Key Points
Approximately 45% of patients with SCD exhibit evidence of accelerated arteriovenous transit on arterial spin labeling MRI.
Patients with SCD with accelerated arteriovenous transit have reduced hemoglobin, and mildly reduced cerebral OEF and oxygen metabolism.
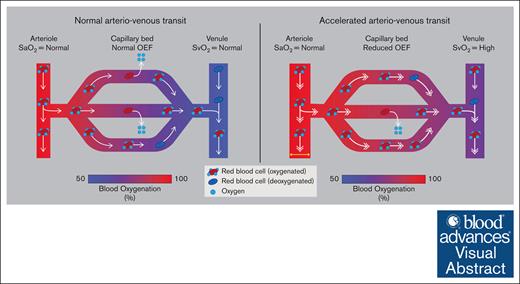
Visual Abstract
Patients with sickle cell disease (SCD) are at elevated risk of silent cerebral infarcts and strokes; however, they frequently lack established stroke risk factors (eg, macrovascular arterial steno-occlusion) and the mechanisms underlying such events are incompletely characterized. This study evaluated cerebral hemometabolism with respect to imaging markers of vascular shunting in 143 participants with SCD, including 73 pediatric (aged 6-17 years) and 70 adult (aged 18-40 years) participants using 3-Tesla brain magnetic resonance imaging (MRI). Vascular shunting was assessed in each patient using a previously published ordinal venous hyperintensity score (VHS) of 0, 1, or 2 on cerebral blood flow-weighted MRI. Participants with VHS of 2, indicative of the most rapid arteriovenous transit, had significantly reduced blood oxygen content (CaO2; 10.90 ± 1.69 mL O2/100 mL blood), oxygen extraction fraction (OEF; 33.52% ± 5.54%), and cerebral metabolic rate of oxygen consumption (CMRO2; 2.91 ± 0.69 mL O2/100 g tissue per minute) compared with their counterparts with VHS = 0 (CaO2 = 12.42 ± 1.58 mL O2/100 mL blood; OEF = 39.03% ± 3.80%; CMRO2 = 3.77 ± 0.84 mL O2/100 g tissue per minute) or VHS = 1 (CaO2 = 11.86 ± 1.73 mL O2/100 mL blood; OEF = 36.37% ± 5.11%; CMRO2 = 3.59 ± 0.78 mL O2/100 g tissue per minute). Both pediatric and adult patients with SCD presenting with greater imaging evidence of vascular shunting had mildly reduced OEF and CMRO2. These findings highlight that imaging markers of vascular shunting are associated with significant, albeit mild, evidence of reduced OEF and CMRO2 in patients with SCD.
Introduction
Sickle cell disease (SCD) encompasses all sickle genotypes and represents the most common genetically inherited blood disorder, whereby formation of abnormal hemoglobin S (HbS) leads to red cell sickling, hemolysis, and correspondingly reduced arterial blood oxygen content (CaO2).1 The sequelae of these phenomena collectively comprise elevated risk for cerebrovascular events including silent cerebral infarcts (SCIs)2 and stroke.3 Importantly, the presence of even mild cerebrovascular injury is clinically meaningful in this population: although SCIs do not cause focal neurological findings, patients remain at risk for progressive infarction,4 and cerebral infarcts are present in approximately half of persons with SCD by age 30 years.5
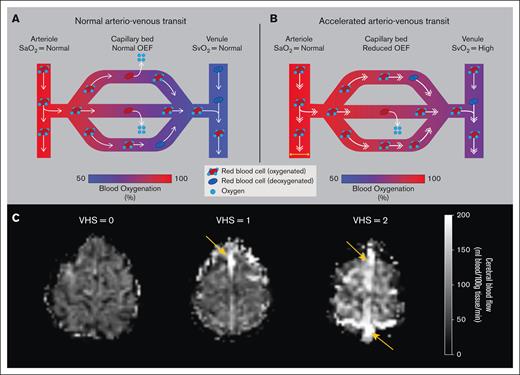
Despite heightened rates of cerebrovascular events, the mechanisms that lead to cerebral infarction in SCD are not well understood. Only a small percentage of persons with SCD have conventional stroke risk factors for stroke such as hypertension and arterial vasculopathy6; instead, cerebral infarction is more likely a result of vascular and metabolic changes at the tissue level secondary to inadequate parenchymal hemodynamic compensatory mechanisms. Because of reduced CaO2 (mL O2/100 mL blood) associated with chronic hemolytic anemia, a greater burden is placed on compensatory changes in cerebral hemodynamics such as cerebral blood flow (CBF; mL blood/100 g tissue per minute) and possibly oxygen extraction fraction (OEF; oxygen consumed/oxygen delivered) to maintain a sufficient cerebral metabolic rate of oxygen consumption (CMRO2; mL O2/100 g tissue per minute). In the setting of moderate SCD disease severity, sufficient oxygen substrate to maintain CMRO2 is often supplied through autoregulatory increases in CBF.7-9 Such hyperemia can be associated with high blood flow velocities and corresponding rapid arteriovenous transit times. When this effect is severe, reduced red cell residency time within the capillaries may lead to reduced oxygen offloading efficiency per unit time (Figure 1A-B), and thus altered OEF, as has been shown in other populations.10,11
A schematic of the hypothesized physiological mechanism for reduced capillary oxygen offloading secondary to accelerated arteriovenous transit. (A) Under healthy conditions, normal arteriovenous transit allows for sufficient oxygen offloading in the capillaries. (B) Under altered hyperemic conditions such as in chronic anemia, compensatory accelerated CBF may shorten the red cell residency time within the capillaries, due to rapid arteriovenous transit, thus resulting in reduced oxygen offloading and OEF. (C) Representative examples of a participant from each VHS on ASL perfusion-weighted imaging. For VHS of 0, no hyperintensity is observed in the superior sagittal sinus nor straight sinus. For VHS of 1, a region of focal hyperintensity (orange arrow) is observed in the superior sagittal sinus but does not persist spatially to the level of the torcula. For VHS of 2, diffuse hyperintensity (orange arrows) is observed along the superior sagittal sinus across multiple axial slices and extends to the torcula. SaO2, arterial oxygen saturation; SvO2, venous oxygen saturation.
