Key Points
Concomitant neurodevelopmental disorder was highly variable among patients, ranging from absent to cognitive and physical impairment.
Patients with extramedullary symptoms also presented at younger age and often suffered from severe infections, needing higher G-CSF doses.
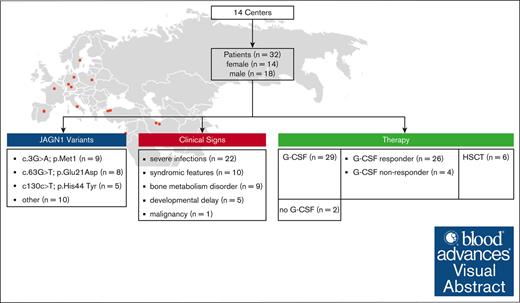
Visual Abstract
Jagunal-homolog1 (JAGN1) is an endoplasmic reticulum–resident protein, which is part of the early secretory pathway and granulocyte colony-stimulating factor (CSF; G-CSF) receptor–mediated signaling. Autosomal recessively inherited variants in JAGN1 lead to congenital neutropenia, early-onset bacterial infections, aphthosis, and skin abscesses due to aberrant differentiation and maturation of neutrophils. Bone metabolism disorders and syndromic phenotype, including facial features, short stature, and neurodevelopmental delay, have been reported. Allogeneic hematopoietic stem cell transplantation (alloHSCT) is a treatment option for patients who respond poorly to therapy with G-CSF and those who suffer from complicated infections. In a retrospective multicenter study, data from 32 patients with JAGN1 deficiency were collected to describe the disease, perform phenotype-genotype analysis, and evaluate treatment modalities. Patients presented with 9 homozygous mutations in JAGN1. All patients experienced infectious complications. Twelve patients presented with short stature and facial features. Neurodevelopmental delay was observed in 4 patients from 3 families. Variant c.3G>A p.Met1, found in 9 patients, was never connected to extramedullary symptoms, except for short stature in 1 patient. Patients with the variants c.63G>T, p.Glu21Asp and c130c>T p.His44 Tyr presented more often with syndromic facial features and bone metabolism disorders. Six patients underwent allogeneic stem cell transplantation due to therapy-refractory neutropenia and severe infections, 1 received the graft because of myelodysplastic syndrome and secondary acute myeloid leukemia. Two patients had to undergo a second transplantation because of autologous reconstitution. One patient who did not undergo transplantation died at age 5 years due to pancolitis and septicemia. All 31 other patients were alive and healthy at the last follow-up.
Introduction
Severe congenital neutropenias (SCNs) are a heterogeneous group of inherited disorders characterized by impaired maturation and differentiation of neutrophils, leading to reduced neutrophil counts of varying extent and an elevated risk for bacterial infections, such as those affecting the ear-nose-throat system, gingivitis, pneumonia, skin infections, and abscesses. The causes of congenital neutropenia include mutations in genes affecting several cell components, such as neutrophil receptors (granulocyte colony-stimulating factor [G-CSF] receptor, C-X-C motif chemokine receptor 4 [CXCR4], and C-X-C motif chemokine receptor 2 [CXCR2]), mitochondria (HCLS1-associated protein X-1 [HAX1], tafazzin [TAZ], adenylate kinase 2 [AK2], and CLBP), endosomal trafficking (adaptor-related protein complex 3 subunit beta 1 [AP3B1]; lysosomal trafficking regulator [LYST]; member RAS oncogene family [RAB27A]; p14; vacuolar protein sorting 45 homolog [VPS45]; T-cell immune regulator, ATPase H+ transporting V0 subunit a3 [TCIRG1]; and E74-like ETS transcription factor 1 [ELF1]), nucleus (growth factor independent 1 transcription repressor [GFI1], globin transcription factor 1 [GATA1], GATA2, partner of Sld five 1 [PSF1], signal recognition particle 54 [SRP54], SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily D member 2 [SMARCD2], U6 small nuclear ribonucleic acid [snRNA] biogenesis phosphodiesterase 1 [USB1], and DnaJ heat shock protein family [Hsp40] member C21 [DNAJC21]), transmembrane proteins (cluster of differentiation 40 [CD40] and cluster of differentiation 40 ligand [CD40L]), the cytoskeleton (Wiskott-Aldrich syndrome protein actin nucleation promoting factor [WAS], WAS/WASL-interacting protein family member 1 [WIPF1], and HAX1), extracellular enzymes (adenosine deaminase 2 [ADA2]), neutrophil granules (ELAs), ribosomes (Shwachman-Bodian-Diamond syndrome [SBDS]), and the endoplasmic reticulum (glucose-6-phosphate transporter 1 [G6PT1], glucose-6-phosphatase catalytic subunit 3 [G6PC3], Jagunal-homolog1 [JAGN1], and vacuolar protein sorting 13 homolog B [VPS13B]; supplemental Figure 1). Among the well-known neutropenias, deficiencies in ELANE,1 HAX1,2 SBDS,3 GATA2,4 and G6PC35 can cause severe infectious complications and carry a risk of developing malignancies over time. Allogeneic hematopoietic stem cell transplantation (alloHSCT) is indicated for patients who are nonresponsive to G-CSF, require high doses of G-CSF, and show disease progression to myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML). JAGN1 deficiency was described in 2013 as a variable clinical phenotype SCN that includes failure to thrive, developmental delay, and skeletal abnormalities. JAGN1 is an endoplasmic reticulum–resident protein that functions in the early secretory pathway and is critical for differentiating and maintaining human neutrophils.6 Several smaller cohorts and case reports have been published.7-10 It was postulated that JAGN1 deficiency accounts for ∼10% of all patients with congenital neutropenia, with variable disease intensity. A zebrafish model was introduced using the CRISPR-Cas9 technique to introduce mutations in the zebrafish jagn1b gene. The results revealed that interference with Jagn1b expression reduced the number of neutrophils without affecting early myelopoiesis or the number of monocytes/macrophages. Even though authors found enhanced apoptosis and activated unfolded protein response in jagn1b morphants, the exact molecular pathomechanisms leading to neutropenia with bone involvement in JAGN1 deficiency remain unclear.11
This study presents comprehensive data on the clinical phenotype of 32 patients with JAGN1 deficiency from 14 centers, focusing on a possible genotype-phenotype correlation of syndromic and neurodevelopmental manifestations.
