186 (7 RCTs)20,22,23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 24/594 (4.0) | 34/592 (5.7) | RR 1.43 (0.85-2.41) | Study population | |
| 40 per 1000 | 17 more per 1000 (from 6 fewer to 56 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000† | 6 more per 1000 (from 2 fewer to 20 more) | ||||||||||
| Nonfatal PE (follow-up: mean 35 mo) | |||||||||||
| 1366 (8 RCTs)20-23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 48/686 (7.0) | 31/680 (4.6) | RR 0.66 (0.42-1.02) | Study population | |
| 70 per 1000 | 24 fewer per 1000 (from 41 fewer to 1 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 36 per 1000 (16, 17)‡ | 12 fewer per 1000 (from 21 fewer to 1 more) | ||||||||||
| DVT (follow-up: mean 33 mo) | |||||||||||
| 1418 (9 RCTs)20-24,26-29 | Not serious | Not serious | Not serious | Serious∗ | none | ⨁⨁⨁◯ MODERATE | 81/711 (11.4) | 69/707 (9.8) | RR 0.85 (0.63-1.14) | Study population | |
| 114 per 1000 | 17 fewer per 1000 (from 42 fewer to 16 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 44 per 1000 (16, 17)‡ | 7 fewer per 1000 (from 16 fewer to 6 more) | ||||||||||
| Major bleeding (follow-up: mean 33 mo) | |||||||||||
| 2581 (9 RCTs)20-23,25-29 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 11/1307 (0.8) | 36/1274 (2.8) | RR 2.02 (1.02-3.98) | Study population | |
| 8 per 1000 | 8 more per 1000 (from 0 fewer to 24 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 21 per 1000 (15)§ | 21 more per 1000 (from 0 more to 63 more) | ||||||||||
The CI around absolute estimates does not rule out substantial benefit or substantial harm.
We used the median in the short duration treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 1.4%.
A meta-analysis18 reported that the cumulative incidence of recurrent VTE was 16% at 2 years for patients with unprovoked VTE, and because the median follow-up of our included studies was 24 months, we calculated the baseline risk to be 8% per patient-year. Assuming that 45% of the VTE events were PE and 55% were DVT,17 we estimated that, for patients with unprovoked VTE, the risk for PE recurrence was 3.6 per 100 patient-years and DVT recurrence was 4.4 per 100 patient-years.
A meta-analysis of 13 prospective cohort studies and 56 randomized trials15 showed that, in people with VTE, the risk for major bleeding during a 6-month treatment with anticoagulants was 2.1%. Assuming a risk for major bleeding of close to zero after anticoagulant discontinuation, we estimated an annualized risk for major bleeding of 2.1%.
Summary of the effects of shorter (3-6 months) vs longer (greater than 6 months) duration of anticoagulation therapy for primary treatment in patients with VTE provoked by chronic risk factors
| Certainty of evidence assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With short-term anticoagulation (3 to 6 mo) . | With long-term anticoagulation (>6 mo) . | Risk with short-term anticoagulation (3 to 6 mo) . | Risk difference with long-term anticoagulation (>6 mo) . | ||||||||
| Mortality (follow-up: mean 37 mo) | |||||||||||
| 1186 (7 RCTs)20,22,23,26-29 | Not serious | Not serious | Not serious | Serious∗ | none | ⨁⨁⨁◯ MODERATE | 24/594 (4.0) | 34/592 (5.7) | RR 1.43 (0.85-2.41) | Study population | |
| 40 per 1000 | 17 more per 1000 (from 6 fewer to 56 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000† | 6 more per 1000 (from 2 fewer to 20 more) | ||||||||||
| Nonfatal PE (follow-up: mean 35 mo) | |||||||||||
| 1366 (8 RCTs)20-23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 48/686 (7.0) | 31/680 (4.6) | RR 0.66 (0.42-1.02) | Study population | |
| 70 per 1000 | 24 fewer per 1000 (from 41 fewer to 1 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 19 per 1000 (9, 10)‡ | 6 fewer per 1000 (from 11 fewer to 0 more) | ||||||||||
| DVT (follow-up: mean 33 mo) | |||||||||||
| 1418 (9 RCTs)20-24,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 81/711 (11.4) | 69/707 (9.8) | RR 0.85 (0.63-1.14) | Study population | |
| 114 per 1000 | 17 fewer per 1000 (from 42 fewer to 16 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 23 per 1000 (9, 10)‡ | 3 fewer per 1000 (from 9 fewer to 3 more) | ||||||||||
| Major bleeding (follow-up: mean 33 mo) | |||||||||||
| 2581 (9 RCTs)20-23,25-29 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 11/1307 (0.8) | 36/1274 (2.8) | RR 2.02 (1.02-3.98) | Study population | |
| 8 per 1000 | 8 more per 1000 (from 0 fewer to 24 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 21 per 1000 (13)§ | 21 more per 1000 (from 0 more to 63 more) | ||||||||||
| Certainty of evidence assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With short-term anticoagulation (3 to 6 mo) . | With long-term anticoagulation (>6 mo) . | Risk with short-term anticoagulation (3 to 6 mo) . | Risk difference with long-term anticoagulation (>6 mo) . | ||||||||
| Mortality (follow-up: mean 37 mo) | |||||||||||
| 1186 (7 RCTs)20,22,23,26-29 | Not serious | Not serious | Not serious | Serious∗ | none | ⨁⨁⨁◯ MODERATE | 24/594 (4.0) | 34/592 (5.7) | RR 1.43 (0.85-2.41) | Study population | |
| 40 per 1000 | 17 more per 1000 (from 6 fewer to 56 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000† | 6 more per 1000 (from 2 fewer to 20 more) | ||||||||||
| Nonfatal PE (follow-up: mean 35 mo) | |||||||||||
| 1366 (8 RCTs)20-23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 48/686 (7.0) | 31/680 (4.6) | RR 0.66 (0.42-1.02) | Study population | |
| 70 per 1000 | 24 fewer per 1000 (from 41 fewer to 1 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 19 per 1000 (9, 10)‡ | 6 fewer per 1000 (from 11 fewer to 0 more) | ||||||||||
| DVT (follow-up: mean 33 mo) | |||||||||||
| 1418 (9 RCTs)20-24,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 81/711 (11.4) | 69/707 (9.8) | RR 0.85 (0.63-1.14) | Study population | |
| 114 per 1000 | 17 fewer per 1000 (from 42 fewer to 16 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 23 per 1000 (9, 10)‡ | 3 fewer per 1000 (from 9 fewer to 3 more) | ||||||||||
| Major bleeding (follow-up: mean 33 mo) | |||||||||||
| 2581 (9 RCTs)20-23,25-29 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 11/1307 (0.8) | 36/1274 (2.8) | RR 2.02 (1.02-3.98) | Study population | |
| 8 per 1000 | 8 more per 1000 (from 0 fewer to 24 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 21 per 1000 (13)§ | 21 more per 1000 (from 0 more to 63 more) | ||||||||||
The CI around the absolute estimates does not rule out substantial benefit or substantial harm.
