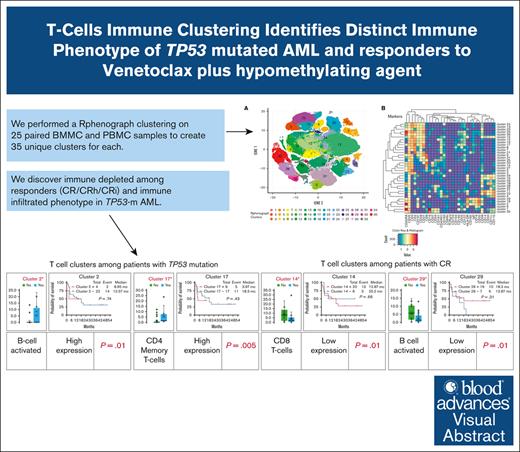
Visual Abstract
TO THE EDITOR:
The advent of venetoclax (VEN)-based therapy has revolutionized the treatment of patients with acute myeloid leukemia (AML), with improvement in remission rates from historical figures of 10% to 20% with single-agent hypomethylating agents (HMAs) to 60% to 70% with HMA plus VEN combination therapy.1 However, remission durations are short-lived in patients with high-risk AML.2,3 As our knowledge of the AML genomic landscape has expanded, effective and more tolerable targeted therapies are available to improve outcomes.4-6 However, the majority of relapsed/refractory patients with AML do not have targetable mutations and have a poor prognosis.7 Although immunotherapy is an appealing possibility, patients with AML are known to have abnormalities in their innate and adaptive immunity that can cause immune suppression and exhaustion.8-11 Accordingly, better understanding of the interaction between the immune system and AML is required for the development of effective AML immunotherapies.
The HMA plus VEN combination therapy leads to increased T-cell effector function, rendering the leukemic cells more susceptible to T-cell–mediated cytotoxicity.12TP53, apart from its role as a tumor suppressor, is also involved in immune responses and inflammation.13 The TP53 mutation (TP53-m) in AML is a poor prognostic marker and is commonly seen in older patients with AML who have poor response to conventional therapies.14 Flotetuzumab is an investigational immunotherapy, which in a phase 1/1b study demonstrated complete responses in patients with TP53-m AML and showed that the responses were correlated with increased expression of T-cell effector and interferon gamma–related genes.15 These encouraging results motivated the initiation of further studies to assess the immune profile in patients with TP53-m AML.16
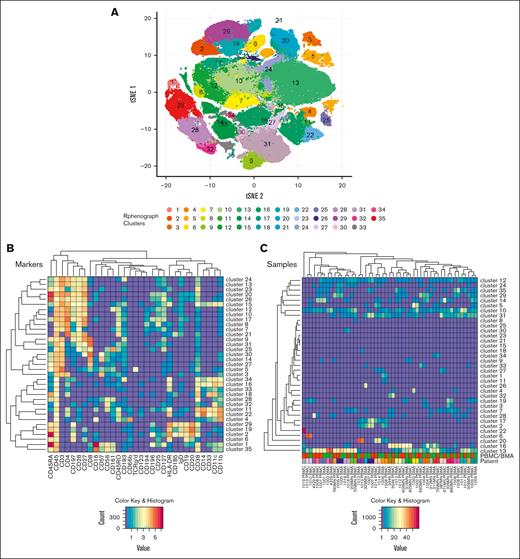
We conducted an investigator-initiated study to evaluate baseline immune profiles by mass cytometry–based cytometry by time-of-flight (CyTOF) analysis in 26 newly diagnosed patients with AML receiving HMA plus VEN combination therapy. Details on the methods performed are outlined in the supplemental Material. We performed a RphenoGraph clustering on 25 paired bone marrow (BM) and peripheral blood (PB) mononuclear cell (BMMC and PBMC) samples to identify 35 unique immune cell clusters for each (Figure 1; supplemental Tables 1 and 2). Differences in the fractional cluster sizes were then compared in 2 patient subgroups: those with and without TP53-m and those who had or had not achieved complete remission with partial hematologic recovery or incomplete hematological recovery (CR/CRh/CRi) as per European LeukemiaNet (ELN) 2022 criteria.17 Statistical significance of differences in the clusters was examined using Student t test. Spearman correlation (R) test was performed to evaluate the correlation between PBMCs and BMMCs. R value >0.7 suggests strong correlation, and P value shows how likely the correlation coefficient was observed due to chance.
BMMC and PBMC immune cell phenotyping. CyTOF analysis was performed on 17 paired BMMC and PBMC samples using the CyTOF kit. (A) Thirty-five unique clusters were identified with 31 markers using the RphenoGraph clustering algorithm. Results were mapped onto a tSNE plot. (B) Relative expression of all 31 markers used to generate the 35 clusters. (C) Heat maps show the relative mean intensity of expression for each of the 31 markers and clusters. tSNE, t-distributed stochastic neighbor embedding.