Patients and methods
This multicenter retrospective study included all patients with JAGN1 deficiency registered in the European Society for Blood and Marrow Transplant (EBMT)/European Society for Immunodeficiencies (ESID) registries and the French Neutropenia Registry. Chart-based data were gathered via case report forms by the responsible clinician, in addition to previously published data by December 2023. Data on alloHSCT (indications, donor choices, types of conditioning regimens, and outcomes) were specifically obtained from the centers.
The study was approved by the local ethics committee of Frankfurt Goethe University (Institutional review board approval number 2021-449). The responsible physician diagnosed JAGN1 deficiency based on genetic confirmation. Written informed consent according to the Declaration of Helsinki was obtained from the parents and patients.
Immune monitoring
Immune reconstitution after alloHSCT was performed according to the protocol of the treatment center. Immune phenotyping data were collected at diagnosis and after alloHSCT for patients who underwent transplant. Quantitative donor chimerism analyses were performed according to the protocol of the treating center.
Statistical analysis
All analyses were conducted using statistical R software version 4.3.3 (R Project for Statistical Computing; www.r-project.org/). We used Fisher exact test or the Mann-Whitney-Wilcoxon test to compare the distributions of syndromic features and other characteristics among pediatric patients with JAGN1 deficiency. Kendall correlation analysis explored the relationships between related features, including binary and ordered variables. One sample proportion was examined using the 2-sided proportion test with Yates continuity correction due to small sample size.
All tests were 2-tailed; a P value <.05 was considered to indicate statistical significance.
Results
Patient characteristics
This cohort included 32 patients with JAGN1 deficiency from 14 centers. Males accounted for 56% of the cohort. The median age at clinical diagnosis was 8 months (range, 0-120; Table 1). The median age at last follow-up was 16 years. Twelve patients were aged ≥20 years, with the oldest patient alive being 67 years.
Patient characteristics
| . | Total (N = 32) . | Syndromic features . | P value . | |
|---|---|---|---|---|
| No (n = 20) . | Yes (n = 12) . | |||
| Gender, male, n (%) | 18 (56) | 13 (65) | 5 (42) | |
| Age at diagnosis, median (range), mo | 8 (0-120) | 19 (0-120) | 6 (0-60) | .225 |
| Mutation, n (%) | .089 | |||
| c.3G>A; p.Met1 | 9 (28) | 8 (40) | 1 (8) | |
| c.63G>T; p.Glu21Asp | 8 (25) | 5 (25) | 3 (25) | |
| c130c>T; p.His44 Tyr | 5 (16) | 2 (10) | 3 (25) | |
| c.40G>A; p.Gly14Ser | 2 (6) | 2 (10) | 0 (0) | |
| c.485A>G; p.Gln162Arg | 2 (6) | 0 (0) | 2 (17) | |
| c.35_43delCCGACGGCA; p.Thr12_Gly14 | 1 (3) | 0 (0) | 1 (8) | |
| c. 297C>G p. Tyr99∗ | 1 (3) | 1 (5) | 0 (0) | |
| c. 59G>A p. Arg20Glu/ c.59G > A; p.R20Q | 3 (9) | 2 (10) | 1 (8) | |