A schematic of the hypothesized physiological mechanism for reduced capillary oxygen offloading secondary to accelerated arteriovenous transit. (A) Under healthy conditions, normal arteriovenous transit allows for sufficient oxygen offloading in the capillaries. (B) Under altered hyperemic conditions such as in chronic anemia, compensatory accelerated CBF may shorten the red cell residency time within the capillaries, due to rapid arteriovenous transit, thus resulting in reduced oxygen offloading and OEF. (C) Representative examples of a participant from each VHS on ASL perfusion-weighted imaging. For VHS of 0, no hyperintensity is observed in the superior sagittal sinus nor straight sinus. For VHS of 1, a region of focal hyperintensity (orange arrow) is observed in the superior sagittal sinus but does not persist spatially to the level of the torcula. For VHS of 2, diffuse hyperintensity (orange arrows) is observed along the superior sagittal sinus across multiple axial slices and extends to the torcula. SaO2, arterial oxygen saturation; SvO2, venous oxygen saturation.
Previous studies from multiple groups have suggested that hyperintense venous signal (Figure 1C) on arterial spin labeling (ASL) magnetic resonance imaging (MRI) may reflect vascular compartment shunting and may be associated with infarction risk; patients with SCD that present with hyperintense venous signal were shown to have increased cervical arterial blood flow velocities,12 reduced OEF,13,14 and CMRO2,14 and lower cognitive performance.15 However, important gaps in the literature include the characterization of clinical and cerebral hemodynamic measures with respect to this imaging phenomenon in both pediatric and adult patients with SCD. In addition to vascular compartment shunting, it is also possible that spatial shunting occurs via altered capillary flow patterns due to differences in resistance pathways secondary to SCD pathology; however, this possibility has not yet been investigated.
The purpose of this study was to (1) quantify imaging evidence of vascular shunting across pediatric and adult participants with SCD, (2) characterize this phenomenon in the context of independent measures of hemodynamics and metabolism, and (3) explore how vascular shunting relates to potential spatial perfusion heterogeneity. Findings are intended to further inform the mechanisms by which compensatory hyperemia, in the setting of anemia, influences brain physiology.
Materials and methods
Participants
Persons (aged 6-40 years) with SCD, defined as phenotype sickle human Hb (HbSS) or HbSβ0-thalassemia (HbSβ0), were recruited from the clinical services of a comprehensive SCD clinic. All participants provided informed, written consent or assent for this prospective, cross-sectional study in accordance with the Vanderbilt University Medical Center institutional review board and the Declaration of Helsinki of 1975 and its amendments. Exclusionary criteria for all participants included pregnancy or other contraindications to high-quality 3-Tesla MRI such as dental braces, intracranial clips, implantable devices that were 3.0T prohibitive, or metallic foreign body in the eyes; and infarct spanning greater than one-third of the middle cerebral artery territory on MRI. Treatment regimen was recorded, and eligible participants could be on hydroxyurea and/or blood transfusion treatment; in the case of blood transfusions, all participants underwent MRI scanning late (at least 3 weeks) in their transfusion cycle when their hematocrit was near nadir.
Neurological examination and hematologic measures
All participants with SCD had a detailed clinical history and underwent a neurological examination by a board-certified stroke neurologist (L.C.J.). Total Hb level and HbS percentage, were measured via venipuncture within 7 days of the scan. Arterial oxygen saturation (Ya) values were obtained with pulse oximetry at the time of imaging.
MRI acquisition
MRI data were acquired at 3.0T (Philips Healthcare, Best, The Netherlands) using body coil radiofrequency transmission and sensitivity-encoding phased-array reception. Standard noncontrast anatomical head and neck MRI and angiography were performed for tissue segmentation and characterization of prior infarct and vasculopathy extent including 3-dimensional (3D) T1-weighted, 2D T2-weighted fluid-attenuated inversion recovery (FLAIR), diffusion-weighted, and intracranial time-of-flight imaging (specific sequences and parameters listed in supplemental Materials).
