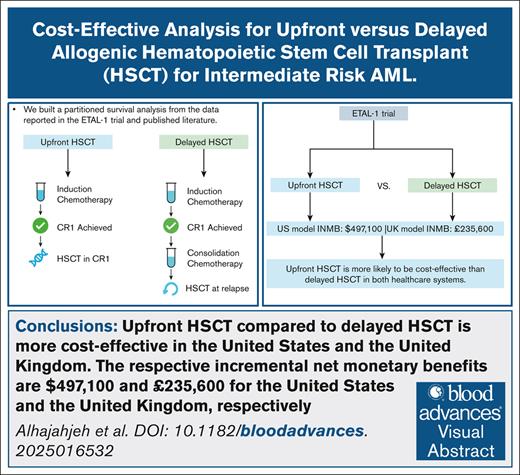
Key Points
Upfront HSCT for intermediate-risk AML is always cost-effective in the United States and the United Kingdom, compared with delayed HSCT.
The respective INMBs are $497 100 and £235 600, respectively, for the United States and the United Kingdom.
Visual Abstract
The ETAL-1 trial demonstrated that upfront allogeneic hematopoietic stem cell transplant (HSCT) improved disease-free survival, but not overall survival, when compared with consolidation chemotherapy followed by “delayed” HSCT on relapse in patients with intermediate-risk acute myeloid leukemia (AML). However, the health-economic implications of upfront HSCT compared with delayed HSCT are unknown. We developed a partitioned survival analysis model using derived survival data, probabilities of salvage treatments, utilities, and costs from the ETAL-1 trial and published literature. The primary outcome was the incremental net monetary benefit (INMB) from the perspective of the United States and United Kingdom health care systems, at all accepted willingness-to-pay (WTP) thresholds: $50 000 to $150 000 per quality-adjusted life year (QALY), and £20 000 to £30 000 per QALY, respectively. The respective INMBs favored upfront HSCT, and were $497 100 (95% confidence interval [CI], $259 800-$719 600) and £235 600 (95% CI, £166 800-£298 500) at WTP thresholds of $150 000 per QALY and £30 000 per QALY. Across deterministic sensitivity analyses, no model input changed the conclusion that upfront HSCT is the cost-effective strategy in either jurisdiction. Probabilistic sensitivity analysis showed that upfront HSCT was cost-effective in 100% of iterations, and was less costly and more effective (ie, “dominant”) in >90% of iterations in both health care systems. In conclusion, we conducted a partitioned survival analysis based on the ETAL-1 trial, and showed that proceeding to HSCT in first remission is the cost-effective strategy in the care of patients with intermediate-risk AML in both the United States and the United Kingdom, as compared with delayed HSCT.
Introduction
Hematopoietic stem cell transplant (HSCT) has demonstrated the best survival benefit for patients with acute myeloid leukemia (AML).1 However, due to the increased risk of morbidity and mortality with HSCT, an individualized treatment approach carefully balancing patient and disease characteristics is needed.2,3 The European LeukemiaNet (ELN) classifies patients with AML into 3 distinct risk categories: favorable, intermediate, and adverse, based on their molecular and cytogenetic abnormalities.4,5 Treatment recommendations vary by risk group, with patients with favorable-risk AML generally managed with chemotherapy alone, and patients with adverse-risk AML advised to undergo HSCT in first remission if they are transplant eligible.2,6,7 However, the optimal treatment strategy for intermediate-risk AML remains less defined, highlighting a critical gap in clinical guidance.2,8
The ETAL-1 trial, a randomized phase 3 study, provides essential insights into the management of intermediate-risk AML in patients aged <60 years.6 This trial compared outcomes between HSCT in first remission and consolidation chemotherapy (CCT) followed by HSCT upon relapse (ie, delayed HSCT).6 The results demonstrated a clear advantage in 2-year disease-free survival (DFS) with upfront HSCT, while overall survival (OS) was comparable between the 2 treatment approaches.6 Despite these clinical findings, the cost-effectiveness of immediate HSCT in first remission vs delayed HSCT following relapse remains unknown, but is of significant interest given the high costs of transplant and posttransplant care.
Our study aims to address this gap by evaluating the cost-effectiveness of “upfront” HSCT (ie, HSCT in first remission) compared with a strategy of CCT followed by “delayed” HSCT upon relapse for patients with intermediate-risk AML. By incorporating AML-specific clinical outcomes, costs, and quality of life data, this research seeks to provide guidance for optimizing treatment decisions in intermediate-risk AML in both the United States and the United Kingdom.
Methods
Model overview
We developed a partitioned survival analysis model to assess the cost-effectiveness of upfront HSCT compared with delayed HSCT for patients with intermediate-risk AML in their first remission.4 This analysis was based on data from the ETAL-1 trial.6 The cohort’s mean enrollment age was 48.2 years, with all participants being newly diagnosed with AML, and treated initially with 7+3 induction chemotherapy.6 Among the 96 patients analyzed, 50 underwent HSCT (upfront HSCT), while 46 received CCT during their first remission followed by HSCT in cases of relapse (delayed HSCT).6 Notably, 15.8% of patients in the HSCT group received a single cycle of high-dose cytarabine as consolidation therapy prior to HSCT, while none of the patients received >1 cycle. The remainder proceeded to HSCT directly.6
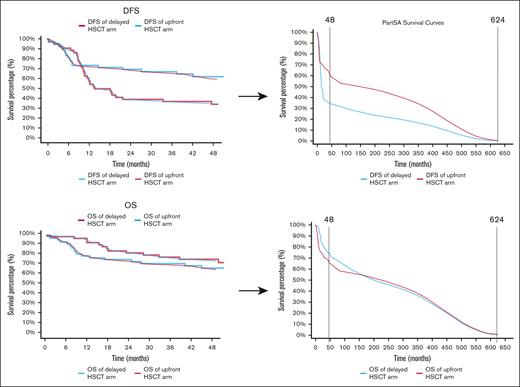
The model was built using survival data from the ETAL-1 trial, reconstructed from Kaplan-Meier survival curves available in the study’s supplementary materials for DFS and OS based on the ELN 2017 risk criteria.6,9 These parameters informed time-varying hazards that reflected both relapse rates and mortality over the trial’s 50-month follow-up period (Figure 1A-B).
Observed and projected DFS and OS for upfront vs delayed HSCT for patients with intermediate-risk AML. (A) DFS curves for upfront and delayed HSCT. These Kaplan-Meier curves compare the DFS of patients with intermediate-risk AML treated with upfront HSCT vs delayed HSCT over a follow-up period of 48 months. The blue line represents DFS for the delayed HSCT group, while the red line represents DFS for the HSCT group. (B) OS curves for upfront and delayed HSCT. These Kaplan-Meier curves show the OS of patients with intermediate-risk AML treated with upfront HSCT vs delayed HSCT over a follow-up period of 48 months. The blue line corresponds to the delayed HSCT arm, and the red line represents the HSCT group. (C) PSA of DFS beyond 48 months. This PSA model extends DFS projections up to 624 months (∼52 years), comparing outcomes for delayed HSCT (blue) and upfront HSCT (red). The dashed vertical lines mark significant time points at 48 months (transition from observed to modeled data), and the extrapolated end point at 624 months. This figure predicts long-term DFS trends based on survival probabilities. (D) PSA of OS beyond 48 months. This PSA model extrapolates OS projections for delayed HSCT (blue) and upfront HSCT (red) up to 624 months. As in panel C, dashed vertical lines denote the 48-month cutoff between observed and modeled data, along with the extrapolated end point at 624 months. This analysis provides insight into the potential long-term survival benefits of each treatment.