We used the median in the short-duration treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 1.4%.
A meta-analysis of 10 cohort studies and 5 randomized trials16 reported that the rate of VTE recurrence was 4.2% per patient-year for patients with a chronic risk factor. Assuming that 45% of the VTE events were PE and 55% were DVT,17 we estimated that, for patients with VTE provoked by chronic risk factors, the risk for PE recurrence was 1.89 per 100 patient-years and DVT recurrence was 2.31 per 100 patient-years.
A meta-analysis of 13 prospective cohort studies and 56 randomized trials15 showed that, in people with VTE, the risk for major bleeding during a 6-months treatment with anticoagulants was 2.1%. Assuming a risk for major bleeding of close to zero after anticoagulant discontinuation, we estimated an annualized risk for major bleeding of 2.1%.
Summary of the effects of shorter (3-6 months) vs longer (greater than 6 months) duration of anticoagulation therapy for primary treatment in patients with a VTE provoked by transient risk factors
| Certainty of evidence assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With short-term anticoagulation (3 to 6 mo) . | With long-term anticoagulation (>6 mo) . | Risk with short-term anticoagulation (3 to 6 mo) . | Risk difference with long-term anticoagulation (>6 mo) . | ||||||||
| Mortality (follow up: mean 37 mo) | |||||||||||
| 1186 (7 RCTs)20,22,23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 24/594 (4.0) | 34/592 (5.7) | RR 1.43 (0.85-2.41) | Study population | |
| 40 per 1000 | 17 more per 1000 (from 6 fewer to 56 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000† | 6 more per 1000 (from 2 fewer to 20 more) | ||||||||||
| Nonfatal PE (follow-up: mean 35 mo) | |||||||||||
| 1366 (8 RCTs)20-23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 48/686 (7.0) | 31/680 (4.6) | RR 0.66 (0.42-1.02) | Study population | |
| 70 per 1000 | 24 fewer per 1000 (from 41 fewer to 1 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 15 per 1000 (9, 10)‡ | 5 fewer per 1000 (from 9 fewer to 0 more) | ||||||||||
| DVT (follow-up: mean 33 mo) | |||||||||||
| 1418 (9 RCTs)20-24,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 81/711 (11.4) | 69/707 (9.8) | RR 0.85 (0.63-1.14) | Study population | |
| 114 per 1000 | 17 fewer per 1000 (from 42 fewer to 16 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 18 per 1000 (9, 10)‡ | 3 fewer per 1000 (from 7 fewer to 3 more) | ||||||||||
| Major bleeding (follow-up: mean 33 mo) | |||||||||||
| 2581 (9 RCTs)20-23,25-29 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 11/1307 (0.8) | 36/1274 (2.8) | RR 2.02 (1.02-3.98) | Study population | |
| 8 per 1000 | 8 more per 1000 (from 0 fewer to 24 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 21 per 1000 (13)§ | 21 more per 1000 (from 0 more to 63 more) | ||||||||||
| Certainty of evidence assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With short-term anticoagulation (3 to 6 mo) . | With long-term anticoagulation (>6 mo) . | Risk with short-term anticoagulation (3 to 6 mo) . | Risk difference with long-term anticoagulation (>6 mo) . | ||||||||
| Mortality (follow up: mean 37 mo) | |||||||||||
| 1186 (7 RCTs)20,22,23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 24/594 (4.0) | 34/592 (5.7) | RR 1.43 (0.85-2.41) | Study population | |
| 40 per 1000 | 17 more per 1000 (from 6 fewer to 56 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000† | 6 more per 1000 (from 2 fewer to 20 more) | ||||||||||
| Nonfatal PE (follow-up: mean 35 mo) | |||||||||||
| 1366 (8 RCTs)20-23,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 48/686 (7.0) | 31/680 (4.6) | RR 0.66 (0.42-1.02) | Study population | |
| 70 per 1000 | 24 fewer per 1000 (from 41 fewer to 1 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 15 per 1000 (9, 10)‡ | 5 fewer per 1000 (from 9 fewer to 0 more) | ||||||||||
| DVT (follow-up: mean 33 mo) | |||||||||||
| 1418 (9 RCTs)20-24,26-29 | Not serious | Not serious | Not serious | Serious∗ | None | ⨁⨁⨁◯ MODERATE | 81/711 (11.4) | 69/707 (9.8) | RR 0.85 (0.63-1.14) | Study population | |
| 114 per 1000 | 17 fewer per 1000 (from 42 fewer to 16 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 18 per 1000 (9, 10)‡ | 3 fewer per 1000 (from 7 fewer to 3 more) | ||||||||||
| Major bleeding (follow-up: mean 33 mo) | |||||||||||
| 2581 (9 RCTs)20-23,25-29 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 11/1307 (0.8) | 36/1274 (2.8) | RR 2.02 (1.02-3.98) | Study population | |
| 8 per 1000 | 8 more per 1000 (from 0 fewer to 24 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 21 per 1000 (13)§ | 21 more per 1000 (from 0 more to 63 more) | ||||||||||
CI around absolute estimates does not rule out substantial benefit or substantial harm.