For CBF assessments, 2D pseudocontinuous ASL (pCASL) MRI was performed to evaluate gray matter CBF and assess dural venous signal intensity. Given the ∼7-year duration of the study, there were 2 2D pCASL sequences used in this study with similar, albeit slightly different, parameters (supplemental Materials); the possibility of the study findings varying with sequence used was investigated in supplemental Table 1. Both variations had a common spatial resolution of 3.0 × 3.0 × 7.0 mm and consisted of 20 blocks of alternating acquisitions, with and without spin labeling.
For OEF assessments, T2-relaxation-under-spin-tagging (TRUST)16 MRI was performed. For each session, 2 repeated acquisitions of TRUST were acquired in which the venous blood water was labeled with a sinc-gauss inversion applied to supratentorial parenchyma and superior sagittal sinus. During a delay time of 1022 milliseconds, a nonselective T2-preparation module was applied to provide variable T2-weighting with effective echo times (eTEs) of 0, 40, 80, and 160 milliseconds (averages = 3 per eTE). An image was then acquired (repetition time/echo time = 1978/3.6 milliseconds; spatial resolution = 3.4 × 3.4 × 5.0 mm) at the level of the posterior aspect of the superior sagittal sinus, ∼20 mm above the torcula.
MRI analysis
MRI and magnetic resonance angiography findings were independently reviewed by at least 2 board-certified neuroradiologists (L.T.D., S.P., and D.M.) and disagreements were resolved by consensus. SCIs were defined using standard criteria as an area of signal hyperintensity on T2-weighted FLAIR imaging of ≥3 mm in diameter in 1 imaging plane and visible in at least 2 planes, normal neurological examination, and no history of stroke-like symptoms.17 Strokes were determined by history consistent with clinical stroke and corresponding MRI findings including lesions of hyperintense signal on FLAIR and hypointense on T1-weighted MRI approaching cerebrospinal fluid signal.
CBF was quantified from the CBF-weighted pCASL data using a previously published kinetic model18; full model parameters are provided in the supplemental Materials. CBF maps were then transformed into native T1-weighted space using the M0 image for coregistration and subsequently nonlinearly transformed into Montreal Neurological Institute's 152 brain template (MNI-152) standard space. Mean and standard deviation CBF was estimated using a segmented MNI-152 GM mask,19 and the spatial coefficient of variation (sCoV)20 was calculated.
To assess the degree of venous hyperintensity score (VHS), we used an ordinal scoring system based on CBF-weighted pCASL data (Figure 1C).12 Three reviewers (L.C.J., L.T.D., and M.J.D.) independently assessed the pCASL data for VHS and artifact according to previously published scoring criteria.12,21 Each reviewer assessed the presence of a hyperintense venous signal in the major dural sinuses including the superior sagittal sinus, inferior sagittal sinus, and the straight sinus, and assigned a score of 0 (no venous hyperintensity), 1 (focal hyperintensity of the anterior or posterior aspects of the superior sagittal sinus), or 2 (diffuse hyperintensity of the superior sagittal sinus extending to the torcula). A final VHS was assigned based on reviewer consensus and used in all subsequent analyses.
For metabolic assessment, the OEF (percent) was quantified from TRUST data and hematologic measures. First, the TRUST data were pairwise subtracted and the venous blood water T2 (1/transverse relaxation rate [R2]) was quantified in the superior sagittal sinus by monoexponential curve fitting of the data across the 4 eTEs. The calculated venous T2 and measured hematocrit were then converted to venous oxygen saturation (Yv) according to 4 available T2-Yv-Hb calibration models: the bovine,22 normal human adult Hb,23 HbSS,24 and fetal human Hb (HbF) model.25 For completeness and to enable comparison with prior publications that used different models, the values from the normal human adult Hb model are presented in “Results” given its calibration in human blood over a wide Hb range23 and conflicting evidence on the relevance of Hb-subtype to blood water T226; however, results from all models are presented in supplemental Table 2 and discussed. The CaO2 was calculated from measured Hb levels using the formula as , in which Ca,Hb is the oxygen carrying capacity of Hb (1.34 mL O2/g Hb), [Hb] the measured Hb level via venipuncture, and Ya the arterial oxygen saturation acquired from pulse oximetry. In this model, we assume that all oxygen delivered to the brain is from Hb rather than from oxygen dissolved in plasma (≈0.003 × PaO2); this has been investigated previously and is commonly assumed.8 The global CMRO2 (mL O2/100 g per minute) was then calculated according to the Fick principle as .
Statistical analyses
Demographic, clinical, and imaging parameters were summarized with the mean and standard deviation for continuous variables, and with count or percentage for categorical variables. We evaluated significant differences in the distribution of VHS between pediatric vs adult participants using a χ2 test of independence. We further used χ2 tests of independence to assess significant differences in the distribution of sex and infarct type (none, SCI, or stroke) between both the 3 VHS subgroups and pediatric vs adult participants.