Observed and projected DFS and OS for upfront vs delayed HSCT for patients with intermediate-risk AML. (A) DFS curves for upfront and delayed HSCT. These Kaplan-Meier curves compare the DFS of patients with intermediate-risk AML treated with upfront HSCT vs delayed HSCT over a follow-up period of 48 months. The blue line represents DFS for the delayed HSCT group, while the red line represents DFS for the HSCT group. (B) OS curves for upfront and delayed HSCT. These Kaplan-Meier curves show the OS of patients with intermediate-risk AML treated with upfront HSCT vs delayed HSCT over a follow-up period of 48 months. The blue line corresponds to the delayed HSCT arm, and the red line represents the HSCT group. (C) PSA of DFS beyond 48 months. This PSA model extends DFS projections up to 624 months (∼52 years), comparing outcomes for delayed HSCT (blue) and upfront HSCT (red). The dashed vertical lines mark significant time points at 48 months (transition from observed to modeled data), and the extrapolated end point at 624 months. This figure predicts long-term DFS trends based on survival probabilities. (D) PSA of OS beyond 48 months. This PSA model extrapolates OS projections for delayed HSCT (blue) and upfront HSCT (red) up to 624 months. As in panel C, dashed vertical lines denote the 48-month cutoff between observed and modeled data, along with the extrapolated end point at 624 months. This analysis provides insight into the potential long-term survival benefits of each treatment.
Beyond the trial period, mortality rates for the delayed HSCT arm were calculated by combining age-specific mortality rates of the general United States population with the excess risk of non-acute promyelocytic leukemia AML-related mortality, extending to 216 months after diagnosis. After this point, the mortality risk converged with that of the general population, as excess mortality due to AML after this time is not thought to statistically differ from the general population.10,11
In contrast, for the upfront HSCT group, mortality rates combined general United States population age-specific mortality with post-HSCT–specific mortality up to 84 months after transplant.10,12 This included 48-month follow-up data from the ETAL-1 trial, followed by an additional 36 months of mortality data informed from a large cohort study that evaluated late relapse (after 48 months) of patients with AML after HSCT. As AML relapse >7 years after transplant is rare, and prior studies did not show a statistically significant survival difference compared with the general population, we used an age-specific United States population-based mortality rate starting after month 84 of the model10,12 (Figure 1C-D).
The base-case model was run over a lifetime horizon. In scenario analyses, we also evaluated shorter-term cost-effectiveness by examining a 10-year time horizon.
Model inputs
Cost inputs for both upfront HSCT and delayed HSCT were derived from published literature, and included direct health care costs, such as inpatient, outpatient, emergency department, pharmacy expenses, and supportive care expenses (eg, blood transfusion and antimicrobial use) as reported for treatment regimens used in the ETAL-1 trial.13-17 For salvage therapy costs in the delayed HSCT arm, we used data from the ETAL-1 trial’s supplementary materials to guide the percentage of patients who received each type of salvage therapy after relapse.6 Specifically for the delayed HSCT arm, 48.8% proceeded directly to HSCT without reinduction therapy, while 43.9% and 7.3% received high-intensity chemotherapy (HIC) and low-intensity chemotherapy (LIC) reinduction therapies, respectively, prior to transplantation. Due to the lack of detailed data about subsequent therapy after relapse for the upfront HSCT cohort, we used literature estimates to determine the probabilities and costs associated with each subsequent salvage therapy.18-21 To align with clinical practice in the United States, we noted that 24% of patients in the HSCT arm had FMS-like tyrosine kinase 3 (FLT3) mutations for which we assumed use of gilteritinib as next line of treatment in line with the United States Food and Drug Administration label.3 Patients without a FLT3 mutation received salvage treatment with azacitidine + venetoclax, other LIC, HIC or HSCT directly following published literature. Lastly, we assumed any patients who were not eligible for or declined salvage therapy, including FLT3 inhibitors, azacitidine + venetoclax, HSCT, LIC, or HIC, would receive best supportive care as palliative treatment reflecting real-world practice and commonly used assumptions in cost-effectiveness analysis studies.22-24 Additionally, lifetime HSCT costs included supportive care expenses and costs associated with graft-versus-host disease (GVHD), based on values reported in the literature for both health care perspectives.14,16,25-28
Utility values, expressed in terms of quality-adjusted life years (QALYs), were derived from published literature, considering both patients with and without chronic GVHD.26,29,30 While the ETAL-1 trial reported no statistically significant difference in quality of life between the 2 arms, we modeled our utility values as follows. During the first remission period, patients in the delayed HSCT arm were assigned the utility of being in remission. In contrast, for the upfront HSCT arm, utilities while in first complete remission (CR1) were weighted based on the presence or absence of GVHD. Once disease relapse occurred, all patients, regardless of their initial treatment, were modeled using utility values associated with relapsed AML. Additionally, to capture the difference in patients’ quality of life during the treatment period, specific utility values at the treatment period 1 to 6 months, and 6 to 12 months were applied.29,30 All costs were adjusted for inflation to 2022 United States dollars, and 2022 United Kingdom pounds using the medical component of the consumer price index, and the average exchange price (1.24) for 2022 between United Kingdom pounds and United States dollars was used.31,32 A yearly discount rate of 3% was applied to costs and utilities in both models.32-35 Detailed inputs from the United States and the United Kingdom health care perspective are summarized in Table 1 and Table 2, respectively.
Cost, clinical variables, probabilities, and utilities (United States health care system model)
| Model variable . | Upfront HSCT . | Delayed HSCT . | Reference . | ||
|---|---|---|---|---|---|
| Base-case scenario . | Distribution used in PSAs . | Base-case scenario . | Distribution used in PSAs . | ||
| Primary treatment | |||||
| Cost of HSCT∗ | $1 012 100 | Gamma (400, 2739.36) | N/A | N/A | |
| Cost of CCT∗ | N/A | N/A | $82 300 | Gamma (400, 205.72) | 13 |
| Cost of 1 cycle of HiDAC before HSCT∗ | $27 300 | Gamma (400, 67.16) | N/A | N/A | 36 |