| c.330_331delTT; p.Ser111Hisfs∗72 | 1 (3) | 0 (0) | 1 (8) | |
| Neutrophil counts at diagnosis | .383 | |||
| Median (range), count/μl | 303 (100-2600) | 340 (100-2600) | 295 (100-1170) | |
| Neutrophils <500, n (%) | 22 (71) | 13 (68) | 9 (75) | .694 |
| Not available | 1 | 1 | 0 | |
| Mild infections, n (%) | 32 (100) | 20 (100) | 12 (100) | |
| Severe Infections, n (%) | 21 (66) | 10 (50) | 11 (92) | .016 |
| Bone metabolism disorder, n (%) | 8 (25) | 3 (15) | 5 (42) | .092 |
| Developmental delay, n (%) | 7 (22) | 0 (0) | 7 (58) | <.001 |
| Malignancy, n (%) | 1 (3) | 0 (0) | 1 (8) | .190 |
| G-CSF treatment, n (%) | ||||
| Never required | 2 (6) | 2 (11) | 0 (0) | |
| Start treatment | 29 (91) | 17 (85) | 12 (100) | |
| Not available | 1 | 1 | 0 | |
| HSCT, n (%) | 6 (19) | 3 (15) | 3 (25) | .483 |
| Age at last FU, median (range), mo | 192 (0-804) | 162 (43-804) | 192 (0-348) | .484 |
| . | Total (N = 32) . | Syndromic features . | P value . | |
|---|---|---|---|---|
| No (n = 20) . | Yes (n = 12) . | |||
| Gender, male, n (%) | 18 (56) | 13 (65) | 5 (42) | |
| Age at diagnosis, median (range), mo | 8 (0-120) | 19 (0-120) | 6 (0-60) | .225 |
| Mutation, n (%) | .089 | |||
| c.3G>A; p.Met1 | 9 (28) | 8 (40) | 1 (8) | |
| c.63G>T; p.Glu21Asp | 8 (25) | 5 (25) | 3 (25) | |
| c130c>T; p.His44 Tyr | 5 (16) | 2 (10) | 3 (25) | |
| c.40G>A; p.Gly14Ser | 2 (6) | 2 (10) | 0 (0) | |
| c.485A>G; p.Gln162Arg | 2 (6) | 0 (0) | 2 (17) | |
| c.35_43delCCGACGGCA; p.Thr12_Gly14 | 1 (3) | 0 (0) | 1 (8) | |
| c. 297C>G p. Tyr99∗ | 1 (3) | 1 (5) | 0 (0) | |
| c. 59G>A p. Arg20Glu/ c.59G > A; p.R20Q | 3 (9) | 2 (10) | 1 (8) | |
| c.330_331delTT; p.Ser111Hisfs∗72 | 1 (3) | 0 (0) | 1 (8) | |
| Neutrophil counts at diagnosis | .383 | |||
| Median (range), count/μl | 303 (100-2600) | 340 (100-2600) | 295 (100-1170) | |
| Neutrophils <500, n (%) | 22 (71) | 13 (68) | 9 (75) | .694 |
| Not available | 1 | 1 | 0 | |
| Mild infections, n (%) | 32 (100) | 20 (100) | 12 (100) | |
| Severe Infections, n (%) | 21 (66) | 10 (50) | 11 (92) | .016 |
| Bone metabolism disorder, n (%) | 8 (25) | 3 (15) | 5 (42) | .092 |
| Developmental delay, n (%) | 7 (22) | 0 (0) | 7 (58) | <.001 |
| Malignancy, n (%) | 1 (3) | 0 (0) | 1 (8) | .190 |
| G-CSF treatment, n (%) | ||||
| Never required | 2 (6) | 2 (11) | 0 (0) | |
| Start treatment | 29 (91) | 17 (85) | 12 (100) | |
| Not available | 1 | 1 | 0 | |
| HSCT, n (%) | 6 (19) | 3 (15) | 3 (25) | .483 |
| Age at last FU, median (range), mo | 192 (0-804) | 162 (43-804) | 192 (0-348) | .484 |
P values were determined using Fisher exact test or the Wilcoxon-Mann-Whitney test, as appropriate.
FU, follow-up.
We identified 9 distinct homozygous variants in the JAGN1 gene. Most of them were missense variants. c.3G>A p.Met1 was the most common variant in 9 patients from 4 different pedigrees, followed by c.63G>T p.Glu21Asp in 8 patients from 8 pedigrees and the mutation c130c>T p.His44 Tyr in 5 patients from 4 pedigrees. Compound heterozygous variants were not observed.