The mean of 2 venous blood R2 values for sequential TRUST measurements during the same MRI scan was calculated and used for subsequent analyses. We evaluated the repeatability of these measurements by calculating the Spearman R and intraclass correlation coefficient (ICC), for which ICC of 0.50 to 0.75 was considered moderate, ICC of 0.75 to 0.90 was considered good, and ICC of >0.90 was considered excellent repeatability.
To understand changes in clinical and hemodynamic imaging parameters with degree of VHS in persons with SCD, we used 2-way analysis of variance (ANOVA) tests to assess the effect of degree of VHS, age group (pediatric vs adult), and potential interaction effects between these 2 factors on each parameter. For those parameters and factors found statistically significant (P < .05), we subsequently used post hoc Scheffe tests to directly assess group mean differences within the factor of interest. For those parameters with a significant interaction effect, we interpreted the post hoc comparisons within the context of the interaction effect. In an exploratory analysis, we used 1-way ANOVA tests to assess these relationships in pediatric and adult participants separately.
Results
Demographics
A total of 143 participants with SCD (aged 18.66 ± 8.79 years; 46.85% male) were enrolled; 51.05% were pediatric (aged 11.40 ± 3.67 years; 42.47% male) and 48.95% were adult (aged 26.22 ± 5.60 years; 51.43% male) participants. Participant demographic, clinical, and imaging parameters are summarized in Table 1. All participants were Black with either HbSS or HbSβ0 phenotype. Of the 143 total participants, 22 participants (15.38%) were categorized with a VHS of 0 (ie, no imaging evidence of accelerated vascular transit), 56 participants (39.16%) categorized with VHS of 1 (ie, mild imaging evidence of accelerated vascular transit), and 65 participants (45.46%) categorized with VHS of 2 (ie, substantial imaging of accelerated vascular transit). VHS distribution was significantly different between pediatric and adult participants (χ2 = 25.012; P < .001), with pediatric participants having the greatest frequency of VHS of 2. Sex was not different between VHS subgroups (χ2 = 0.260; P = .878) or between pediatric and adult cohorts (χ2 = 0.821; P = .365). Infarct and stroke prevalence was not different between VHS subgroups (χ2 = 4.630; P = .327) or between pediatric and adult cohorts (χ2 = 4.515; P = .105).
Summary of demographic, clinical, and imaging features
| . | Pediatric . | Adult . | Total . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VHS = 0 . | VHS = 1 . | VHS = 2 . | Total . | VHS = 0 . | VHS = 1 . | VHS = 2 . | Total . | VHS = 0 . | VHS = 1 . | VHS = 2 . | Total . | |
| Demographic and clinical features | ||||||||||||
| n | 8 | 17 | 48 | 73 | 14 | 39 | 17 | 70 | 22 | 56 | 65 | 143 |
| Age, y, mean (SD) | 12.12 (4.51) | 12.82 (3.59) | 10.78 (3.47) | 11.40 (3.67) | 29.37 (6.29) | 25.18 (5.01) | 26.01 (5.67) | 26.22 (5.60) | 23.09 (10.17) | 21.43 (7.34) | 14.76 (7.90) | 18.66 (8.79) |
| Sex, male, n (%) | 4 (50.00) | 6 (35.29) | 21 (43.75) | 31 (42.47) | 7 (50.00) | 21 (53.85) | 8 (47.06) | 36 (51.43) | 11 (50.0) | 27 (48.2) | 29 (44.6) | 67 (46.9) |