| Probability of HiDAC before HSCT∗ | 0.16 | Beta-PERT (0.13, 0.19) | N/A | N/A | 6 |
| Salvage treatment | |||||
| Overall probability of HSCT after relapse† | 0.139 | N/A | 1 | Beta-PERT (0.8, 1) | 6,18,19 |
| Probability of only HSCT after relapse† | 0 | Beta-PERT (0.12, 0.18) | 0.49 0.0513‡ | Beta-PERT (0.39, 0.59) Beta-PERT (0.04, 0.06)‡ | 6,18 |
| Cost of HIC plus HSCT after relapse∗ | $1 191 900 | NA | $1 191 900 | NA | 14,37 |
| Probability of HIC plus HSCT after relapse† | 0.532 | Beta-PERT (0.425, 0.638) | 0.43 0.532‡ | Beta-PERT (0.34, 0.51) Beta-PERT (0.425, 0.638)‡ | 6,19 |
| Cost of LIC plus HSCT after relapse∗ | $1 075 400 | NA | $1 075 400 | NA | 14,37 |
| Probability of LIC plus HSCT after relapse† | 0.468 | Beta-PERT (0.374,0.562) | 0.08 0.468‡ | Beta-PERT (0.07, 0.1) Beta-PERT (0.374, 0.562) | 6,19 |
| Cost of HIC only after relapse∗ | $179 800 | Gamma (400, 449.51) | $179 800 | Gamma (400, 449.51) | 37 |
| Probability of HIC only after relapse† | 0.179 | Beta-PERT (0.143, 0.215) | 0 0.179‡ | N/A Beta-PERT (0.143, 0.215)‡ | 6,18,19,37 |
| Cost of LIC only after relapse∗ | $63 300 | Gamma (400, 158.16) | $63 300 | Gamma (400, 158.16) | 37 |
| Probability of LIC only after relapse† | 0.212 | Beta-PERT (0.170, 0.255) | 0 0.212‡ | N/A Beta-PERT (0.170, 0.255)‡ | 6,18,19,37 |
| Cost of AZA + VEN only after relapse∗ | $81 100 | Gamma (400, 202.73) | $81 100 | Gamma (400, 202.73) | 37 |
| Probability of AZA + VEN only after relapse† | 0.096 | Beta-PERT (0.077, 0.115) | 0 0.096‡ | N/A Beta-PERT (0.077, 0.115)‡ | 6,18,19,37 |
| Cost of FLT3i only after relapse∗ | $45 200 | Gamma (400, 112.95) | $45 200 | Gamma (400, 112.95) | 37 |
| Probability of FLT3i only after relapse† | 0.24 | Beta-PERT (0.179, 0.268) | 0 0.24‡ | N/A Beta-PERT (0.179, 0.268)† | 6 |
| Cost of BSC only after relapse∗ | $74 300 | Gamma (400, 185.76) | $74 300 | Gamma (400, 185.76) | 38 |
| Probability of BSC only after relapse† | 0.134 | Beta-PERT (0.120, 0.180) | 0 0.134‡ | N/A Beta-PERT (0.120, 0.180)† | 6,18,19,37 |
| Cost of supportive care | |||||
| Cost of terminal care∗ | $178 400 | Gamma (400, 446.04) | $178 400 | Gamma (400, 446.04) | 14 |
| GVHD | |||||
| Probability of cGVHD after 2 years | 0.42 | Beta-PERT (0.34, 0.50) | N/A | N/A | 26 |
| Survival parameter | |||||
| HR OS | 1.74 (1.39-2.09) | N/A | Ref | N/A | 6 |
| HR DFS | 0.45 (0.36-0.54) | N/A | Ref | N/A | 6 |
| Utilities | |||||
| During CCT | N/A | N/A | 0.58 | Beta-PERT (0.464, 0.696) | 27 |
| After CCT | N/A | N/A | 0.85 | Beta-PERT (0.688, 1) | 27 |
| During HSCT | 0.613 | Beta-PERT (0.49, 0.736) | 0.597 | Beta-PERT (0.478, 0.716) | 26 |
| After 6 months form HSCT until 1 year | 0.81 | Beta-PERT (0.648, 0.972) | 0.81 | Beta-PERT (0.648, 0.972) | 26 |
| Relapse | 0.53 | Beta-PERT (0.424, 0.636) | 0.53 | Beta-PERT (0.424, 0.636) | 26 |
| Model variable . | Upfront HSCT . | Delayed HSCT . | Reference . | ||
|---|---|---|---|---|---|
| Base-case scenario . | Distribution used in PSAs . | Base-case scenario . | Distribution used in PSAs . | ||
| Primary treatment | |||||
| Cost of HSCT∗ | $1 012 100 | Gamma (400, 2739.36) | N/A | N/A | |
| Cost of CCT∗ | N/A | N/A | $82 300 | Gamma (400, 205.72) | 13 |
| Cost of 1 cycle of HiDAC before HSCT∗ | $27 300 | Gamma (400, 67.16) | N/A | N/A | 36 |
| Probability of HiDAC before HSCT∗ | 0.16 | Beta-PERT (0.13, 0.19) | N/A | N/A | 6 |
| Salvage treatment | |||||
| Overall probability of HSCT after relapse† | 0.139 | N/A | 1 | Beta-PERT (0.8, 1) | 6,18,19 |
| Probability of only HSCT after relapse† | 0 | Beta-PERT (0.12, 0.18) | 0.49 0.0513‡ | Beta-PERT (0.39, 0.59) Beta-PERT (0.04, 0.06)‡ | 6,18 |
| Cost of HIC plus HSCT after relapse∗ | $1 191 900 | NA | $1 191 900 | NA | 14,37 |
| Probability of HIC plus HSCT after relapse† | 0.532 | Beta-PERT (0.425, 0.638) | 0.43 0.532‡ | Beta-PERT (0.34, 0.51) Beta-PERT (0.425, 0.638)‡ | 6,19 |
| Cost of LIC plus HSCT after relapse∗ | $1 075 400 | NA | $1 075 400 | NA | 14,37 |
| Probability of LIC plus HSCT after relapse† | 0.468 | Beta-PERT (0.374,0.562) | 0.08 0.468‡ | Beta-PERT (0.07, 0.1) Beta-PERT (0.374, 0.562) | 6,19 |
| Cost of HIC only after relapse∗ | $179 800 | Gamma (400, 449.51) | $179 800 | Gamma (400, 449.51) | 37 |
| Probability of HIC only after relapse† | 0.179 | Beta-PERT (0.143, 0.215) | 0 0.179‡ | N/A Beta-PERT (0.143, 0.215)‡ | 6,18,19,37 |
| Cost of LIC only after relapse∗ | $63 300 | Gamma (400, 158.16) | $63 300 | Gamma (400, 158.16) | 37 |
| Probability of LIC only after relapse† | 0.212 | Beta-PERT (0.170, 0.255) | 0 0.212‡ | N/A Beta-PERT (0.170, 0.255)‡ | 6,18,19,37 |
| Cost of AZA + VEN only after relapse∗ | $81 100 | Gamma (400, 202.73) | $81 100 | Gamma (400, 202.73) | 37 |
| Probability of AZA + VEN only after relapse† | 0.096 | Beta-PERT (0.077, 0.115) | 0 0.096‡ | N/A Beta-PERT (0.077, 0.115)‡ | 6,18,19,37 |
| Cost of FLT3i only after relapse∗ | $45 200 | Gamma (400, 112.95) | $45 200 | Gamma (400, 112.95) | 37 |
| Probability of FLT3i only after relapse† | 0.24 | Beta-PERT (0.179, 0.268) | 0 0.24‡ | N/A Beta-PERT (0.179, 0.268)† | 6 |
| Cost of BSC only after relapse∗ | $74 300 | Gamma (400, 185.76) | $74 300 | Gamma (400, 185.76) | 38 |
| Probability of BSC only after relapse† | 0.134 | Beta-PERT (0.120, 0.180) | 0 0.134‡ | N/A Beta-PERT (0.120, 0.180)† | 6,18,19,37 |
| Cost of supportive care | |||||
| Cost of terminal care∗ | $178 400 | Gamma (400, 446.04) | $178 400 | Gamma (400, 446.04) | 14 |
| GVHD | |||||
| Probability of cGVHD after 2 years | 0.42 | Beta-PERT (0.34, 0.50) | N/A | N/A | 26 |
| Survival parameter | |||||
| HR OS | 1.74 (1.39-2.09) | N/A | Ref | N/A | 6 |
| HR DFS | 0.45 (0.36-0.54) | N/A | Ref | N/A | 6 |
| Utilities | |||||
| During CCT | N/A | N/A | 0.58 | Beta-PERT (0.464, 0.696) | 27 |
| After CCT | N/A | N/A | 0.85 | Beta-PERT (0.688, 1) | 27 |
| During HSCT | 0.613 | Beta-PERT (0.49, 0.736) | 0.597 | Beta-PERT (0.478, 0.716) | 26 |
| After 6 months form HSCT until 1 year | 0.81 | Beta-PERT (0.648, 0.972) | 0.81 | Beta-PERT (0.648, 0.972) | 26 |
| Relapse | 0.53 | Beta-PERT (0.424, 0.636) | 0.53 | Beta-PERT (0.424, 0.636) | 26 |
AZA + VEN, azacytidine + venetoclax; BSC, best supportive care; cGVHD, chronic graft-versus-host disease; FLT3i, FLT3 inhibitor; HIC, high-intensity chemotherapy (cytarabine in combination with daunorubicin, doxorubicin, etoposide, idarubicin, mitoxantrone, cladribine, or clofarabine); HiDAC, high-dose cytarabine; HR, hazard ratio; LIC, low-intensity chemotherapy (monotherapy of azacitidine, cytarabine, decitabine, gemtuzumab, glasdegib, and ivosidenib); N/A, not applicable.
Adjusted for inflation until the end of 2022.
Calculated depending on data derived from the original trial and literature variables.
This value was used only in sensitivity analysis, and is a secondary value from the literature for probability of certain salvage therapy regimens for patients who did not undergo salvage.