BMMC and PBMC immune cell phenotyping. CyTOF analysis was performed on 17 paired BMMC and PBMC samples using the CyTOF kit. (A) Thirty-five unique clusters were identified with 31 markers using the RphenoGraph clustering algorithm. Results were mapped onto a tSNE plot. (B) Relative expression of all 31 markers used to generate the 35 clusters. (C) Heat maps show the relative mean intensity of expression for each of the 31 markers and clusters. tSNE, t-distributed stochastic neighbor embedding.
The median age at diagnosis was 74 years (range, 53-88); 10 patients (38.5%) had secondary AML, and 15 patients (58%) had adverse-risk disease as per ELN 2022 criteria (supplemental Table 3).17 Among patients with TP53-m (n = 6), the median age was 75.5 years (range, 65-85), 50% (n = 3) of patients had therapy-related AML, and all had high-risk cytogenetics. All patients with TP53-m AML had multihit TP53-m.18 Details regarding responses to HMA plus VEN combination therapy are discussed in detail in the supplemental Material.
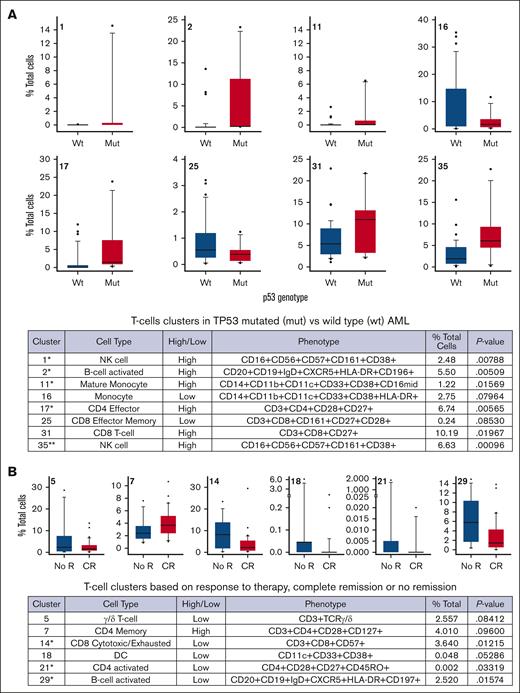
We found significant differences in immune cells cluster abundance, with increased numbers of activated B cells (cluster 2; P = .01), effector memory CD4+ T cells (cluster 17; P = .005), central memory CD8+ T cells (cluster 31; P = .01), and 2 natural killer–cell–rich clusters (cluster 1 [P = .005] and cluster 35 [P = .0009]) among patients with TP53-m AML (Figure 2A). Among patients who achieved CR (n = 12 [57%]), their baseline PBMCs and BMMCs showed significant decreases in the proportion of terminal effector CD8 T cells (cluster 14; P = .01), dendritic cells (cluster 18; P = .05), central memory CD4 T cells (cluster 21; P = .03), and B-cell–activated phenotypes (cluster 29; P = .01; Figure 2B).
T-cell clusters of significance. (A) p53 status identifies differential frequencies of immune populations derived from either BMMCs or PBMCs. BMMC and PBMC samples were segregated based on p53 genotype. (B) BMMC and PBMC samples were segregated based on response to therapy, CR, or no R. Cluster number is in the upper left of each plot. Eight clusters trended toward significance or were significant (∗P < .05 by t test; ∗∗P < .05 by t test with Bonferroni correction). CR, complete remission; DC, dendritic cells; mut, mutation; NK cell, natural killer cell; no R, no remission; wt, wild type.
T-cell clusters of significance. (A) p53 status identifies differential frequencies of immune populations derived from either BMMCs or PBMCs. BMMC and PBMC samples were segregated based on p53 genotype. (B) BMMC and PBMC samples were segregated based on response to therapy, CR, or no R. Cluster number is in the upper left of each plot. Eight clusters trended toward significance or were significant (∗P < .05 by t test; ∗∗P < .05 by t test with Bonferroni correction). CR, complete remission; DC, dendritic cells; mut, mutation; NK cell, natural killer cell; no R, no remission; wt, wild type.