Disease characteristics
Blood counts at diagnosis were available for 31 patients. Severe neutropenia with absolute neutrophil count (ANC) <500/μL was observed in 22 patients (71%), whereas 19 patients (29%) had ANC >500/μL. Absolute lymphocyte count was available for 31 patients (97%) within the age-matched reference range, except for 1 patient (3%) whose absolute lymphocyte count was reduced. Immunoglobulin (Ig) levels were available for 21 patients (66%), within the normal range for 16 patients (76%), and reduced for 5 patients (24%). Early-onset and recurrent infections of the ear-nose-throat system, such as otitis media and bronchitis, were observed in all patients. The infections were severe in 21 patients (66%) and consisted of pneumonia, mastoiditis, abscesses of the skin and mucosa, periodontitis, chronic gingivitis, mouth ulcers, and omphalitis. One patient died at age 5 years of pancolitis and sepsis, despite treatment with G-CSF. Furthermore, 8 patients (25%) suffered from a variability of bone metabolism disorders, including osteopetrosis-like phenotype, repeated fractures, retardation of bone age, scoliosis, joint pain, and deformities. Moreover, 12 patients (38%) presented a syndromic phenotype with facial features, including an enlargement of the forehead, a wide nasal bridge, a thin upper lip, and a hypoplastic maxilla or mandible. Four of these patients also had short stature, which was not accompanied by bone metabolism disorders. In 7 patients (22%), mild developmental retardation and impaired learning capacity were reported. Other findings included dental malformations, cardiac valve defects, hypospadias, strabismus, and endocrinological disorders (supplemental Table 1).
Distribution of disease-related features according to each variant is shown in Figure 1. Among patients carrying the c130c>T p.His44 Tyr variant, 3 of 5 patients (from 2 families) presented facial features including a narrow forehead, broad nasal bridge, hypoplastic maxilla, and a thin upper lip. Additionally, 2 siblings showed an impaired learning capacity and a speech delay. Mutation c.63G>T p.Glu21Asp identified in 8 patients from 8 pedigrees was linked to severe periodontitis, with teeth loss in 3 patients and neurodevelopmental delay in 2 patients. In contrast, mutation c.3G>A p.Met1 identified in 9 patients from 4 pedigrees was never associated to any facial features or neurological findings, but short stature was noticed once. The difference was statistically significant (11.1%; 95% confidence interval [CI], 0-49.3; P = .045).
Clinical features by gene variants. We present the clinical features of the patients in our study cohort according to their genetic variants. The y-axis displays patient IDs, and the x-axis displays the clinical features. The black background indicates the presence of features, and the white background indicates the absence of features. The background filled with lines represents missing values. Pat, patient.
Clinical features by gene variants. We present the clinical features of the patients in our study cohort according to their genetic variants. The y-axis displays patient IDs, and the x-axis displays the clinical features. The black background indicates the presence of features, and the white background indicates the absence of features. The background filled with lines represents missing values. Pat, patient.
Relationships between disease-related features
Syndromic facial features were correlated with developmental delay, bone metabolism disorders, severe infections, and younger age at diagnosis, indicating that these patients were clinically more severely affected by JAGN1 deficiency (Figure 2). Table 1 presents the characteristics of patients with and without syndromic facial features.
Correlogram plot of patient characteristics and clinical features. The plot shows the correlation matrix between variables. The size and intensity of the circle represent the correlation between each pair of variables. Patients with syndromic features correlated with developmental delay, severe infections, and bone metabolism disorders, as well as female sex and a younger age at diagnosis. m, male.
Correlogram plot of patient characteristics and clinical features. The plot shows the correlation matrix between variables. The size and intensity of the circle represent the correlation between each pair of variables. Patients with syndromic features correlated with developmental delay, severe infections, and bone metabolism disorders, as well as female sex and a younger age at diagnosis. m, male.
Treatment and anti-infectious prophylaxis
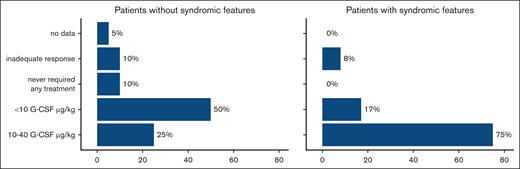
The most appropriate treatment regimen for each patient was chosen by the responsible clinician based on the individual severity of symptoms. Most of the patients received G-CSF as their first-line therapy (29/32). Two patients never required any treatment, and the follow-up information was missing for 1 patient. Three patients did not respond to G-CSF treatment; therefore, their therapy was stopped, and 2 of them had to undergo alloHSCT. The G-CSF dose varied from 5 to 40 μg/kg. Therapy was interrupted in 3 other patients due to the occurrence of severe infections, despite normalized neutrophil count, and development of MDS/secondary AML in 1 patient, driving them to alloHSCT. All patients with syndromic features started first-line G-CSF therapy. One patient did not respond to the therapy, and in 2 patients, the treatment was interrupted because they underwent transplantation. At the time of analysis, 75% of the patients with syndromic features received G-CSF at a dose of ≥10 μg/kg, which was significantly greater than that of patients who did not present with syndromic features. In this group, only 25% of the patients received a dose ≥10 μg/kg (P = .027; Figure 3).
G-CSF therapy in JAGN1-deficient patients with or without syndromic features. The plot shows the details of G-CSF treatment in the study cohort. Patients with syndromic features needed significantly higher doses of G-CSF than those without. In the group with syndromic features, 75% of the patients received 10 to 40 μg of G-CSF, whereas in the group without syndromic features, only 25% received high doses of 10 to 40 μg of G-CSF (P = .027).