Cost, clinical variables, probabilities, and utilities (United Kingdom health care system model)
| Model variable . | Upfront HSCT . | Delayed HSCT . | Reference . | ||
|---|---|---|---|---|---|
| Base-case scenario . | Distribution used in PSAs . | Base-case scenario . | Distribution used in PSAs . | ||
| Primary treatment | |||||
| Cost of HSCT for the first 2 y∗ | £78 800 ($97 700) | Gamma (100, 977.30) | N/A | N/A | 17 |
| Probability of cGVHD after 2 y | 0.42 | Beta-PERT (0.34, 0.50) | N/A | N/A | 26 |
| Annual supportive care after 2 years from HSCT without cGVHD | £11 800 ($14 700) | Gamma (100, 146.64) | N/A | N/A | 16 |
| Annual supportive care after 2 years from HSCT with cGVHD | £24 200 ($30 000) | Gamma (100, 299.90) | N/A | N/A | 16 |
| Cost of CCT∗ | N/A | N/A | £11 700 ($14 500) | Gamma (100, 144.58) | 15 |
| Cost of 1 cycle of HiDAC before HSCT∗ | £2900 ($3600) | Gamma (100, 36.14) | N/A | N/A | 15 |
| Probability of HiDAC before HSCT∗ | 0.16 | Beta-PERT (0.13, 0.19) | N/A | N/A | 6 |
| Supportive care during CCT | N/A | N/A | £7000 ($8700) | (100, 86.65) | 15 |
| Annual supportive care for CCT after 6 months | N/A | N/A | £3900 ($4800) | Gamma (100, 47.98) | 15 |
| Salvage treatments | |||||
| Overall probability of HSCT after relapse† | 0.342 | N/A | 1 | Beta-PERT (0.8, 1) | 19 |
| Probability of only HSCT after relapse† | 0.0513 | Beta-PERT (0.04, 0.06) | 0.49 0.0513‡ | Beta-PERT (0.39, 0.59) Beta-PERT (0.04, 0.06)† | 6,19 |
| Cost of HIC plus HSCT after relapse∗ | £126 400 ($156 700) | NA | £126 400 ($156 700) | NA | 17,39 |
| Probability of HIC plus HSCT after relapse† | 0.2907 | Beta-PERT (0.23, 0.35) | 0.43 0.2907‡ | Beta-PERT (0.34, 0.51) Beta-PERT (0.23, 0.35)† | 6,19 |
| Cost of LIC plus HSCT after relapse∗ | £115 500 ($143 200) | NA | £115 500 ($143 200) | NA | 17,39 |
| Probability of LIC plus HSCT after relapse† | 0 | N/A | 0.08 0‡ | Beta-PERT (0.07, 0.1) N/A | 6,19 |
| Cost of HIC only after relapse∗ | £47 500 ($59 000) | Gamma (100, 589.58) | £47 500 ($59 000) | Gamma (100, 589.58) | 39 |
| Probability of HIC only after relapse† | 0.23 | Beta-PERT (0.18, 0.28) | 0 0.23‡ | N/A Beta-PERT (0.18, 0.28)∗ | 6,19 |
| Cost of LIC only after relapse∗ | £36 700 ($45 500) | Gamma (100, 454.94) | £36 700 ($45 500) | Gamma (100, 454.94) | 39 |
| Probability of LIC only after relapse† | 0.3 | Beta-PERT (0.24, 0.36) | 0 0.3‡ | N/A Beta-PERT (0.24, 0.36)∗ | 6,19 |
| Cost of BSC only after relapse∗ | £4700 ($5800) | Gamma (100, 57.92) | £4700 ($5800) | Gamma (100, 57.92) | 39 |
| Probability of BSC only after relapse† | 0.128 | Beta-PERT (0.03, 0.045) | 0 0.128‡ | N/A Beta-PERT (0.11, 0.17)∗ | 6,19 |
| Cost of supportive care | |||||
| Cost of terminal care | £16 700 ($20 700) | Gamma (100, 206.79) | £16 700 ($20 700) | Gamma (100, 206.79) | 15 |
| GVHD | |||||
| Probability of cGVHD after 2 y | 0.42 | Beta-PERT (0.34, 0.50) | N/A | N/A | 26 |
| Survival parameter | |||||
| HR OS | 1.74 (1.39-2.09) | N/A | Ref | N/A | 6 |
| HR DFS | 0.45 (0.36-0.54) | N/A | Ref | N/A | 6 |
| Utilities | |||||
| During CCT | N/A | N/A | 0.58 | Beta-PERT (0.464, 0.696) | 27 |
| After CCT | N/A | N/A | 0.85 | Beta-PERT (0.688, 1) | 27 |
| During HSCT | 0.613 | Beta-PERT (0.49, 0.736) | 0.597 | Beta-PERT (0.478, 0.716) | 26 |
| After 6 months from HSCT until 1 y | 0.81 | Beta-PERT (0.648, 0.972) | 0.81 | Beta-PERT (0.648, 0.972) | 26 |
| Relapse | 0.53 | Beta-PERT (0.424, 0.636) | 0.53 | Beta-PERT (0.424, 0.636) | 26 |
| Model variable . | Upfront HSCT . | Delayed HSCT . | Reference . | ||
|---|---|---|---|---|---|
| Base-case scenario . | Distribution used in PSAs . | Base-case scenario . | Distribution used in PSAs . | ||
| Primary treatment | |||||
| Cost of HSCT for the first 2 y∗ | £78 800 ($97 700) | Gamma (100, 977.30) | N/A | N/A | 17 |
| Probability of cGVHD after 2 y | 0.42 | Beta-PERT (0.34, 0.50) | N/A | N/A | 26 |
| Annual supportive care after 2 years from HSCT without cGVHD | £11 800 ($14 700) | Gamma (100, 146.64) | N/A | N/A | 16 |
| Annual supportive care after 2 years from HSCT with cGVHD | £24 200 ($30 000) | Gamma (100, 299.90) | N/A | N/A | 16 |
| Cost of CCT∗ | N/A | N/A | £11 700 ($14 500) | Gamma (100, 144.58) | 15 |
| Cost of 1 cycle of HiDAC before HSCT∗ | £2900 ($3600) | Gamma (100, 36.14) | N/A | N/A | 15 |
| Probability of HiDAC before HSCT∗ | 0.16 | Beta-PERT (0.13, 0.19) | N/A | N/A | 6 |
| Supportive care during CCT | N/A | N/A | £7000 ($8700) | (100, 86.65) | 15 |
| Annual supportive care for CCT after 6 months | N/A | N/A | £3900 ($4800) | Gamma (100, 47.98) | 15 |
| Salvage treatments | |||||
| Overall probability of HSCT after relapse† | 0.342 | N/A | 1 | Beta-PERT (0.8, 1) | 19 |
| Probability of only HSCT after relapse† | 0.0513 | Beta-PERT (0.04, 0.06) | 0.49 0.0513‡ | Beta-PERT (0.39, 0.59) Beta-PERT (0.04, 0.06)† | 6,19 |
| Cost of HIC plus HSCT after relapse∗ | £126 400 ($156 700) | NA | £126 400 ($156 700) | NA | 17,39 |
| Probability of HIC plus HSCT after relapse† | 0.2907 | Beta-PERT (0.23, 0.35) | 0.43 0.2907‡ | Beta-PERT (0.34, 0.51) Beta-PERT (0.23, 0.35)† | 6,19 |
| Cost of LIC plus HSCT after relapse∗ | £115 500 ($143 200) | NA | £115 500 ($143 200) | NA | 17,39 |
| Probability of LIC plus HSCT after relapse† | 0 | N/A | 0.08 0‡ | Beta-PERT (0.07, 0.1) N/A | 6,19 |
| Cost of HIC only after relapse∗ | £47 500 ($59 000) | Gamma (100, 589.58) | £47 500 ($59 000) | Gamma (100, 589.58) | 39 |
| Probability of HIC only after relapse† | 0.23 | Beta-PERT (0.18, 0.28) | 0 0.23‡ | N/A Beta-PERT (0.18, 0.28)∗ | 6,19 |
| Cost of LIC only after relapse∗ | £36 700 ($45 500) | Gamma (100, 454.94) | £36 700 ($45 500) | Gamma (100, 454.94) | 39 |
| Probability of LIC only after relapse† | 0.3 | Beta-PERT (0.24, 0.36) | 0 0.3‡ | N/A Beta-PERT (0.24, 0.36)∗ | 6,19 |
| Cost of BSC only after relapse∗ | £4700 ($5800) | Gamma (100, 57.92) | £4700 ($5800) | Gamma (100, 57.92) | 39 |
| Probability of BSC only after relapse† | 0.128 | Beta-PERT (0.03, 0.045) | 0 0.128‡ | N/A Beta-PERT (0.11, 0.17)∗ | 6,19 |
| Cost of supportive care | |||||
| Cost of terminal care | £16 700 ($20 700) | Gamma (100, 206.79) | £16 700 ($20 700) | Gamma (100, 206.79) | 15 |
| GVHD | |||||
| Probability of cGVHD after 2 y | 0.42 | Beta-PERT (0.34, 0.50) | N/A | N/A | 26 |
| Survival parameter | |||||
| HR OS | 1.74 (1.39-2.09) | N/A | Ref | N/A | 6 |
| HR DFS | 0.45 (0.36-0.54) | N/A | Ref | N/A | 6 |
| Utilities | |||||