Although not statistically significant, overall survival was decreased among patients demonstrating increase in cluster 2 (B-cell–activated phenotype [high]; 8.95 vs 12.57 months; P = .74), cluster 11 (mature monocyte phenotype [high]; 2.83 vs 18.83 months; P = .19), and cluster 17 (effector memory CD4 T cells [high]; 3.97 vs 18.3 months; P = .43; supplemental Figure 2). Overall survival was numerically higher but not statistically significant among patients with increases in cluster 5 (gamma-delta T cells [low]; 18.4 vs 3.9 months; P = .28) and cluster 29 (B-cell activated [low]; 18.3 vs 12.87 months; P = .31; supplemental Figure 3).
We performed Pearson correlation (R) between PBMC and BMMC clustering to find whether results are comparable between PB and BM testing. We found a strong correlation between PBMCs and BMMCs among 26 of the 35 clusters (supplemental Figure 4). Significant correlation was seen in clusters that showed significance in TP53-m AML (cluster 17 [R = 0.86; P ≤ .001], cluster 31 [R = 0.86; P ≤ .001], and cluster 35 [R = 0.88; P ≤ .001]). Similarly, strong correlation was seen in clusters that showed significance among patients achieving CR (cluster 14 [R = 0.90; P ≤ .001] and cluster 29 [R = 0.96; P ≤ .001]).
In this single-institutional study using CyTOF analysis, we found strong immunophenotypic correlation between diagnostic PBMC and BMMC samples. Moreover, we have seen novel immune cell clusters among patients who achieved CR and those with TP53-m AML.
AML is a heterogeneous disease in which tumor morphology, immunophenotyping, and molecular aberrations help in diagnosis and prognostication. Before the advent of genomic sequencing, immunophenotyping had been used as a powerful tool for diagnosis and prognostication in AML19 and is still being used widely for the evaluation of measurable residual disease.20 Although flow cytometry is routinely used for immunophenotyping, CyTOF offers an advantage because it has the ability to detect more markers per sample, which can aid in better understanding of the clonal architecture of AML, which is highly heterogeneous. Apart from its tumor suppressor function, TP53 gene is also involved in immune responses and inflammation.13 More importantly, studies have shown that higher tumor mutational burden and higher expression of T-cell effector and interferon gamma–related genes are associated with favorable responses to immunotherapy in TP53-m hematological as well as solid malignancies.15,21 Similar to earlier reports with conventional immunophenotyping,22 using CyTOF, we have observed higher expression of natural killer cells, CD4 effectors cells, and CD8 T-cell clusters in TP53-m AML. To the best of our knowledge, this is the first study showing distinct immune cell clustering among patients with TP53-m AML using CyTOF.
The immunophenotyping technique is evolving, and now, data are suggesting concordance between BM and PB flow cytometric analysis.23,24 Similarly, we have observed significant concordance between CyTOF analysis done from PBMCs and BMMCs. The results are encouraging because the analysis from BM aspirates may be limited by the invasiveness of the procedure. It is known that the development of AML is a result of immune suppression and senescence.9,10 Similarly, in our analysis excluding TP53-m AML, we have observed significantly lower expression of CD8 cytotoxic, CD4-activated, and B-cell–activated cell clusters from diagnostic PBMC and BMMC samples. Although in vitro and vivo studies have shown that VEN plus HMA combination therapy can enhance T-cell effector function and cytotoxicity without causing T-cell apoptosis,12 our study did not include the subsequent remission PBMC/BMMC samples to assess changes in immune profile after HMA plus VEN combination therapy, which is a notable limitation of the study.
In summary, we have shown novel immune cell clustering in patients with TP53-m AML. Secondly, it is commonly thought that immunophenotyping using BMMCs may be functionally more relevant than PBMCs, but our findings of a strong correlation between the 2 highlight the utility of PBMC samples for performing CyTOF. Future analysis with large sample size is needed to confirm our findings.
The study was approved by the institutional review board. This trial was registered at www.clinicaltrials.gov as #NCT06279572.
Acknowledgments: The authors thank the patients for consenting to this investigator-initiated study.
This study is supported by Mayo Clinic Cancer Center, Cancer Center Support Grant (grant number P30 CA015083).
Contribution: T.B. and M.S. designed the study, performed analysis, and wrote the paper; K.L.K., M.U., and D.C. performed CyTOF analysis; K.D.P. interpreted the CyTOF data and performed the analysis; J.F., H.M., N.G., S.H.K., M.R.L., A.A., A.A.-K., H.A., A.T., and M.P. contributed patients; and all authors reviewed and approved the final draft of the paper.
Conflict-of-interest disclosure: T.B. served on an advisory board for Pfizer, Takeda, and MorphoSys. The authors declare no competing financial interests.
Correspondence: Talha Badar, Division of Hematology & Medical Oncology, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224; email: badar.talha@mayo.edu.
References
Author notes
Data are available on reasonable request from the corresponding author, Talha Badar (badar.talha@mayo.edu).
The full-text version of this article contains a data supplement.