| Total Hb, g/dL, mean (SD) | 9.92 (0.96) | 9.58 (1.23) | 8.51 (1.20) | 8.91 (1.30) | 9.42 (1.24) | 9.07 (1.27) | 8.38 (1.31) | 8.97 (1.30) | 9.60 (1.15) | 9.22 (1.27) | 8.48 (1.22) | 8.94 (1.30) |
| HbS fraction, %, mean (SD)∗ | 66.81 (14.95) | 64.11 (16.64) | 66.88 (20.38) | 66.28 (18.90) | 70.77 (22.79) | 70.67 (14.32) | 53.24 (26.04) | 66.39 (20.40) | 69.11 (19.48) | 68.81 (15.14) | 63.42 (22.54) | 66.33 (19.56) |
| Previous SCI, n (%) | 4 (50.00) | 4 (23.53) | 12 (25.00) | 20 (27.40) | 7 (50.00) | 15 (38.46) | 5 (29.41) | 27 (38.57) | 11 (50.0) | 19 (33.9) | 17 (26.2) | 47 (32.9) |
| Previous overt stroke, n (%) | 1 (12.5) | 1 (5.88) | 3 (6.25) | 5 (6.85) | 1 (7.14) | 5 (12.82) | 3 (17.65) | 9 (12.86) | 2 (9.1) | 6 (10.7) | 6 (9.2) | 14 (9.8) |
| Treatment | ||||||||||||
| None, n (%) | 0 (0.00) | 2 (11.76) | 3 (6.25) | 5 (6.85) | 0 (0.00) | 2 (5.13) | 2 (11.76) | 4 (5.71) | 0 (0.00) | 4 (7.15) | 5 (7.69) | 9 (6.29) |
| Hydroxyurea, n (%) | 6 (75.00) | 10 (58.82) | 35 (72.92) | 51 (69.86) | 12 (85.71) | 31 (79.49) | 8 (47.06) | 51 (72.86) | 18 (81.81) | 41 (73.21) | 43 (66.15) | 102 (71.33) |
| Transfusion, n (%) | 2 (25.00) | 5 (29.41) | 10 (20.83) | 17 (23.29) | 2 (14.29) | 6 (15.38) | 7 (41.18) | 15 (21.14) | 4 (18.18) | 11 (19.64) | 17 (26.15) | 31 (22.38) |
| Hemodynamic features | ||||||||||||
| Arterial oxygenation, %, mean (SD) | 97.88 (2.23) | 96.65 (2.27) | 96.20 (2.86) | 96.49 (2.69) | 95.64 (2.32) | 95.57 (2.35) | 94.89 (3.58) | 95.42 (2.67) | 96.45 (2.49) | 95.90 (2.36) | 95.86 (3.09) | 95.97 (2.72) |
| CaO2, mL O2/100 mL blood, mean (SD) | 13.02 (1.34) | 12.41 (1.65) | 19.98 (1.66) | 11.53 (1.79) | 12.08 (1.65) | 11.62 (1.73) | 10.67 (1.79) | 11.48 (1.78) | 12.42 (1.58) | 11.86 (1.73) | 10.90 (1.69) | 11.51 (1.78) |
| Gray matter CBF mean, mL blood/100 g tissue per minute, mean (SD) | 70.11 (22.93) | 73.03 (17.80) | 78.25 (16.48) | 76.14 (17.55) | 83.19 (17.93) | 89.84 (17.65) | 90.61 (17.55) | 88.70 (17.64) | 78.43 (20.39) | 84.74 (19.19) | 81.48 (17.51) | 82.29 (18.63) |
| Gray matter CBF SD, mL blood/100 g tissue per minute, mean (SD) | 24.58 (9.28) | 24.01 (5.79) | 25.14 (4.37) | 24.82 (5.34) | 31.35 (5.60) | 31.79 (5.21) | 31.79 (5.22) | 31.70 (5.22) | 28.89 (7.69) | 29.43 (6.45) | 26.88 (5.43) | 28.19 (6.29) |
| Gray matter CBF sCoV, %, mean (SD) | 34.56 (3.62) | 33.01 (2.78) | 32.54 (3.52) | 32.87 (3.39) | 38.50 (7.13) | 35.91 (5.26) | 35.96 (8.34) | 36.44 (6.48) | 37.07 (6.30) | 35.03 (4.81) | 33.43 (5.37) | 34.62 (5.43) |
| Whole-brain OEF, %, mean (SD) | 37.64 (3.63) | 34.52 (4.83) | 32.82 (5.50) | 33.75 (5.35) | 39.82 (3.78) | 37.17 (5.08) | 35.49 (5.31) | 37.30 (5.05) | 39.03 (3.80) | 36.37 (5.11) | 33.52 (5.54) | 35.48 (5.49) |
| Whole-brain CMRO2, mL O2/100 g tissue per minute, mean (SD) | 3.38 (1.00) | 3.04 (0.58) | 2.76 (0.62) | 2.89 (0.68) | 3.92 (0.71) | 3.80 (0.74) | 3.36 (0.72) | 3.72 (0.75) | 3.77 (0.84) | 3.59 (0.78) | 2.91 (0.69) | 3.31 (0.83) |
| . | Pediatric . | Adult . | Total . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VHS = 0 . | VHS = 1 . | VHS = 2 . | Total . | VHS = 0 . | VHS = 1 . | VHS = 2 . | Total . | VHS = 0 . | VHS = 1 . | VHS = 2 . | Total . | |
| Demographic and clinical features | ||||||||||||
| n | 8 | 17 | 48 | 73 | 14 | 39 | 17 | 70 | 22 | 56 | 65 | 143 |