| During CCT | N/A | N/A | 0.58 | Beta-PERT (0.464, 0.696) | 27 |
| After CCT | N/A | N/A | 0.85 | Beta-PERT (0.688, 1) | 27 |
| During HSCT | 0.613 | Beta-PERT (0.49, 0.736) | 0.597 | Beta-PERT (0.478, 0.716) | 26 |
| After 6 months from HSCT until 1 y | 0.81 | Beta-PERT (0.648, 0.972) | 0.81 | Beta-PERT (0.648, 0.972) | 26 |
| Relapse | 0.53 | Beta-PERT (0.424, 0.636) | 0.53 | Beta-PERT (0.424, 0.636) | 26 |
HIC, high-intensity chemotherapy (cytarabine in combination with daunorubicin, doxorubicin, etoposide, idarubicin, mitoxantrone, cladribine, or clofarabine); HiDAC, high-dose cytarabine; LIC, low-intensity chemotherapy (monotherapy of azacitidine, cytarabine, decitabine, gemtuzumab, glasdegib, and ivosidenib).
Adjusted for inflation until the end of 2022.
Calculated depending on data derived from the original trial and literature variables.
This value is a secondary value from the literature for probability of patients who did not undergo salvage HSCT as documented in the ETAL-1 trail.
Model outputs
The primary model output was the incremental net monetary benefit (INMB), a reformulation of the incremental cost-effectiveness ratio that is not subject to instability (ie, because it is not a ratio). The INMB was calculated by multiplying each accepted willingness-to-pay (WTP) threshold across all accepted WTP thresholds, set at $50 000, $100 000, and $150 000 per QALY for the United States health care perspective and at £20 000 and £30 000 per QALY for the United Kingdom health care perspective, by the incremental effectiveness, defined as the difference in utility between the upfront HSCT arm and delayed HSCT arms.35,40,41 The incremental cost, calculated as the cost difference between the 2 arms, was then subtracted from these results.31,32 A positive INMB (ie, >$0) for a pairwise strategy comparison indicates that that strategy is the cost-effective strategy at a given WTP threshold.
Threshold, deterministic, and PSAs
We conducted threshold analyses to evaluate the threshold probability of undergoing salvage HSCT for patients in the delayed HSCT arm that would favor the alternative strategy as the cost-effective strategy. All model input parameters were varied ±20% across deterministic sensitivity analyses. Uncertainty in the model was then propagated across all parameters simultaneously via probabilistic sensitivity analyses (PSAs) with 10 000 iterations.23,24,42 Cost inputs were parameterized with gamma distributions, while probabilities and utilities were parameterized with Beta distribution-program evaluation and review technique (Beta-PERT) distributions.22
Results
Model validation
The reconstructed survival curves closely matched ETAL-1 trial data, supporting the validity of our model. In the delayed HSCT arm, the median DFS was 18.3 months, with a 2-year DFS rate of 38.9%, which closely matched the original study’s reported rate of 39%. In addition, the upfront HSCT arm did not reach the median DFS, with a 2-year DFS rate of 69.4%, aligning well with the 70% reported in the ETAL-1 trial (Figure 1).6
Base-case and scenario analysis: United States
In the base-case analysis for the United States, the total discounted costs and QALYs for upfront HSCT and delayed HSCT were $1 645 900 and $2 017 900, and 9.74 and 8.90 QALYs, respectively. These yielded an INMB of $497 100 (95% confidence interval [CI] $259 800-$719 600) for upfront HSCT compared with delayed HSCT at a WTP threshold of $150 000 per QALY (Table 3). At WTP thresholds of $100 000 and $50 000 per QALY, the INMB was $455 100 and $413 100, respectively.
Base-case results and PSA (United States health care perspective)
| Strategy . | Cost (95% CI) (USD) . | Incremental cost (95% CI) (USD) . | Effectiveness (95% CI) (QALY) . | Incremental effectiveness (95% CI) (QALY) . | INMB (95% CI) (USD/QALY) . | % Cost-effectiveness at WTP threshold of $50 000 . | % Cost-effectiveness at WTP threshold of $100 000 . | % Cost-effectiveness at WTP threshold of $150 000 . |
|---|---|---|---|---|---|---|---|---|
| United States model (lifetime horizon) | ||||||||
| Upfront HSCT | $1 645 900 ($1 435 600-$1 869 900) | $371 100 ($227 300-$482 100) | 9.74 (8.75-10.72) | 0.84 (−0.39 to 2.16) | $497 100 ($259 800-$719 600) | 100 | 100 | 100 |
| Delayed HSCT | $2 017 000 ($1 790 900-$2 236 600) | 8.90 (8.01-9.70) | 0 | 0 | 0 | |||
| INMB at thresholds of $50 000, $100 000, and $150 000 per QALY (95% CI) $413 100 ($248 600-$542 200), $455 100 ($262 200-$620 700), and $497 100 ($259 800-$719 600) | ||||||||
| United States model (10-year horizon) | ||||||||
| Upfront HSCT | $1 589 000 ($1 379 700-$1 813 000) | $132 900 (–$13 400 to $224 800) | 4.74 (4.41-5.09) | 0.30 (−0.18 to 0.82) | $178 000 ($13 400-$298 800) | 97.51 | 98.23 | 98.43 |
| Delayed HSCT | $1 722 000 ($1 484 200-$1 927 400) | 4.44 (4.01-4.83) | 2.49 | 1.77 | 1.57 | |||
| INMB at thresholds of $50 000, $100 000, and $150 000 per QALY (95% CI) $147 900 (−$7200 to $252 400), $162 900 (−$4000 to $272 500), and $178 000 ($13 400-$298 800) | ||||||||
| Strategy . | Cost (95% CI) (USD) . | Incremental cost (95% CI) (USD) . | Effectiveness (95% CI) (QALY) . | Incremental effectiveness (95% CI) (QALY) . | INMB (95% CI) (USD/QALY) . | % Cost-effectiveness at WTP threshold of $50 000 . | % Cost-effectiveness at WTP threshold of $100 000 . | % Cost-effectiveness at WTP threshold of $150 000 . |
|---|---|---|---|---|---|---|---|---|
| United States model (lifetime horizon) | ||||||||
| Upfront HSCT | $1 645 900 ($1 435 600-$1 869 900) | $371 100 ($227 300-$482 100) | 9.74 (8.75-10.72) | 0.84 (−0.39 to 2.16) | $497 100 ($259 800-$719 600) | 100 | 100 | 100 |
| Delayed HSCT | $2 017 000 ($1 790 900-$2 236 600) | 8.90 (8.01-9.70) | 0 | 0 | 0 | |||
| INMB at thresholds of $50 000, $100 000, and $150 000 per QALY (95% CI) $413 100 ($248 600-$542 200), $455 100 ($262 200-$620 700), and $497 100 ($259 800-$719 600) | ||||||||
| United States model (10-year horizon) | ||||||||
| Upfront HSCT | $1 589 000 ($1 379 700-$1 813 000) | $132 900 (–$13 400 to $224 800) | 4.74 (4.41-5.09) | 0.30 (−0.18 to 0.82) | $178 000 ($13 400-$298 800) | 97.51 | 98.23 | 98.43 |
| Delayed HSCT | $1 722 000 ($1 484 200-$1 927 400) | 4.44 (4.01-4.83) | 2.49 | 1.77 | 1.57 | |||
| INMB at thresholds of $50 000, $100 000, and $150 000 per QALY (95% CI) $147 900 (−$7200 to $252 400), $162 900 (−$4000 to $272 500), and $178 000 ($13 400-$298 800) | ||||||||
Costs have been rounded to the nearest hundred.