We used the median in the short duration treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 1.4%.
A meta-analysis of 10 cohort studies and 5 randomized trials16 reported that the rate of VTE recurrence was 3.3% per patient-year for patients with a transient risk factor. Assuming that 45% of the VTE events were PE and 55% were DVT,17 we estimated that, for patients with VTE provoked by transient risk factors, the risk for PE recurrence was 1.49 per 100 patient-years and DVT recurrence was 1.81 per 100 patient-years.
A meta-analysis of 13 prospective cohort studies and 56 randomized trials15 showed that, in people with VTE, the risk for major bleeding during 6 months of treatment with anticoagulants was 2.1%. Assuming a risk for major bleeding of close to zero after anticoagulant discontinuation, we estimated an annualized risk for major bleeding of 2.1%.
PE
PE was reported in 8 VKA trials20-23,26-29 with 1366 patients and a mean follow-up of 35 months. Overall, 48 of the 686 patients (7.0%) assigned to the shorter duration (3-6 months) arm and 31 of 680 patients (4.6%) assigned to the longer duration arm developed a PE. When compared with shorter duration (3-6 months) therapy, the longer (>6 months) duration of anticoagulation therapy probably reduced the incidence of nonfatal PE (RR, 0.66; 95% CI, 0.42-1.02; Figure 2; ARR, 12 fewer per 1000 [95% CI, 21 fewer to 1 more] for patients with unprovoked VTE; 6 fewer per 1000 [95% CI, 11 fewer to 0 more] for patients with VTE provoked by chronic risk factors; and 5 fewer per 1000 [95% CI, 9 fewer to 0 more] for patients with VTE provoked by transient risk factors; moderate certainty; Tables 2, 3, and 4).
DVT
DVT was reported in 9 VKA trials20-24,26-29 with 1418 patients and a mean follow-up of 33 months. Overall, 81 of the 711 patients (11.4%) assigned to the shorter duration (3-6 months) arm and 69 of 707 patients (9.8%) assigned to the longer duration arm developed a DVT event. When compared with the shorter duration (3-6 months) arm, the longer duration (>6 months) of anticoagulation probably reduced the incidence of DVT (RR, 0.85; 95% CI, 0.63-1.14; Figure 2; ARR, 7 fewer per 1000 [95% CI, 16 fewer to 6 more] for patients with unprovoked VTE; 3 fewer per 1000 [95% CI, 9 fewer to 3 more] for patients with VTE provoked by chronic risk factors; and 3 fewer per 1000 [95% CI, 7 fewer to 3 more] for patients with VTE provoked by transient risk factors; moderate certainty; Tables 2, 3, and 4).
MAJOR BLEEDING
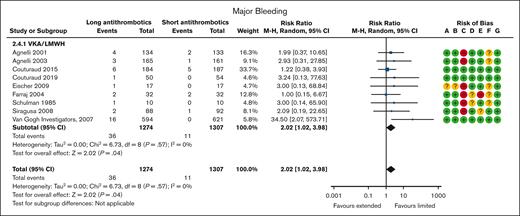
Major bleeding was reported in 9 VKA/LMWH trials20-23,25-29 with 2581 patients and a mean follow-up of 33 months. Overall, 11 of the 1307 patients (0.8%) assigned to the shorter duration (3-6 months) arm and 36 of 1274 patients (2.8%) assigned to the longer duration arm suffered a major bleeding event. When compared with the shorter duration (3-6 months) arm, a longer (>6 months) duration of anticoagulation therapy increased major bleeding (RR, 2.02; 95% CI, 1.02-3.98; Figure 2; ARR, 21 more per 1000 [95% CI, 0 more to 63 more] for patients with unprovoked VTE, VTE provoked by chronic risk factors, or VTE provoked by transient risk factors; high certainty; Tables 2, 3, and 4).
Secondary prevention
ALL-CAUSE MORTALITY
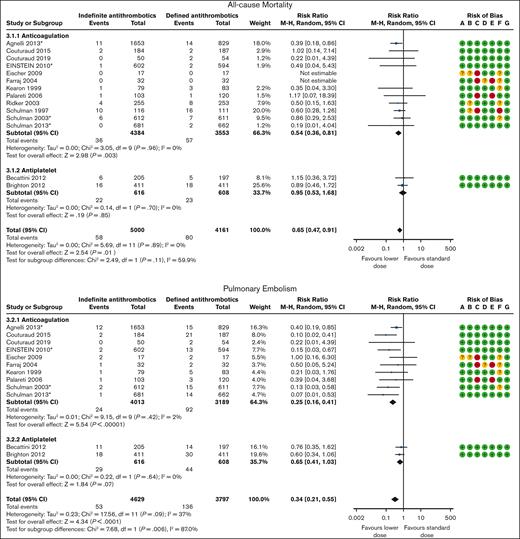
Mortality was reported in 4 DOAC,31,32,36,38 8 VKA/LMWH,26-29,33-35,37 and 2 antiplatelet trials39,40 with 9161 patients and a mean follow-up of 22 months. Overall, 80 of the 4161 patients (1.9%) assigned to discontinued treatment died as opposed to 58 of 5000 patients (1.2%) assigned to continuing treatment indefinitely (RR, 0.65; 95% CI, 0.47-0.91; Figure 3). The pooled effect estimate was stratified by type of antithrombotic treatment. Anticoagulants and antiplatelet therapy showed different effect estimates. We therefore presented the results and rated the certainty of the evidence separately. When compared with discontinuing treatment, indefinite anticoagulation therapy was associated with decreased all-cause mortality (RR, 0.54; 95% CI, 0.36-0.81; ARR, 3 fewer per 1000 [95% CI, 4 fewer to 1 fewer] for patients with unprovoked VTE or VTE provoked by chronic risk factors; high certainty; Tables 5 and 6). When compared with discontinuing treatment, indefinite antiplatelet therapy may be associated with decreased all-cause mortality (RR, 0.95; 95% CI, 0.53-1.68; ARR, 1 fewer per 1000 [95% CI, 7 fewer to 10 more] for patients with unprovoked VTE or VTE provoked by chronic risk factors; low certainty; Tables 5 and 6).