| Age, y, mean (SD) | 12.12 (4.51) | 12.82 (3.59) | 10.78 (3.47) | 11.40 (3.67) | 29.37 (6.29) | 25.18 (5.01) | 26.01 (5.67) | 26.22 (5.60) | 23.09 (10.17) | 21.43 (7.34) | 14.76 (7.90) | 18.66 (8.79) |
| Sex, male, n (%) | 4 (50.00) | 6 (35.29) | 21 (43.75) | 31 (42.47) | 7 (50.00) | 21 (53.85) | 8 (47.06) | 36 (51.43) | 11 (50.0) | 27 (48.2) | 29 (44.6) | 67 (46.9) |
| Total Hb, g/dL, mean (SD) | 9.92 (0.96) | 9.58 (1.23) | 8.51 (1.20) | 8.91 (1.30) | 9.42 (1.24) | 9.07 (1.27) | 8.38 (1.31) | 8.97 (1.30) | 9.60 (1.15) | 9.22 (1.27) | 8.48 (1.22) | 8.94 (1.30) |
| HbS fraction, %, mean (SD)∗ | 66.81 (14.95) | 64.11 (16.64) | 66.88 (20.38) | 66.28 (18.90) | 70.77 (22.79) | 70.67 (14.32) | 53.24 (26.04) | 66.39 (20.40) | 69.11 (19.48) | 68.81 (15.14) | 63.42 (22.54) | 66.33 (19.56) |
| Previous SCI, n (%) | 4 (50.00) | 4 (23.53) | 12 (25.00) | 20 (27.40) | 7 (50.00) | 15 (38.46) | 5 (29.41) | 27 (38.57) | 11 (50.0) | 19 (33.9) | 17 (26.2) | 47 (32.9) |
| Previous overt stroke, n (%) | 1 (12.5) | 1 (5.88) | 3 (6.25) | 5 (6.85) | 1 (7.14) | 5 (12.82) | 3 (17.65) | 9 (12.86) | 2 (9.1) | 6 (10.7) | 6 (9.2) | 14 (9.8) |
| Treatment | ||||||||||||
| None, n (%) | 0 (0.00) | 2 (11.76) | 3 (6.25) | 5 (6.85) | 0 (0.00) | 2 (5.13) | 2 (11.76) | 4 (5.71) | 0 (0.00) | 4 (7.15) | 5 (7.69) | 9 (6.29) |
| Hydroxyurea, n (%) | 6 (75.00) | 10 (58.82) | 35 (72.92) | 51 (69.86) | 12 (85.71) | 31 (79.49) | 8 (47.06) | 51 (72.86) | 18 (81.81) | 41 (73.21) | 43 (66.15) | 102 (71.33) |
| Transfusion, n (%) | 2 (25.00) | 5 (29.41) | 10 (20.83) | 17 (23.29) | 2 (14.29) | 6 (15.38) | 7 (41.18) | 15 (21.14) | 4 (18.18) | 11 (19.64) | 17 (26.15) | 31 (22.38) |
| Hemodynamic features | ||||||||||||
| Arterial oxygenation, %, mean (SD) | 97.88 (2.23) | 96.65 (2.27) | 96.20 (2.86) | 96.49 (2.69) | 95.64 (2.32) | 95.57 (2.35) | 94.89 (3.58) | 95.42 (2.67) | 96.45 (2.49) | 95.90 (2.36) | 95.86 (3.09) | 95.97 (2.72) |
| CaO2, mL O2/100 mL blood, mean (SD) | 13.02 (1.34) | 12.41 (1.65) | 19.98 (1.66) | 11.53 (1.79) | 12.08 (1.65) | 11.62 (1.73) | 10.67 (1.79) | 11.48 (1.78) | 12.42 (1.58) | 11.86 (1.73) | 10.90 (1.69) | 11.51 (1.78) |
| Gray matter CBF mean, mL blood/100 g tissue per minute, mean (SD) | 70.11 (22.93) | 73.03 (17.80) | 78.25 (16.48) | 76.14 (17.55) | 83.19 (17.93) | 89.84 (17.65) | 90.61 (17.55) | 88.70 (17.64) | 78.43 (20.39) | 84.74 (19.19) | 81.48 (17.51) | 82.29 (18.63) |
| Gray matter CBF SD, mL blood/100 g tissue per minute, mean (SD) | 24.58 (9.28) | 24.01 (5.79) | 25.14 (4.37) | 24.82 (5.34) | 31.35 (5.60) | 31.79 (5.21) | 31.79 (5.22) | 31.70 (5.22) | 28.89 (7.69) | 29.43 (6.45) | 26.88 (5.43) | 28.19 (6.29) |
| Gray matter CBF sCoV, %, mean (SD) | 34.56 (3.62) | 33.01 (2.78) | 32.54 (3.52) | 32.87 (3.39) | 38.50 (7.13) | 35.91 (5.26) | 35.96 (8.34) | 36.44 (6.48) | 37.07 (6.30) | 35.03 (4.81) | 33.43 (5.37) | 34.62 (5.43) |
| Whole-brain OEF, %, mean (SD) | 37.64 (3.63) | 34.52 (4.83) | 32.82 (5.50) | 33.75 (5.35) | 39.82 (3.78) | 37.17 (5.08) | 35.49 (5.31) | 37.30 (5.05) | 39.03 (3.80) | 36.37 (5.11) | 33.52 (5.54) | 35.48 (5.49) |
| Whole-brain CMRO2, mL O2/100 g tissue per minute, mean (SD) | 3.38 (1.00) | 3.04 (0.58) | 2.76 (0.62) | 2.89 (0.68) | 3.92 (0.71) | 3.80 (0.74) | 3.36 (0.72) | 3.72 (0.75) | 3.77 (0.84) | 3.59 (0.78) | 2.91 (0.69) | 3.31 (0.83) |
Data shown are mean (SD) unless otherwise noted.