USD, United States Dollar.
In a scenario analysis examining a shorter 10-year time horizon, the total discounted costs and QALYs for upfront HSCT and delayed HSCT were $1 589 000 and $1 722 000, and 4.74 and 4.44 QALYs, respectively. This yielded an INMB of $178 900 (95% CI, $13 400-$298 800) at a WTP threshold of $150 000 per QALY (Table 3). At WTP thresholds of $100 000 and $50 000 per QALY, the INMB values were $162 900 and $147 900, respectively.
Base-case and scenario analysis: United Kingdom
In the base-case analysis of the United Kingdom model, the total discounted costs and QALYs for upfront HSCT and delayed HSCT were £335 800 and £551 100, and 9.74 and 8.90 QALYs, respectively. These resulted in an INMB of £235 600 (95% CI, £166 800-£298 500) for upfront HSCT compared with delayed HSCT at a WTP threshold of £30 000 per QALY. The INMB value was £228 871 at a WTP threshold of £20 000 per QALY (Table 4).
Base-case results and PSA (United Kingdom health care perspective)
| Strategy . | Cost (95% CI) (UK pound) . | Incremental cost (95% CI) (UK pound) . | Effectiveness (95% CI) (QALY) . | Incremental effectiveness (95% CI) (QALY) . | INMB (95% CI) (UK pound/QALY) . | % Cost-effectiveness at WTP threshold of $20 000 . | % Cost-effectiveness at WTP threshold of $30 000 . |
|---|---|---|---|---|---|---|---|
| United Kingdom model (lifetime horizon) | |||||||
| Upfront HSCT | £335 800 (£303 700-£369 800) | £215 300 (£152 300-£270 600) | 9.74 (8.75-10.72) | 0.84 (−0.39 to 2.16) | £235 600 (£166 800-£298 500) | 100 | 100 |
| Delayed HSCT | £551 100 (£487 000-£609 000) | 8.90 (8.01-9.70) | 0 | 0 | |||
| INMB at thresholds of £20 000 and £30 000 per QALY (95% CI) £187 200 (£164 000-£287 300) and £235 600 (£166 800-£298 500) | |||||||
| United Kingdom model (10-year horizon) | |||||||
| Upfront HSCT | £231 900 (£208 000-£256 800) | £120 100 (£83 900-£148 600) | 4.74 (4.40-5.08) | 0.30 (−0.18 to 0.82) | £127 300 (£89 800-£158 600) | 100 | 100 |
| Delayed HSCT | £352 000 (£313 500-£384 000) | 4.44 (4.01-4.83) | 0 | 0 | |||
| INMB at thresholds of £20 000 and £30 000 per QALY (95% CI) £187 200 (£164 000-£287 300) and £235 600 (£166 800-£298 500) | |||||||
| Strategy . | Cost (95% CI) (UK pound) . | Incremental cost (95% CI) (UK pound) . | Effectiveness (95% CI) (QALY) . | Incremental effectiveness (95% CI) (QALY) . | INMB (95% CI) (UK pound/QALY) . | % Cost-effectiveness at WTP threshold of $20 000 . | % Cost-effectiveness at WTP threshold of $30 000 . |
|---|---|---|---|---|---|---|---|
| United Kingdom model (lifetime horizon) | |||||||
| Upfront HSCT | £335 800 (£303 700-£369 800) | £215 300 (£152 300-£270 600) | 9.74 (8.75-10.72) | 0.84 (−0.39 to 2.16) | £235 600 (£166 800-£298 500) | 100 | 100 |
| Delayed HSCT | £551 100 (£487 000-£609 000) | 8.90 (8.01-9.70) | 0 | 0 | |||
| INMB at thresholds of £20 000 and £30 000 per QALY (95% CI) £187 200 (£164 000-£287 300) and £235 600 (£166 800-£298 500) | |||||||
| United Kingdom model (10-year horizon) | |||||||
| Upfront HSCT | £231 900 (£208 000-£256 800) | £120 100 (£83 900-£148 600) | 4.74 (4.40-5.08) | 0.30 (−0.18 to 0.82) | £127 300 (£89 800-£158 600) | 100 | 100 |
| Delayed HSCT | £352 000 (£313 500-£384 000) | 4.44 (4.01-4.83) | 0 | 0 | |||
| INMB at thresholds of £20 000 and £30 000 per QALY (95% CI) £187 200 (£164 000-£287 300) and £235 600 (£166 800-£298 500) | |||||||
Costs have been rounded to the nearest hundred.
In a scenario analysis examining a shorter 10-year time horizon, the total discounted costs and QALYs for upfront HSCT and delayed HSCT were £231 900 and £352 000, as well as 4.74 and 4.44 QALYs, respectively. This yielded an INMB of £129 300 (95% CI, £89 800-£158 600) for the upfront HSCT compared with the delayed HSCT at a WTP threshold of £30 000 per QALY (Table 4). At a WTP threshold of £20 000 per QALY, the INMB value was £126 100.
Threshold analyses: United States and United Kingdom
There was no threshold in the probability of undergoing salvage HSCT in the delayed HSCT arm that would make delayed HSCT the cost-effective strategy in the base-case analyses for both the United States and United Kingdom, as well as the short time-horizon in the United Kingdom. This threshold was only reached in the short time-horizon in the United States (<67.4%).
Deterministic and PSAs: United States health care model
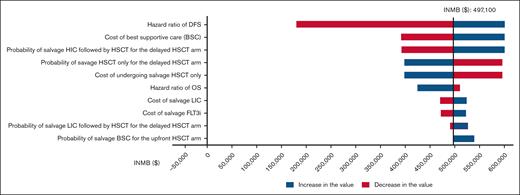
One-way sensitivity analyses for the United States health care model revealed that no model input parameter variation could change the conclusion that upfront HSCT is the cost-effective strategy (ie, none led to a negative INMB; Figure 2). Upfront HSCT was the cost-effective strategy in 100% of 10 000 iterations across all WTP thresholds (Figure 3). Furthermore, upfront HSCT dominated delayed HSCT (ie, it was both less costly and more effective) in 100% of the iterations across all the WTP thresholds (Table 3).
Tornado diagram of the 10 variables with the greatest influence on cost-effectiveness for the United States health care model. The bars in red indicate an increase in the value, while the blue bars indicate a decrease in the value of the given variable, and the vertical dashed line is the point where delayed HSCT becomes cost-effective. None of the variable changes was able to make delayed HSCT more likely cost-effective compared with upfront HSCT (ie, the INMB does not become <$0). FLT3i, FLT3 Inhibitor.
Tornado diagram of the 10 variables with the greatest influence on cost-effectiveness for the United States health care model. The bars in red indicate an increase in the value, while the blue bars indicate a decrease in the value of the given variable, and the vertical dashed line is the point where delayed HSCT becomes cost-effective. None of the variable changes was able to make delayed HSCT more likely cost-effective compared with upfront HSCT (ie, the INMB does not become <$0). FLT3i, FLT3 Inhibitor.