G-CSF therapy in JAGN1-deficient patients with or without syndromic features. The plot shows the details of G-CSF treatment in the study cohort. Patients with syndromic features needed significantly higher doses of G-CSF than those without. In the group with syndromic features, 75% of the patients received 10 to 40 μg of G-CSF, whereas in the group without syndromic features, only 25% received high doses of 10 to 40 μg of G-CSF (P = .027).
Stem cell transplantation
Six patients (6/32 [19%]) received alloHSCT between age 9 and 228 months, with a median of 78 months. They were offered alloHSCT because of the failure of G-CSF treatment and recurrent infections (n = 2), MDS/secondary AML (n = 1), or persistent infections despite normalized neutrophil count on G-CSF (n = 3). Two patients received a second transplantation due to graft failure and autologous reconstitution (Table 2).
Transplant characteristics and outcomes
| ID . | Indication for HSCT . | Sex, age at HSCT (y) . | Stem cell source . | Donor . | Conditioning regimen . | Serotherapy . | GVHD prophylaxis . | Neutrophil engraftment (>500/μL), d . | Thrombocyte engraftment (unsupported, >20 000/μL), d . | T cells at day 100 (CD3+/μL) . | Chimerism . | GVHD . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Immunodeficiency, G-CSF nonresponder | m, 1.6 | BM | MSD | FLU (5.2 mg/kg), TT (5mg/kg), MEL (4.6 mg/kg) | ATG Fresenius (60 mg/kg) | CSA | +17 | _ | 593 | Autologous | _ |
| 1 | Autologous reconstitution | m, 2.5 | BM | MSD | BU (12.8 mg/kg), CY (120 mg/kg) | ATG Fresenius (60 mg/kg) | CSA | +15 | +56 | 242 | Mixed (30% autologous) | _ |
| 2 | MDS/AML M2 | f, 19 | BM | MUD | BU (8.75 mg/kg), CY (120 mg/kg) | Campath (1,8 mg/kg) | MTX, CSA | +15 | +15 | 832 | Complete | _ |
| 12 | Infections, severe periodontitis, G-CSF nonresponder | m, 11.3 | PBSC | Haplo, brother | FLU (150 mg/m2), TREO (42 g/m2), TT (5 mg/kg) | none | MTX, CSA, Post-Cy day +3 / +4 (50 mg/kg), MMF | +21 | +25 | n.a. | Complete | _ |
| 14_1 | Severe infections | m, 10.3 | PBSC | Haplo, mother | BU (15,5 mg/kg)∗, CY(54 mg/kg), TT(9 mg/kg) | ATG Fresenius (30 mg/kg) | MMF | _ | _ | _ | Autologous | _ |
| 14_2 | Graft failure | m, 10.3 | PBSC | Haplo, father | FLU (120 mg/m2), CY (60 mg/kg), TT (5 mg/kg), TBI (2 × 2 Gy), rituximab (2 × 375 mg/m2), bortezomib (2 × 1.3 mg/m2) | ATG thymoglobulin (4.5 mg/kg) | MMF | +19 | +12 | n.a. | _ | |
| 19 | Severe infections | m, 3.3 | BM | MSD | FLU (160 mg/m2), TREO (42 g/m2), TT (5 mg/kg) | ATG Fresenius (15 mg/kg) | MTX, CSA | +19 | +25 | 231 | Complete | _ |
| 27 | Neutropenia, severe infections, aspergillosis | m, 0.8 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| ID . | Indication for HSCT . | Sex, age at HSCT (y) . | Stem cell source . | Donor . | Conditioning regimen . | Serotherapy . | GVHD prophylaxis . | Neutrophil engraftment (>500/μL), d . | Thrombocyte engraftment (unsupported, >20 000/μL), d . | T cells at day 100 (CD3+/μL) . | Chimerism . | GVHD . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Immunodeficiency, G-CSF nonresponder | m, 1.6 | BM | MSD | FLU (5.2 mg/kg), TT (5mg/kg), MEL (4.6 mg/kg) | ATG Fresenius (60 mg/kg) | CSA | +17 | _ | 593 | Autologous | _ |
| 1 | Autologous reconstitution | m, 2.5 | BM | MSD | BU (12.8 mg/kg), CY (120 mg/kg) | ATG Fresenius (60 mg/kg) | CSA | +15 | +56 | 242 | Mixed (30% autologous) | _ |
| 2 | MDS/AML M2 | f, 19 | BM | MUD | BU (8.75 mg/kg), CY (120 mg/kg) | Campath (1,8 mg/kg) | MTX, CSA | +15 | +15 | 832 | Complete | _ |
| 12 | Infections, severe periodontitis, G-CSF nonresponder | m, 11.3 | PBSC | Haplo, brother | FLU (150 mg/m2), TREO (42 g/m2), TT (5 mg/kg) | none | MTX, CSA, Post-Cy day +3 / +4 (50 mg/kg), MMF | +21 | +25 | n.a. | Complete | _ |
| 14_1 | Severe infections | m, 10.3 | PBSC | Haplo, mother | BU (15,5 mg/kg)∗, CY(54 mg/kg), TT(9 mg/kg) | ATG Fresenius (30 mg/kg) | MMF | _ | _ | _ | Autologous | _ |
| 14_2 | Graft failure | m, 10.3 | PBSC | Haplo, father | FLU (120 mg/m2), CY (60 mg/kg), TT (5 mg/kg), TBI (2 × 2 Gy), rituximab (2 × 375 mg/m2), bortezomib (2 × 1.3 mg/m2) | ATG thymoglobulin (4.5 mg/kg) | MMF | +19 | +12 | n.a. | _ | |
| 19 | Severe infections | m, 3.3 | BM | MSD | FLU (160 mg/m2), TREO (42 g/m2), TT (5 mg/kg) | ATG Fresenius (15 mg/kg) | MTX, CSA | +19 | +25 | 231 | Complete | _ |
| 27 | Neutropenia, severe infections, aspergillosis | m, 0.8 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
Complete donor chimerism was defined as donor chimerism ≥99%.