Secondary prevention: indefinite vs discontinued antithrombotic therapy. ∗Trials using DOAC. Risk of bias legend: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias.
Secondary prevention: indefinite vs discontinued antithrombotic therapy. ∗Trials using DOAC. Risk of bias legend: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); G, other bias.
Summary of the effects of indefinite vs discontinued antithrombotic therapy for secondary prevention in patients with unprovoked VTE
| Certainty assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With discontinuation of antithrombotic therapy . | With indefinite antithrombotic therapy . | Risk with discontinuation of antithrombotic therapy . | Risk difference with indefinite antithrombotic therapy . | ||||||||
| Mortality: anticoagulants (follow-up: mean 21 mo) | |||||||||||
| 7937 (12 RCTs) 26-29,31-38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 57/3553 (1.6) | 36/4384 (0.8) | RR 0.54 (0.36-0.81) | Study population: 19 mo | |
| 16 per 1000 | 7 fewer per 1000 (from 10 fewer to 3 fewer) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 7 per 1000∗ | 3 fewer per 1000 (from 4 fewer to 1 fewer) | ||||||||||
| Mortality: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 23/608 (3.8) | 22/616 (3.6) | RR 0.95 (0.53-1.68) | Study population | |
| 38 per 1000 | 2 fewer per 1000 (from 18 fewer to 26 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000‡ | 1 fewer per 1000 (from 7 fewer to 10 more) | ||||||||||
| Nonfatal PE-anticoagulants (follow up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 92/3189 (2.9) | 24/4013 (0.6) | RR 0.25 (0.16-0.41) | Study population | |
| 29 per 1000 | 22 fewer per 1000 (from 24 fewer to 17 fewer) | ||||||||||
| Annualized risk for PE | |||||||||||
| 36 per 1000 (16, 17)§ | 27 fewer per 1000 (from 30 fewer to 21 fewer) | ||||||||||
| Nonfatal PE: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 44/608 (7.2) | 29/616 (4.7) | RR 0.65 (0.411.03) | Study population | |
| 72 per 1000 | 25 fewer per 1000 (from 42 fewer to 2 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 36 per 1000 (16, 17)§ | 13 fewer per 1000 (from 21 fewer to 1 more) | ||||||||||
| Deep venous thrombosis (DVT) - anticoagulants (follow up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 198/3189 (6.2) | 33/4013 (0.8) | RR 0.15 (0.10-0.21) | Study population | |
| 62 per 1000 | 53 fewer per 1000 (from 56 fewer to 49 fewer) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 44 per 1000 (16, 17)§ | 37 fewer per 1000 (from 40 fewer to 35 fewer) | ||||||||||
| DVT: antiplatelet agents (follow-up: mean 30 mo) | |||||||||||
| 1347 (3 RCTs)30,39,40 | Not serious | Not serious¶ | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 100/671 (14.9) | 58/676 (8.6) | RR 0.44 (0.17 to 1.13) | Study population | |
| 149 per 1000 | 83 fewer per 1000 (from 124 fewer to 19 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 44 per 1000 (16, 17)§ | 25 fewer per 1000 (from 37 fewer to 6 more) | ||||||||||
| Major bleeding: anticoagulants (follow-up: mean 19 mo) | |||||||||||
| 8210 (13 RCTs)26-29,31-38,41 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 20/3687 (0.5) | 50/4523 (1.1) | RR 1.98 (1.18 to 3.30) | Study population | |
| 5 per 1000 | 5 more per 1000 (from 1 more to 12 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 4 more per 1000 (from 1 more to 9 more) | ||||||||||
| Major bleeding: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 7/608 (1.2) | 9/616 (1.5) | RR 1.28 (0.48-3.41) | Study population | |
| 12 per 1000 | 3 more per 1000 (from 6 fewer to 29 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 1 more per 1000 (from 2 fewer to 10 more) | ||||||||||
| Certainty assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With discontinuation of antithrombotic therapy . | With indefinite antithrombotic therapy . | Risk with discontinuation of antithrombotic therapy . | Risk difference with indefinite antithrombotic therapy . | ||||||||
| Mortality: anticoagulants (follow-up: mean 21 mo) | |||||||||||
| 7937 (12 RCTs) 26-29,31-38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 57/3553 (1.6) | 36/4384 (0.8) | RR 0.54 (0.36-0.81) | Study population: 19 mo | |
| 16 per 1000 | 7 fewer per 1000 (from 10 fewer to 3 fewer) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 7 per 1000∗ | 3 fewer per 1000 (from 4 fewer to 1 fewer) | ||||||||||
| Mortality: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 23/608 (3.8) | 22/616 (3.6) | RR 0.95 (0.53-1.68) | Study population | |
| 38 per 1000 | 2 fewer per 1000 (from 18 fewer to 26 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000‡ | 1 fewer per 1000 (from 7 fewer to 10 more) | ||||||||||
| Nonfatal PE-anticoagulants (follow up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 92/3189 (2.9) | 24/4013 (0.6) | RR 0.25 (0.16-0.41) | Study population | |
| 29 per 1000 | 22 fewer per 1000 (from 24 fewer to 17 fewer) | ||||||||||
| Annualized risk for PE | |||||||||||
| 36 per 1000 (16, 17)§ | 27 fewer per 1000 (from 30 fewer to 21 fewer) | ||||||||||
| Nonfatal PE: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 44/608 (7.2) | 29/616 (4.7) | RR 0.65 (0.411.03) | Study population | |
| 72 per 1000 | 25 fewer per 1000 (from 42 fewer to 2 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 36 per 1000 (16, 17)§ | 13 fewer per 1000 (from 21 fewer to 1 more) | ||||||||||