SD, standard deviation; VHS, venous hyperintensity score.
Total N = 135; pediatric, n = 70; adult, n = 65.
Repeatability of OEF-weighted measurement
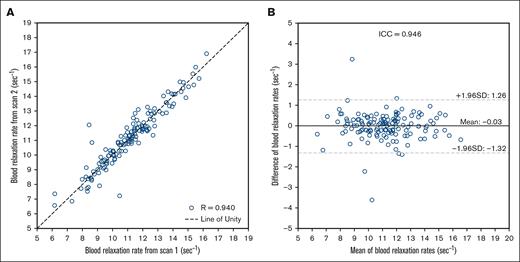
Although repeatability of CBF methodology is well characterized in the literature,18,27 OEF-weighted TRUST repeatability assessments in SCD are more limited and therefore all TRUST scans were repeated once. One participant was removed from this analysis because of a scanner malfunction in the second run of the TRUST sequence. For all other participants, the 2 quantified venous relaxation rates (R2) demonstrated excellent repeatability with an ICC of 0.946 and a Spearman correlation of 0.940 (Figure 2).
Summary plots of the repeatability of TRUST MRI method in SCD. (A) A scatterplot of relaxation rates (R2) from sequential TRUST scans for each subject (n = 142) is shown. Spearman correlation analysis of the sequential R2 measurements from TRUST demonstrates significant correlation (R = 0.940; P < .001). (B) A Bland-Altman plot portrays the mean R2 of the sequential TRUST scans and the difference between scans for each participant. ICC analysis demonstrates excellent repeatability (ICC = 0.946) of the TRUST scan in persons with SCD. SD, standard deviation.
Summary plots of the repeatability of TRUST MRI method in SCD. (A) A scatterplot of relaxation rates (R2) from sequential TRUST scans for each subject (n = 142) is shown. Spearman correlation analysis of the sequential R2 measurements from TRUST demonstrates significant correlation (R = 0.940; P < .001). (B) A Bland-Altman plot portrays the mean R2 of the sequential TRUST scans and the difference between scans for each participant. ICC analysis demonstrates excellent repeatability (ICC = 0.946) of the TRUST scan in persons with SCD. SD, standard deviation.
Clinical and hematologic findings
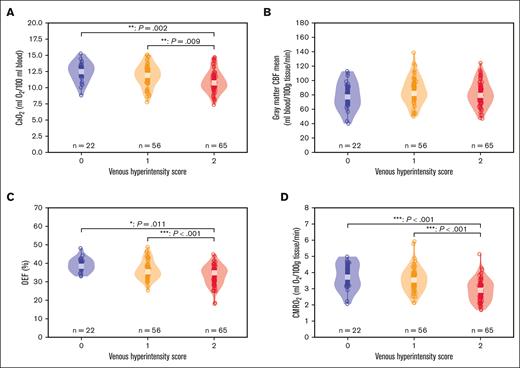
Table 2 summarizes the results from 2-way ANOVA analyses for main and interaction effects of VHS and age group (pediatric vs adult) on the clinical and imaging parameters; additionally, representative images and measures from 2 case examples are highlighted in Figure 3. Total Hb (F(2,137) = 9.334; P < .001) and CaO2 (Figure 4A; F(2,137) = 8.746; P < .001) were significantly different between VHS groups; specifically, both hematologic measures were reduced in participants with SCD with imaging evidence suggestive of rapid arteriovenous transit, defined as VHS = 2, compared with their counterparts with VHS = 0 (total Hb: Scheffe P = .001; CaO2: Scheffe P = .002) or VHS = 1 (total Hb: Scheffe P = .005; CaO2: Scheffe P = .009). Arterial oxygenation (F(1,137) = 7.692; P = .006) was significantly different between age groups; specifically arterial oxygenation was lower in adult participants than in pediatric participants (Scheffe P = .019). Eight participants (5.59%) did not have measured HbS percentages and were subsequently excluded from analysis with HbS percentage. Of the remaining participants, there was a significant interaction effect between VHS and age group on HbS percentage (F(2,129) = 3.472; P = .034).