ICE scatterplots comparing upfront HSCT and delayed HSCT at a WTP threshold of $150 000 per QALY. The green dots represent iterations favoring upfront HSCT, and red dots represent iterations where delayed HSCT is preferred. 100% of the iterations fall below the WTP threshold, indicating upfront HSCT is cost-effective compared with delayed HSCT. ICE, incremental cost-effectiveness.
ICE scatterplots comparing upfront HSCT and delayed HSCT at a WTP threshold of $150 000 per QALY. The green dots represent iterations favoring upfront HSCT, and red dots represent iterations where delayed HSCT is preferred. 100% of the iterations fall below the WTP threshold, indicating upfront HSCT is cost-effective compared with delayed HSCT. ICE, incremental cost-effectiveness.
For the short-term model, one-way sensitivity analyses revealed that no model input parameter variation could change the conclusion that upfront HSCT is the cost-effective strategy (supplemental Figure 1). PSAs showed that upfront HSCT was the cost-effective strategy in 98.4% of iterations, and it dominated delayed HSCT (ie, was both more effective and less costly) in 85.3% of iterations, using a WTP threshold of $150 000 per QALY (supplemental Figure 3). At WTP thresholds of $100 000 and $50 000 per QALY, HSCT was cost-effective in 98.2% and 97.5% of the iterations, respectively, and dominated delayed HSCT in 85.3% of the iterations at both WTP thresholds (Table 3).
Deterministic and PSAs: United Kingdom health care model
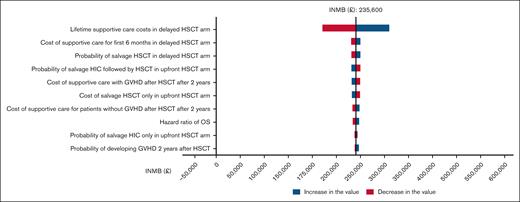
One-way sensitivity analyses for the United Kingdom health care model showed results similar to the United States model, namely that no model input variation changed the conclusion (ie, that upfront HSCT is the cost-effective strategy; Figure 4). PSA demonstrated that upfront HSCT was cost-effective in 100% of 10 000 iterations across all WTP thresholds, and dominated delayed HSCT in 90.9% of the iterations across all the WTP thresholds (Table 4; Figure 5).
Tornado diagram of the 10 variables with the greatest influence on cost-effectiveness for the United Kingdom health care model. The bars in red indicate an increase in the value, while the blue bars indicate a decrease in the value of the given variable, and the vertical dash line is the point where delayed HSCT becomes cost-effective. None of the changes in the input variable were able to make delayed HSCT more likely cost-effective compared with upfront HSCT (ie, the INMB does not become <$0). FLT3i. FLT3 inhibitor.
Tornado diagram of the 10 variables with the greatest influence on cost-effectiveness for the United Kingdom health care model. The bars in red indicate an increase in the value, while the blue bars indicate a decrease in the value of the given variable, and the vertical dash line is the point where delayed HSCT becomes cost-effective. None of the changes in the input variable were able to make delayed HSCT more likely cost-effective compared with upfront HSCT (ie, the INMB does not become <$0). FLT3i. FLT3 inhibitor.
ICE scatterplots comparing upfront HSCT and delayed HSCT at a WTP threshold of £30 000 per QALY. The green dots represent iterations favoring upfront HSCT, and the red dots represent iterations where delayed HSCT is preferred. 100% of the iterations fall below the WTP threshold, indicating that upfront HSCT is cost-effective compared with delayed HSCT. ICE, incremental cost-effectiveness.
ICE scatterplots comparing upfront HSCT and delayed HSCT at a WTP threshold of £30 000 per QALY. The green dots represent iterations favoring upfront HSCT, and the red dots represent iterations where delayed HSCT is preferred. 100% of the iterations fall below the WTP threshold, indicating that upfront HSCT is cost-effective compared with delayed HSCT. ICE, incremental cost-effectiveness.
In the short-term model, the one-way sensitivity analysis showed that no parameter variation changed the conclusion (ie, that upfront HSCT is cost-effective; supplemental Figure 2). PSAs demonstrated that upfront HSCT was cost-effective in 100% of 10 000 iterations across all WTP thresholds, and dominated delayed HSCT in 88.9% of the iterations across all WTP thresholds (Table 4; supplemental Figure 4).
Discussion
To our knowledge, this is the first study to evaluate the cost-effectiveness of upfront HSCT compared with delayed HSCT (ie, CCT at first remission followed by HSCT upon relapse) for patients with intermediate-risk AML within the United States and United Kingdom health care systems.
Our findings show that upfront HSCT dominates delayed HSCT, meaning that it is less costly and more effective for intermediate-risk AML in both the United States and United Kingdom health care settings. While the decision to proceed to HSCT in CR1 is informed by the clinical context (eg, donor availability, disease status after induction and consolidation therapy, patient comorbidities), our results can inform decision-making from a health system perspective. As such, our results clearly demonstrate the health economic advantages of proceeding to HSCT in CR1 for patients with intermediate-risk AML with an available donor rather than delaying transplant until the time of relapse. This advantage is mainly driven by the high rates of disease relapse without transplant, which leads to the excess costs in the delayed HSCT strategy, accrued via the need for reinduction chemotherapy, and higher rates of complications and health care utilization. Data compiled from the Medical Research Council AML10, 12, and 15 trials showed that 60% of patients with intermediate-risk AML who did not undergo an HSCT in CR1 relapsed, and only 54% of the patients who relapsed were able to achieve a second complete remission (CR2).43 The recommendation for HSCT in CR1 for patients with intermediate-risk AML as endorsed by the ELN and National Comprehensive Cancer Network is further supported by a systematic review and meta-analysis showing an improvement inrelapse-free survival (RFS) and OS with HSCT.44 However, it is important to acknowledge that the definition of intermediate risk AML has evolved over time, including during the time when the ETAL-1 trial was conducted, which required us to focus on the subset of patients with ELN 2017 intermediate risk.6
These data also emphasize that a subset of patients with intermediate-risk AML are potentially curable without transplant, and might be able to avoid transplant-associated complications and costs. Identification of these patients at low risk for relapse would be clinically important. Measurable residual disease (MRD) status has been identified as a key prognostic marker for long-term outcomes in general and posttransplant relapse.45-48 Although MRD data are not available from the ETAL-1 trial, incorporating MRD assessment into decision-making regarding HSCT might enable a more individualized treatment decision allowing patients at lower risk for relapse to defer HSCT in CR1. As costs of HSCT are a substantial driver of the economic burden of AML care, refining patient selection based on disease risk offers the potential to reduce costs as well. As our short-term United States model showed that a reduction in the cumulative relapse risk to <32.6% would make deferral of HSCT in CR1 a cost-effective strategy, such an approach to patient selection for HSCT might be considered from a health economic perspective, although identification of patients with intermediate-risk AML with such a low rate of relapse appears clinically challenging. Furthermore, no such threshold was identified in the United Kingdom model and with a lifetime horizon United States model likely due to ongoing costs of supportive care and risk of relapse with delayed HSCT. Although further analyses using other trials or real-world data would be valuable to evaluate how salvage treatment rates and HSCT effectiveness across different clinical scenarios impact outcomes and cost-effectiveness, our threshold analysis showed that the delayed HSCT strategy would only become cost-effective if the overall postrelapse transplant rate dropped below 32.6% in the short-term model, and no such threshold was identified in the long-term model. While this estimate does not account for variations in HSCT efficacy by remission status (eg, CR2 vs refractory disease), such a low overall transplant rate remains well below what is typically observed in routine clinical practice in the United States or United Kingdom, supporting the robustness of our findings favoring upfront HSCT.