| Deep venous thrombosis (DVT) - anticoagulants (follow up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 198/3189 (6.2) | 33/4013 (0.8) | RR 0.15 (0.10-0.21) | Study population | |
| 62 per 1000 | 53 fewer per 1000 (from 56 fewer to 49 fewer) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 44 per 1000 (16, 17)§ | 37 fewer per 1000 (from 40 fewer to 35 fewer) | ||||||||||
| DVT: antiplatelet agents (follow-up: mean 30 mo) | |||||||||||
| 1347 (3 RCTs)30,39,40 | Not serious | Not serious¶ | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 100/671 (14.9) | 58/676 (8.6) | RR 0.44 (0.17 to 1.13) | Study population | |
| 149 per 1000 | 83 fewer per 1000 (from 124 fewer to 19 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 44 per 1000 (16, 17)§ | 25 fewer per 1000 (from 37 fewer to 6 more) | ||||||||||
| Major bleeding: anticoagulants (follow-up: mean 19 mo) | |||||||||||
| 8210 (13 RCTs)26-29,31-38,41 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 20/3687 (0.5) | 50/4523 (1.1) | RR 1.98 (1.18 to 3.30) | Study population | |
| 5 per 1000 | 5 more per 1000 (from 1 more to 12 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 4 more per 1000 (from 1 more to 9 more) | ||||||||||
| Major bleeding: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 7/608 (1.2) | 9/616 (1.5) | RR 1.28 (0.48-3.41) | Study population | |
| 12 per 1000 | 3 more per 1000 (from 6 fewer to 29 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 1 more per 1000 (from 2 fewer to 10 more) | ||||||||||
We used the median in the discontinued treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 0.7%.
The optimal information size was not met and the wide CI around absolute estimates does not rule out substantial benefit or substantial harm, and therefore we downrated the quality by 2 levels for impression.
We used the median in the discontinued treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 1.4%.
A meta-analysis18 reported that the cumulative incidence of recurrent VTE was 16% at 2 years for patients with unprovoked VTE, and because the median follow-up of our included studies was 24 months, we calculated the baseline risk to be 8% per patient-year. Assuming that 45% of the VTE events were PE and 55% were DVT,17 we estimated that, for patients with unprovoked VTE, the risk for PE recurrence was 3.6 per 100 patient-years and DVT recurrence was 4.4 per 100 patient-years.
The CI around absolute estimates does not rule out substantial benefit or substantial harm, and therefore we downrated the quality by 1 level for impression.
Although I2 = 85%, the treatment effects were all in favor of indefinite antithrombotic therapy, and we therefore did not downrate the quality for inconsistency.
We used the median in the discontinued treatment arm from eligible RCTs as the baseline risk and estimated the annualized risk for bleeding to be 0.4%.
Summary of the effects of indefinite vs discontinued antithrombotic therapy for secondary prevention in patients with VTE provoked by chronic risk factors
| Certainty assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With discontinuation of antithrombotic therapy . | With indefinite antithrombotic therapy . | Risk with discontinuation of antithrombotic therapy . | Risk difference with indefinite antithrombotic therapy . | ||||||||
| Mortality: anticoagulants (follow-up: mean 18 mo) | |||||||||||
| 7937 (12 RCTs)26-29,31-38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 57/3553 (1.6) | 36/4384 (0.8) | RR 0.54 (0.36-0.81) | Study population: 19 mo | |
| 16 per 1000 | 7 fewer per 1000 (from 10 fewer to 3 fewer) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 7 per 1000∗ | 3 fewer per 1000 (from 4 fewer to 1 fewer) | ||||||||||
| Mortality: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 23/608 (3.8) | 22/616 (3.6) | RR 0.95 (0.53-1.68) | Study population | |
| 38 per 1000 | 2 fewer per 1000 (from 18 fewer to 26 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000‡ | 1 fewer per 1000 (from 7 fewer to 10 more) | ||||||||||
| Nonfatal PE: anticoagulants (follow-up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 92/3189 (2.9) | 24/4013 (0.6) | RR 0.25 (0.16-0.41) | Study population | |
| 29 per 1000 | 22 fewer per 1000 (from 24 fewer to 17 fewer) | ||||||||||
| Annualized risk for PE | |||||||||||
| 19 per 1000 (9, 10)§ | 14 fewer per 1000 (from 16 fewer to 11 fewer) | ||||||||||
| Nonfatal PE: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 44/608 (7.2) | 29/616 (4.7) | RR 0.65 (0.41-1.03) | Study population | |
| 72 per 1000 | 25 fewer per 1000 (from 42 fewer to 2 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 19 per 1000 (9, 10)§ | 7 fewer per 1000 (from 11 fewer to 1 more) | ||||||||||
| DVT: anticoagulants (follow-up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 198/3189 (6.2) | 33/4013 (0.8) | RR 0.15 (0.10-0.21) | Study population | |
| 62 per 1000 | 53 fewer per 1000 (from 56 fewer to 49 fewer) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 23 per 1000 (9, 10)§ | 20 fewer per 1000 (from 21 fewer to 18 fewer) | ||||||||||
| DVT: antiplatelet agents (follow-up: mean 30 mo) | |||||||||||
| 1347 (3 RCTs)30,39,40 | Not serious | Not serious¶ | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 100/671 (14.9) | 58/676 (8.6) | RR 0.44 (0.17-1.13) | Study population | |
| 149 per 1000 | 83 fewer per 1000 (from 124 fewer to 19 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 23 per 1000 (9, 10)§ | 13 fewer per 1000 (from 19 fewer to 3 fewer) | ||||||||||