BM, bone marrow; CY, cyclophosphamide; f, female; FLU, fludarabine; Haplo, haploidentical; m, male; MEL, melphalane; MSD, matched sibling donor; MTX, methotrexate; MUD, matched unrelated donor; n.a., not available; PBSC, peripheral blood stem cell; postCY, posttransplant cyclophosphamide; TBI, total body irradiation; TREO, treosulfan; TT, thiotepa.
AUC (area under the concentration-vs-time curve) 85 mg x h/L.
HLA typing and donor selection
The donors were either matched siblings (n = 2), haploidentical (n = 2), or 9 of 10 matched unrelated donors (n = 1). For 1 patient, no detailed transplantation data were available. Stem cells were harvested via bone marrow aspiration (n = 4), and in 1 case, peripheral stem cells were mobilized via G-CSF (Table 2).
Conditioning regimen and GVHD prophylaxis
In line with the literature, the European Society for Blood and Marrow Transplant and Stem Cell Transplantation for Immunodeficiencies in Europe reports, conditioning regimens, including total treosulfan dose ≥30 g/m2 body surface and busulfan (BU) ≥8 mg/kg body weight, were considered myeloablative.12 All patients received a myeloablative conditioning regimen. BU was administered to 3 patients (P1, P2, and P14). BU pharmacokinetics were performed in 1 of these patients (P14). BU was dosed based on the average daily area under the concentration-vs-time curve of 85 mg × h/L. The serotherapy included antithymocyte globulin (Fresenius), thymoglobulin, and alemtuzumab. Despite myeloablative treatment, 2 patients (P1 and P14) showed graft failure/autologous engraftment. P1 was reconditioned using BU/cyclophosphamide/antithymocyte globulin and achieved stable engraftment. P14 received a combination of low-dose total body irradiation, fludarabine, and thiotepa, along with bortezomib and rituximab, in addition to thymoglobulin before the second haploidentical HSCT.
Graft-versus-host disease (GVHD) prophylaxis included cyclosporine A (CSA) monotherapy or CSA in combination with methotrexate. In the haploidentical HSCT setting, 1 patient received mycophenolate mofetil for both transplantations, and the other patient received posttransplant cyclophosphamide in addition to CSA, mycophenolate mofetil, and methotrexate (Table 2).
Overall survival and event-free survival
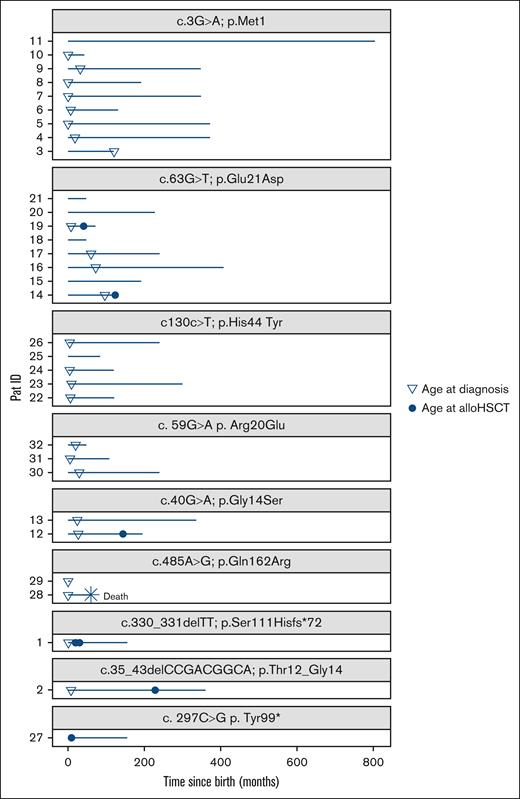
At last follow-up (median, 192 months; range, 6-804), 31 patients (97%) were alive. Twelve patients were aged >20 years; the oldest patient in this cohort was 67 years old. One patient died at age 5 years due to pancolitis with sepsis. She presented with the c.485A>G p.Gln162Arg variant and was diagnosed with severe neutropenia (282 count/μL) immediately after birth. Despite treatment with G-CSF up to 20 μg/kg, she suffered from severe infections as well as bone metabolism disorders and syndromic features.