While the ETAL-1 trial only enrolled patients with an HLA-matched donor available in case of relapse, recent advances in haploidentical HSCT (eg, T-cell receptor alpha-beta [TCRαβ]/CD19 depletion and post-transplant cyclophosphamide) have greatly expanded donor availability, making HSCT a feasible option for most patients.49-51 Furthermore, previous cost-effectiveness analyses from a European perspective suggest that haploidentical HSCT is more likely to be cost-effective compared with matched HSCT.52 Due to the limitations of the ETAL-1 trial, we cannot comment on the impact of different graft sources. However, due to the clear results favoring upfront HSCT (ie, upfront HSCT dominating over delayed HSCT in both United States and United Kingdom models), it is reasonable to assume that an even more cost-effective approach to HSCT using haploidentical donors would not affect the conclusions of our study.51-53
To demonstrate that our observations are not limited to the United States health care system, we also conducted a similar analysis assuming a United Kingdom health care system perspective. In line with multiple other cost-effective studies, the absolute costs of care in the United Kingdom were lower than in the United States.23,24,42,53,54 However, these cost differences affected all aspects of the model, and did not change the ultimate conclusion (ie, that upfront HSCT is the cost-effective strategy). Showing that HSCT in CR1 is cost-effective as compared with delayed HSCT in the United States and the United Kingdom highlights the generalizability of our findings within the high-income country context.
It is important to acknowledge that our study has certain limitations. First, we relied on the published aggregate data from the ETAL-1 trial due to lack of access to individual patient-level data.6 Second, the ETAL-1 trial was prematurely terminated due to low accrual despite demonstrating a statistically significant DFS advantage for upfront HSCT.6 The fact that all patients who relapsed within the delayed HSCT strategy underwent a salvage HSCT at the time of relapse may have contributed to the absence of an OS difference. However, the assumption that 100% of patients with relapsed intermediate-risk AML are able to undergo a salvage HSCT is much higher than the 48% to 70% reported in other studies of patients eligible for intensive salvage therapy.43,55 Although subsequent analyses using other trials or real-world data would be helpful to evaluate the effect of rates of salvage treatment on clinical outcomes and cost-effectiveness, the identified threshold transplant rates (ie, <32.6%) in the delayed HSCT strategy are much lower than any rates seen in routine practice in the United States or United Kingdom, and only apply to the short-term model. Third, utility values, costs, and probabilities were derived from existing literature, restricting our analysis to the data reported in these sources. Nonetheless, PSA was used to address this uncertainty regarding model input parameters, and the PSA demonstrated consistent results across the 2 health care settings. Fourth, in the threshold analysis, we were only able to evaluate changes in the cost of the consolidation approach without changes in the effectiveness. Fifth, our model does not account for individual patient-level factors such as comorbidities, frailty, psychosocial, socioeconomic factors, which can significantly influence treatment eligibility, outcomes, and associated costs. As such, our results only apply to young patients (ie, aged <60 years) with an HLA-matched donor similar to the patients enrolled in the ETAL-1 trial. It is therefore uncertain whether older patients and those with multiple medical comorbidities undergoing HSCT after a reduced-intensity conditioning or from an alternative donor source would derive the same cost-effectiveness benefit from upfront HSCT as observed in our base-case. Thus, further research incorporating these real-world patient characteristics is warranted to validate the generalizability of our findings across more diverse patient populations. Lastly, our analysis was conducted from a United States and United Kingdom health care system perspective, and may not be transferrable to other settings. Nevertheless, given the consistency of our results across 2 distinct health care systems that have significant differences, we anticipate similar trends may apply to health care settings in low- to middle-income countries, increasing the generalizability of our results even further to other parts of the world.
In conclusion, this study provides health economic guidance for managing young (aged <60 years) patients with intermediate-risk AML who were eligible for intensive chemotherapy, as classified by the ELN 2017 criteria, in their first remission. Our findings underscore the significant clinical impact of upfront HSCT, demonstrating its dominance over delayed HSCT, meaning it is both less costly and more effective, in both the United States and United Kingdom health care systems. This conclusion is primarily driven by the high rates of relapse with CCT alone, which ultimately leads to higher lifetime costs for salvage therapies and HSCT. Thus, the lower cost and superior clinical efficacy of upfront HSCT strongly support its adoption as the preferred therapeutic strategy for eligible patients with intermediate-risk AML in their first remission.
Acknowledgments
G.G. is supported by the NOMIS Foundation, Frederick A. DeLuca Foundation, Yale Cancer Center, Yale Bunker Endowment, National Institutes of Health (NIH), and National Heart, Lung, and Blood Institute (grant 1K01 HL175220). This investigation was supported by the NIH Research Grant CA-016359 from the National Cancer Institute. J.P.B. is supported by the Edwards P. Evans Foundation.
The contents of this article are solely the responsibility of the authors, and do not necessarily represent the official views of the funding sources. This manuscript is the result of funding in whole or in part by the NIH. It is subject to the NIH Public Access Policy. Through acceptance of this federal funding, the NIH has been given a right to make this manuscript publicly available in PubMed Central upon the official date of publication, as defined by the NIH.
Authorship
Contribution: A.A. was responsible for building the model, methodology, data collection, and writing the full paper; K.K.P. was responsible for building the model, methodology, and writing the full paper; N.A.P., T.K., J.M.S., L.M., S.S., L.G., S.F.H., and M.S. were responsible for critical review; A.M.Z. was responsible for critical review and supervision; and G.G. and J.P.B. were responsible for building the model, methodology, and supervision.
Conflict-of-interest disclosure: L.M. serves as a consultant for Rigel. M.S. served on the advisory board for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, Rigel, Bristol Myers Squibb (BMS), Sobi, and Syndax; consulted for Boston Consulting and Dedham group; and participated in continuing medical education activity for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. N.A.P. received consulting fees from Pfizer, Agios Pharmaceuticals, Blueprint Medicines, Incyte, Novartis, Celgene/BMS, CTI BioPharma/Sobi, PharmaEssentia, Constellation Pharmaceuticals/MorphoSys, Aptose Biosciences, and AbbVie; and other financial support for serving on an Independent Data Review Committee for Cogent Biosciences. S.F.H. has been a consultant for Celgene, Bayer, Genentech, Pharmacyclics, AbbVie; and has received research funding from DTRM Biopharma, Celgene, and TG Therapeutics. A.M.Z. received research funding (institutional) from Celgene/BMS, AbbVie, Astex, Pfizer, Kura, Medimmune/AstraZeneca, Boehringer Ingelheim, Incyte, Takeda, Novartis, Shattuck Labs, Geron, Foran, and Aprea; has participated in advisory boards and/or had a consultancy with, and received honoraria from, AbbVie, Pfizer, Celgene/BMS, Jazz, Incyte, Agios, Servier, Boehringer-Ingelheim, Novartis, Astellas, Daiichi Sankyo, Geron, Taiho, Seattle Genetics, Otsuka, BeyondSpring, Takeda, Ionis, Amgen, Janssen, Genentech, Epizyme, Syndax, Gilead, Kura, Chiesi, ALX Oncology, BioCryst, Notable, Orum, Mendus, Zentalis, Schrodinger, Regeneron, Syros, and Tyme; and served on clinical trial committees for Novartis, AbbVie, Gilead, Syros, BioCryst, AbbVie, ALX Oncology, Kura, Geron, and Celgene/BMS. The remaining authors declare no competing financial interests.
Correspondence: Jan Philipp Bewersdorf, Section of Hematology, Department of Internal Medicine, Yale University, 333 Cedar St, PO Box 208028, New Haven, CT 06520-8028; email: jan.bewersdorf@yale.edu.
References
Author notes
G.G. and J.P.B. contributed equally to this study as joint senior authors.
Presented in oral form at the annual meeting of the American Society of Hematology, San Diego, CA, 9 December 2024.
Presented as part of the 2025 highlight of the American Society of Hematology, Latin America, Punta del Este, Uruguay, 25 to 26 April 2025.
Original data are available on request from the corresponding author, Jan Philipp Bewersdorf (jan.bewersdorf@yale.edu).
The full-text version of this article contains a data supplement.