| Major bleeding: anticoagulants (follow-up: mean 19 mo) | |||||||||||
| 8210 (13 RCTs)26-29,31-38,41 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 20/3687 (0.5) | 50/4523 (1.1) | RR 1.98 (1.18-3.30) | Study population | |
| 5 per 1000 | 5 more per 1000 (from 1 more to 12 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 4 more per 1000 (from 1 more to 9 more) | ||||||||||
| Major bleeding: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 7/608 (1.2) | 9/616 (1.5) | RR 1.28 (0.48-3.41) | Study population | |
| 12 per 1000 | 3 more per 1000 (from 6 fewer to 29 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 1 more per 1000 (from 2 fewer to 10 more) | ||||||||||
| Certainty assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up . | Risk of bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall certainty of evidence . | Study event rates (%) . | Relative effect (95% CI) . | Anticipated absolute effects . | ||
| With discontinuation of antithrombotic therapy . | With indefinite antithrombotic therapy . | Risk with discontinuation of antithrombotic therapy . | Risk difference with indefinite antithrombotic therapy . | ||||||||
| Mortality: anticoagulants (follow-up: mean 18 mo) | |||||||||||
| 7937 (12 RCTs)26-29,31-38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 57/3553 (1.6) | 36/4384 (0.8) | RR 0.54 (0.36-0.81) | Study population: 19 mo | |
| 16 per 1000 | 7 fewer per 1000 (from 10 fewer to 3 fewer) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 7 per 1000∗ | 3 fewer per 1000 (from 4 fewer to 1 fewer) | ||||||||||
| Mortality: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 23/608 (3.8) | 22/616 (3.6) | RR 0.95 (0.53-1.68) | Study population | |
| 38 per 1000 | 2 fewer per 1000 (from 18 fewer to 26 more) | ||||||||||
| Mortality rate: 1 y | |||||||||||
| 14 per 1000‡ | 1 fewer per 1000 (from 7 fewer to 10 more) | ||||||||||
| Nonfatal PE: anticoagulants (follow-up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 92/3189 (2.9) | 24/4013 (0.6) | RR 0.25 (0.16-0.41) | Study population | |
| 29 per 1000 | 22 fewer per 1000 (from 24 fewer to 17 fewer) | ||||||||||
| Annualized risk for PE | |||||||||||
| 19 per 1000 (9, 10)§ | 14 fewer per 1000 (from 16 fewer to 11 fewer) | ||||||||||
| Nonfatal PE: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 44/608 (7.2) | 29/616 (4.7) | RR 0.65 (0.41-1.03) | Study population | |
| 72 per 1000 | 25 fewer per 1000 (from 42 fewer to 2 more) | ||||||||||
| Annualized risk for PE | |||||||||||
| 19 per 1000 (9, 10)§ | 7 fewer per 1000 (from 11 fewer to 1 more) | ||||||||||
| DVT: anticoagulants (follow-up: mean 17 mo) | |||||||||||
| 7202 (10 RCTs)26-29,31-33,35,36,38 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 198/3189 (6.2) | 33/4013 (0.8) | RR 0.15 (0.10-0.21) | Study population | |
| 62 per 1000 | 53 fewer per 1000 (from 56 fewer to 49 fewer) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 23 per 1000 (9, 10)§ | 20 fewer per 1000 (from 21 fewer to 18 fewer) | ||||||||||
| DVT: antiplatelet agents (follow-up: mean 30 mo) | |||||||||||
| 1347 (3 RCTs)30,39,40 | Not serious | Not serious¶ | Not serious | Serious‖ | None | ⨁⨁⨁◯ MODERATE | 100/671 (14.9) | 58/676 (8.6) | RR 0.44 (0.17-1.13) | Study population | |
| 149 per 1000 | 83 fewer per 1000 (from 124 fewer to 19 more) | ||||||||||
| Annualized risk for DVT | |||||||||||
| 23 per 1000 (9, 10)§ | 13 fewer per 1000 (from 19 fewer to 3 fewer) | ||||||||||
| Major bleeding: anticoagulants (follow-up: mean 19 mo) | |||||||||||
| 8210 (13 RCTs)26-29,31-38,41 | Not serious | Not serious | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 20/3687 (0.5) | 50/4523 (1.1) | RR 1.98 (1.18-3.30) | Study population | |
| 5 per 1000 | 5 more per 1000 (from 1 more to 12 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 4 more per 1000 (from 1 more to 9 more) | ||||||||||
| Major bleeding: antiplatelet agents (follow-up: mean 31 mo) | |||||||||||
| 1224 (2 RCTs)39,40 | Not serious | Not serious | Not serious | Very serious† | None | ⨁⨁◯◯ LOW | 7/608 (1.2) | 9/616 (1.5) | RR 1.28 (0.48-3.41) | Study population | |
| 12 per 1000 | 3 more per 1000 (from 6 fewer to 29 more) | ||||||||||
| Annualized risk for major bleeding | |||||||||||
| 4 per 1000# | 1 more per 1000 (from 2 fewer to 10 more) | ||||||||||
We used the median in the discontinued treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 0.7%.
The optimal information size was not met and the wide CI around the absolute estimates does not rule out substantial benefit or substantial harm, and so we downrate the quality by 2 levels for impression.
We used the median in the discontinued treatment arm from eligible RCTs as the baseline risk and estimated an annualized mortality of 1.4%.
A meta-analysis of 10 cohort studies and 5 randomized trials16 reported that the rate of VTE recurrence was 4.2% per patient-year for patients with a chronic risk factor. Assuming that 45% of the VTE events were PE and 55% were DVT,17 we estimated that, for patients with VTE provoked by chronic risk factors, the risk for PE recurrence was 1.89 per 100 patient-years and DVT recurrence was 2.31 per 100 patient-years.
The CI around the absolute estimates does not rule out substantial benefit or substantial harm, and thus we downrated the quality by 1 level for impression.
Although I2 = 85%, the treatment effects were all in favor of indefinite antithrombotic therapy and we therefore did not rate down the quality for inconsistency.