No alloHSCT-related mortality was reported (median follow-up after alloHSCT, 91.5 months; range, 9-147; Figure 4). Acute and chronic GVHD of the liver was suspected in 1 patient because of transaminitis and a bilateral cataract after transplantation. The patient received steroid treatment for an extended period of 2 years and calcineurin inhibitors for 3 years. All symptoms resolved entirely. Veno-occlusive disease was reported in 1 patient, and 1 patient developed phototoxicity after treatment with voriconazole. One patient experienced reactivation of Epstein-Barr virus without further complications. Further infectious complications after transplantation were not observed (Table 2). One patient underwent a second alloHSCT after autologous reconstitution at 10 months, and 1 patient experienced graft failure 1 month after the first alloHSCT.
Follow-up. The plot shows the follow-up of the study cohort sorted according to the genetic variants. The y-axis displays patient IDs, and the x-axis displays the time since birth in months. At the last follow-up (median, 192 months; range, 6-804), 31 patients were alive. One patient died at age 5 years due to pancolitis with sepsis. Six patients received alloHSCT. No alloHSCT-related mortality was reported (median follow-up after alloHSCT, 91.5 months; range, 9-147). Pat, patient.
Follow-up. The plot shows the follow-up of the study cohort sorted according to the genetic variants. The y-axis displays patient IDs, and the x-axis displays the time since birth in months. At the last follow-up (median, 192 months; range, 6-804), 31 patients were alive. One patient died at age 5 years due to pancolitis with sepsis. Six patients received alloHSCT. No alloHSCT-related mortality was reported (median follow-up after alloHSCT, 91.5 months; range, 9-147). Pat, patient.
Engraftment and immune reconstitution
Neutrophil engraftment (>500/μL) was observed at a median of 18 days (range, 15-21), and patients achieved an unsupported platelet count >20 000/μL at a median of 25 days (range, 12-56). On day 100, the neutrophil count reached a median of 2600/μL (range, 1650/μL to 3000/μL) and 4535/μL (range, 1850/μL to 7100/μL) 1 year after transplantation. On day 100, the median CD3+ T cells was 417/μL (range, 231/μL to 832/μL). At their last follow-up, all patients showed an adequate reconstitution of T cells and presented with age-normal cell counts (Table 2).
Chimerism
Three patients achieved complete donor chimerism within 30 days after transplantation. P1 achieved a mixed donor chimerism of ∼70% after second alloHSCT. Data were missing for P27 (Table 2).
Discussion
In this study, we aimed to assess the genotype-phenotype correlation in a large cohort of JAGN1-deficient patients. We observed substantial disease variability. In total, 7 patients were reported having slight developmental delays and learning disorders. Although we cannot confirm any severe cognitive impairment in JAGN1 deficiency, a variety of skeletal abnormalities and a predisposition to fractures, which might indicate an osteogenesis imperfecta or osteopetrosis-like phenotype, were found in 8 patients (8/32 [25%]). Patients with the JAGN1 variants c.63G>T, p.Glu21Asp and c130c>T p.His44 Tyr presented more often with extramedullary symptoms such as syndromic facial features and bone metabolism disorders than patients with the variant c.3G>A p.Met1 (P = .039). Extramedullary symptoms were found in 3 patients with the variant c.63G>T, p.Glu21Asp (37.5%; 95% CI, 10.2-74.1) and 4 patients carrying the variant c130c>T p.His44 Tyr (60%; 95% CI, 17.0-92.7). In contrast, variant c.3G>A p.Met1, found in 9 patients, was never associated with extramedullary symptoms, except for short stature in 1 patient (11.1%; 95% CI, 0-49.3). These are important clinical aspects for counseling parents and families.
This is similar to the genotype-phenotype correlation observed in HAX1 deficiency. Some mutations (eg, mutations in exon 2) cause isolated neutropenia, whereas other mutations affecting both isoforms A and B can cause neurodevelopmental delay and epilepsy.13
In our cohort, 91% of the patients received G-CSF as a first-line therapy. Nevertheless, at the last follow-up, 3 patients who did not undergo transplantation were alive without G-CSF treatment. One patient with the variant c.3G>A p.Met1 (P3) did not respond to G-CSF, resulting in therapy discontinuation. Other 2 patients with the variants c130c>T p.His44 Tyr (P25) and c.59G > A; p.R20Q (P32) never required any treatment. This highlights again the variability of disease severity.