We used the median in the discontinued treatment arm from eligible RCTs as the baseline risk and estimated the annualized risk for bleeding to be 0.4%.
PE
PE was reported in 4 DOAC,31,32,36,38 6 VKA/LMWH,26-29,33,35 and 2 antiplatelet trials39,40 with 8426 patients and a mean follow-up of 20 months. Overall, 136 of the 3797 patients (3.6%) assigned to discontinue treatment developed a PE event as opposed to 53 of 4629 patients (1.1%) assigned to continue treatment indefinitely (RR, 0.34; 95% CI, 0.21-0.55; Figure 3). Anticoagulants and antiplatelet therapy showed different effect estimates with a significant subgroup effect (P = .006). We therefore evaluated the certainty of the evidence for each subgroup. When compared with discontinuing treatment, indefinite anticoagulation therapy reduced nonfatal PE (RR, 0.25; 95% CI, 0.16-0.41; ARR, 27 fewer per 1000 [95% CI, 30 fewer to 21 fewer] or 14 fewer per 1000 [95% CI, 16 fewer to 11 fewer] for patients with unprovoked VTE or VTE provoked by chronic risk factors, respectively; high certainty; Tables 5 and 6). When compared with discontinuing treatment, indefinite antiplatelet therapy probably reduce nonfatal PE (RR, 0.65; 95% CI, 0.41-1.03; ARR, 13 fewer per 1000 [95% CI, 21 fewer to 1 more] or 7 fewer per 1000 [95% CI, 11 fewer to 1 more] for patients with unprovoked VTE or VTE provoked by chronic risk factors, respectively; moderate certainty; Tables 5 and 6).
DVT
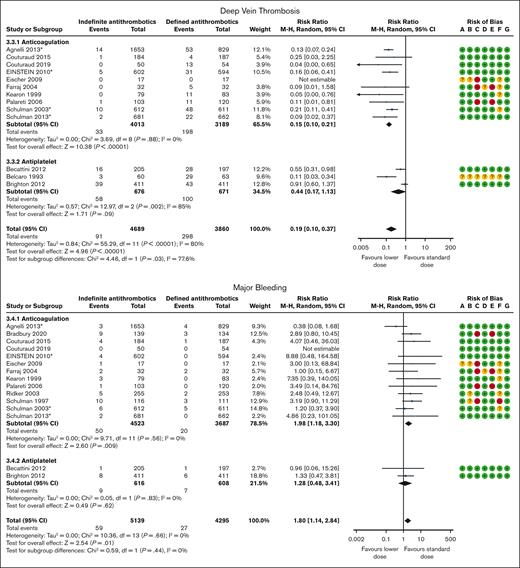
DVT was reported in 4 DOAC,31,32,36,38 6 VKA/LMWH,26-29,33,35 and 3 antiplatelet trials30,39,40 with 8549 patients and a mean follow-up of 20 months. Overall, 298 of the 3860 patients (7.7%) assigned to discontinue treatment developed a DVT event as opposed to 91 of 4689 patients (1.9%) assigned to continue treatment indefinitely (RR, 0.19; 95% CI, 0.10-0.37; Figure 3). Anticoagulants and antiplatelet therapy showed different effect estimates with a significant subgroup effect (P = .03), so we evaluated the certainty of evidence for each subgroup. When compared with discontinuing treatment, indefinite anticoagulation therapy reduced the incidence of DVT (RR, 0.15; 95% CI, 0.10-0.21; ARR, 37 fewer per 1000 [95% CI, 40 fewer to 35 fewer] or 20 fewer per 1000 [95% CI, 21 fewer to 18 fewer] for patients with unprovoked VTE or VTE provoked by chronic risk factors, respectively; high certainty; Tables 5 and 6). When compared with discontinuing treatment, indefinite antiplatelet therapy probably reduce the incidence of DVT (RR, 0.44; 95% CI, 0.17-1.13; ARR, 25 fewer per 1000 [95% CI, 37 fewer to 7 more] or 13 fewer per 1000 [95% CI, 19 fewer to 3 fewer] for patients with unprovoked VTE or VTE provoked by chronic risk factors, respectively; moderate certainty; Tables 5 and 6).
MAJOR BLEEDING
Major bleeding was reported in 4 DOAC,31,32,36,38 9 VKA/LMWH,26-29,33-35,37,41 and 2 antiplatelet trials39,40 with 9434 patients and a mean follow-up of 22 months. Overall, 27 of the 4295 patients (0.6%) assigned to discontinue treatment suffered a major bleeding event as opposed to 59 of 5139 patients (1.1%) assigned to continue treatment indefinitely (RR, 1.80; 95% CI, 1.14-2.84; Figure 3). The pooled effect estimate was stratified by type of antithrombotic treatment. Anticoagulants and antiplatelet therapy showed different effect estimates, and we therefore present the results and rate the certainty of evidence separately. When compared with discontinuing treatment, indefinite anticoagulation therapy increased the incidence of major bleeding events (RR, 1.98; 95% CI, 1.18-3.30; ARR, 4 more per 1000 [95% CI, 1 more to 9 more] for patients with unprovoked VTE or VTE provoked by chronic risk factors; high certainty; Tables 5 and 6). When compared with discontinuing treatment, indefinite antiplatelet therapy may increase the incidence of major bleeding events (RR, 1.28; 95% CI, 0.48-3.41; ARR, 1 more per 1000 [95% CI, 2 fewer to 10 more] for patients with unprovoked VTE or VTE provoked by chronic risk factors; low certainty; Tables 5 and 6).