Patients with extramedullary symptoms also presented at younger age and suffered often from severe infections, indicating that they were more critically affected by the disease. Interestingly, allogeneic stem cell transplantation was only performed in 6 patients, even though several patients presented with severe infections and inflammatory complications. In the majority of the cohort, the disease could be controlled with supportive care and neutrophil stimulation. Although there were no transplant-related complications such as severe infections or severe GVHD reported during alloHSCT, 2 patients suffered a graft failure/autologous engraftment despite receiving myeloablative conditioning and serotherapy. Both patients could be rescued by a second alloHSCT. The European branch of the Severe Chronic Neutropenia International Registry previously reported a 5-year survival rate of 80%14 after alloHSCT in patients with all kinds of SCN. Acute or chronic GVHD and bacterial or viral infections were major risk factors for treatment-related morbidity. In our cohort, only severely affected patients had to undergo alloHSCT. This included 3 patients who did not respond to G-CSF treatment and 1 patient with MDS/AML. Two of the 6 patients who underwent transplant needed a second alloHSCT due to autologous reconstitution and graft failure. AlloHSCT resulted in stable hematopoietic recovery and a cure for infections in all patients without severe treatment-related toxicity. These data are superior to the outcomes reported in previous studies.14,15 We were able to demonstrate the efficacy of G-CSF for JAGN1-deficient patients. G-CSF treatment is an acceptable long-term treatment choice in children, keeping in mind that patients should be monitored for the development of malignancies regularly. Based on our data, one should discuss the role of preemptive alloHSCT for JAGN1-deficient patients at an early age in preventing disease complications and/or progressing to MDS/AML. Furthermore, the high disease activity with severe infections and inflammatory complications might have contributed to the graft failure/rejection in 2 patients; hence, we would postulate that providing an anti-inflammatory pretreatment might be beneficial and could be discussed for severely affected patients. Based on the information we have in this cohort and our experience, both treatment with G-CSF and alloHSCT did not ameliorate bone metabolism disorders.
Rosenberg et al reported on 374 patients with SCN registered in the Severe Chronic Neutropenia International Registry and found a cumulative incidence of 22% for MDS/AML after 15 years on G-CSF.16 An analysis of the French Neutropenia registry among 374 patients reported a cumulative incidence of MDS/AML of 8.1% at 20 years, including patients who did not receive G-CSF.17 An elevated risk of developing hematological abnormalities was reported mainly for patients with ELANE, HAX1, and SBDS mutations but was also found in those with GATA2, G6PC3, and CLPB mutations.18 Due to the risk of hematological complications, careful monitoring of patients on G-CSF and the performance of annual bone marrow evaluations in patients with high-risk mutations are recommended (ELANE, HAX1, and SBDS). To our knowledge, disease progression to MDS and/or AML has not yet been reported in patients with JAGN1 deficiency. In this cohort, 1 patient developed MDS/AML at age 18 years. At the last follow-up, 12 patients were aged >20 years and had received long-term G-CSF. The oldest patient alive and receiving G-CSF was aged 67 years. The risk for MDS/AML seems to be low in JAGN1 deficiency. Nevertheless, we suggest close clinical monitoring of JAGN1-deficient patients on G-CSF stimulation and, if necessary, regular bone marrow aspirates.
In 2021, JAGN1 deficiency was shown to affect B cells through aberrant IgG N-glycosylation. The authors postulated that the defective N-glycosylation in JAGN1 affects humoral immune function in mice and human JAGN1 deficiency.19 In our cohort, 5 patients had decreased Ig levels; however, we did not find a relevant B-cell–related disease phenotype, such as antibody deficiency or Common Variable Immunodeficiency (CVID). Based on our data, we cannot confirm whether patients would benefit from additional Ig replacement treatment.
In summary, this is, to our knowledge, the first comprehensive data analysis of a large JAGN1 cohort; nonetheless, the numbers are still small, and some patients had their last follow-up >2 years ago, which is a limitation of this study. It highlights the need for further close monitoring of affected patients during their adulthood international registry studies.
Acknowledgments
The authors thank all the patients, their families and caregivers, and all the referring colleagues and the nursing staff.
Authorship
Contribution: J.F.-S., E.S.-M., and S. Bakhtiar designed the study and wrote the manuscript; J.F.-S., J.R.H., and S. Bakhtiar collected detailed patient data and wrote the manuscript; A.W., F.H., L.I.G.-G., Z.C., S.S., F.C., S. Baris, A.F., M.A., R.S., M.K.-D., C.Z., C.B.-C., B.B., C.P., G.K., M.S., M.H.A., P.B., B.N., and J.D. provided clinical data, shared the data, assisted in conceptualization, and discussed the results; and all the authors edited and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shahrzad Bakhtiar, Division for Stem Cell Transplantation and Immunology, Department of Pediatrics, Goethe University Frankfurt, Theodor-Stern Kai 7, 60590 Frankfurt am Main, Germany; email: bakhtiar@med.uni-frankfurt.de.
References
Author notes
Original data are available on request from the corresponding author, Shahrzad Bakhtiar (bakhtiar@med.uni-frankfurt.de).
The full-text version of this article contains a data supplement.