Discussion
We systematically reviewed 22 trials that included 11 617 patients to evaluate the effect of the duration of primary treatment and of secondary prevention of VTE. For primary treatment, moderate-certainty evidence indicates that, when compared with a shorter course duration (3-6 months), treating patients with a longer course (>6 months) of VKA/LMWH probably reduces recurrent PE and DVT, but it is also associated with increased mortality, and high-certainty evidence indicates that a longer course (>6 months) of VKA/LMWH increases major bleeding. Thus, using a shorter duration (3-6 months) of anticoagulation might bring more benefit than a longer duration (>6 months) of anticoagulation for the primary treatment of all types of VTEs, that is, until the decision whether the patient should continue with secondary prevention or not, regardless of the type of VTE risk factors. When considering secondary prevention for VTE, high-certainty evidence indicates that indefinite anticoagulation therapy, when compared with discontinuing treatment, is associated with decreased mortality and a reduction in recurrent PE and DVT but with an increased risk for bleeding. Moderate-certainty evidence indicates that indefinite antiplatelet therapy probably reduces the incidence of recurrent PE and DVT and may also be associated with decreased mortality, but it may increase the risk for major bleeding, as supported by low-certainty evidence. Thus, for secondary prevention of VTE, indefinite antithrombotic treatment might be more beneficial than discontinued treatment for patients with unprovoked VTE or VTE provoked by chronic risk factors, but this does not apply to VTE provoked by transient risk factors because they do not require indefinite antithrombotic treatment. The evidence continues to support the current recommendations in the 2020 ASH guidelines.
This systematic review and meta-analysis have several strengths. First, it provides the most comprehensive body of evidence, including patients with provoked and unprovoked VTE, for both primary treatment and secondary prevention. Second, it provides the evidence base that supports the 5 key recommendations in the ASH VTE treatment guidelines and includes updates based on the latest evidence.11 Third, we considered all critical outcomes prioritized by the ASH guideline panel that have extensive clinical and research expertise in VTE treatment. Fourth, we reported both relative and ARR for a variety of patients with VTE (ie, unprovoked or provoked, either caused by transient or chronic risk factors). This could be more informative for patients and clinicians when faced with clinical decision-making for different VTE subgroups. Lastly, we used the GRADE approach to assess the certainty of the evidence and considered the risk of bias of the included studies. The limitations of this review include the absence of a search for gray literature, although we cross-referenced relevant systematic reviews and RCTs, and this may lead to potential publication bias. Second, we did not assess the health economics or considered patients’ values and preferences associated with the duration of anticoagulant therapy, which could offer a different perspective.42 In addition, we did not prospectively register this study, although a meta-epidemiologic study has shown that prospective registration does not significantly affect the overall reporting quality, although it can at least improve methodologic quality.43
Several meta-analyses44-57 have addressed the duration of antithrombotic treatment. None of them, however, clearly distinguished between primary treatment and secondary prevention, which reflects different phases of VTE management and is critical for guiding clinical decision-making. Consequently, they included only 2 to 15 trials, whereas we included 22 unique RCTs of which 10 targeted primary treatment and 16 targeted secondary prevention. Among the current systematic reviews, only 144 focused exclusively on unprovoked VTE and concluded that a shorter course (3-6 months) of anticoagulation was encouraged before determining whether to continue with indefinite treatment; the remaining studies included a heterogeneous patient population but did not provide absolute effect estimates for provoked and unprovoked VTE populations, which may lead to limited applicability in clinical practice. In contrast, our research took into account baseline risks for each type of VTE and converted relative effects into absolute effects, thereby making the results more useful for clinical decision-making.
For primary treatment, readers should be cautious when using the results because all the data on the anticoagulation therapy came from VKA/LMWH trials. Future trials on DOACs or other anticoagulants may alter the balance of benefits and harms or provide higher quality of evidence regarding shorter vs longer courses of anticoagulation in primary treatment. In addition, future trials can report clinical outcomes in patients separately by different types of VTE. This would enable a more direct summary of the evidence for each type of VTE.
In conclusion, when considering the balance of benefits (to prevent recurrent PE and DVT) and harms (major bleeding and mortality), for the primary treatment of VTE (unprovoked, provoked by transient, or chronic risk factors), a shorter duration (3-6 months) is superior to a longer duration (>6 months) of anticoagulant treatment. For secondary prevention of unprovoked VTE and VTE provoked by chronic risk factors, indefinite antithrombotic treatment outperforms discontinued treatment.
Acknowledgments
American Society of Hematology (ASH) funded this research.
In the development of the systematic review, ASH formed the guideline panel, and prioritized the research questions and the outcomes of interest.58 ASH had no involvement in the collection, analysis, and interpretation of the data; and in the decision to approve publication of the manuscript.
Authorship
Contribution: Y.-Q.Z., I.N., and T.L.O. conceived and designed the study; W.W. and G.R. designed and performed the search strategy; Y.-Q.Z., L.C.L., F.A., L.L., J.W., P.Z., S.P., N.S., F.A., C.A.C.G., S.K., A.A., S.A.K., and A.M.P. screened and selected the articles; Y.-Q.Z., L.C.L., F.A., L.L., J.W., P.Z., S.P., N.S., F.A., C.A.C.G., S.K., A.A., and S.A.K. extracted the data; L.C.L, F.A., L.L., Q.H., J.W., P.Z., S.P., N.S., F.A., C.A.C.G., S.K., A.A., S.A.K., and A.M.P. assessed the risk of bias of included trials; A.L., R.K., and Y.-Q.Z. analyzed the data; A.L., Y.-Q.Z., L.C.L., F.A., L.L., Q.H., J.W., P.Z., S.P., N.S., F.A., C.A.C.G., S.K., A.A., and S.A.K. performed the GRADE assessment; A.L., R.K., Y.-Q.Z., and I.N. interpreted the results; A.L. and R.K. drafted the manuscript; the corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted; and all authors critically revised the manuscript, had full access to all the data in the study, and had the final responsibility for the decision to submit the manuscript for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yu-Qing Zhang, Department of Health Research Methods, Evidence, and Impact, McMaster University, HSC 2C, 1200 Main St West, Hamilton, ON L8N 3Z5; email: madisonz1220@gmail.com.
References
Author notes
The statistical code and data set are available on request from the corresponding author, Yu-Qing Zhang (madisonz1220@gmail.com).
The full-text version of this article contains a data supplement.