Results from 2-way ANOVA analyses of differences between the 3 VHS groups, pediatric vs adult participants, and interaction effects
| Variable . | 2-way ANOVA . | Post hoc Scheffe test . | |||||
|---|---|---|---|---|---|---|---|
| VHS (F, P value) . | Age group (F, P value) . | VHS: age group (F, P value) . | 0 vs 1 (P value) . | 0 vs 2 (P value) . | 1 vs 2 (P value) . | Peds vs adults (P value) . | |
| Total Hb (g/dL) | (9.334, <.001) | (2.349, .128) | (0.346, .708) | .465 | .001 | .005 | — |
| HbS fraction (%) | (1.367, .259) | (0.544, .462) | (3.472, .034) | — | — | — | — |
| Arterial oxygenation (%) | (0.434, 0.648) | (7.692, 0.006) | (0.339, 0.713) | — | — | — | .019 |
| CaO2 (mL O2/100 mL blood) | (8.746, <.001) | (3.881, .051) | (0.361, .698) | .419 | .002 | .009 | — |
| Gray matter CBF, mean (mL blood/100 g tissue per minute) | (1.138, .323) | (19.382, <.001) | (0.209, .812) | — | — | — | <.001 |
| Gray matter CBF SD (mL blood/100 g tissue per minute) | (3.629, .029) | (52.356, <.001) | (0.150, .861) | .942 | .429 | .085 | <.001 |
| Gray matter CBF sCoV (%) | (4.400, .014) | (12.162, <.001) | (0.079, .924) | .316 | .024 | .260 | <.001 |
| Whole-brain OEF (%) | (11.160, <.001) | (7.665, .006) | (0.019, .982) | .124 | <.001 | .011 | <.001 |
| Whole-brain CMRO2 (mL O2/100 g tissue per minute) | (18.372, <.001) | (26.416, <.001) | (0.230, .795) | .718 | <.001 | <.001 | <.001 |
| Variable . | 2-way ANOVA . | Post hoc Scheffe test . | |||||
|---|---|---|---|---|---|---|---|
| VHS (F, P value) . | Age group (F, P value) . | VHS: age group (F, P value) . | 0 vs 1 (P value) . | 0 vs 2 (P value) . | 1 vs 2 (P value) . | Peds vs adults (P value) . | |
| Total Hb (g/dL) | (9.334, <.001) | (2.349, .128) | (0.346, .708) | .465 | .001 | .005 | — |
| HbS fraction (%) | (1.367, .259) | (0.544, .462) | (3.472, .034) | — | — | — | — |
| Arterial oxygenation (%) | (0.434, 0.648) | (7.692, 0.006) | (0.339, 0.713) | — | — | — | .019 |
| CaO2 (mL O2/100 mL blood) | (8.746, <.001) | (3.881, .051) | (0.361, .698) | .419 | .002 | .009 | — |
| Gray matter CBF, mean (mL blood/100 g tissue per minute) | (1.138, .323) | (19.382, <.001) | (0.209, .812) | — | — | — | <.001 |
| Gray matter CBF SD (mL blood/100 g tissue per minute) | (3.629, .029) | (52.356, <.001) | (0.150, .861) | .942 | .429 | .085 | <.001 |
| Gray matter CBF sCoV (%) | (4.400, .014) | (12.162, <.001) | (0.079, .924) | .316 | .024 | .260 | <.001 |
| Whole-brain OEF (%) | (11.160, <.001) | (7.665, .006) | (0.019, .982) | .124 | <.001 | .011 | <.001 |
| Whole-brain CMRO2 (mL O2/100 g tissue per minute) | (18.372, <.001) | (26.416, <.001) | (0.230, .795) | .718 | <.001 | <.001 | <.001 |
If the variable were significantly different (P < .05) between the VHS group or age group, subsequent Scheffe tests were performed to determine direct pairwise differences between groups for the appropriate factor.
Peds, pediatrics; SD, standard deviation.
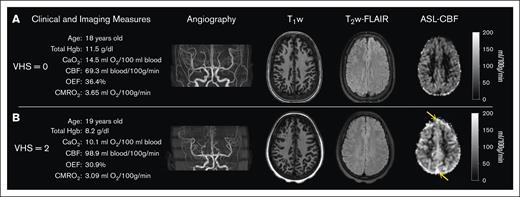
Case examples. Demographic, clinical, and imaging measures are presented alongside angiography, T1-weighted, T2-FLAIR, and CBF images for 2 age-matched individuals with SCD and (A) VHS of 0 and (B) VHS of 2 on ASL MRI. The individual with VHS of 2 (B) had lower total Hb and arterial blood oxygen content; and displayed elevated CBF, reduced OEF, and reduced CMRO2 compared with the individual with VHS of 0 (A). Orange arrows on panel B indicate evidence of signal hyperintensity in the superior sagittal sinus on ASL MRI.
Case examples. Demographic, clinical, and imaging measures are presented alongside angiography, T1-weighted, T2-FLAIR, and CBF images for 2 age-matched individuals with SCD and (A) VHS of 0 and (B) VHS of 2 on ASL MRI. The individual with VHS of 2 (B) had lower total Hb and arterial blood oxygen content; and displayed elevated CBF, reduced OEF, and reduced CMRO2 compared with the individual with VHS of 0 (A). Orange arrows on panel B indicate evidence of signal hyperintensity in the superior sagittal sinus on ASL MRI.
Comparing cerebral hemodynamic measures across VHS subgroups. Box and violin plots are shown for all participants comparing CMRO2 and its various components across 3 subgroups (post hoc Scheffe test; P < .05): VHS = 0 (n = 22), VHS = 1 (n = 56), and VHS = 2 (n = 65). (A) CaO2 was significantly reduced in the VHS = 2 group compared with VHS = 0 (P = .002) and VHS = 1 (P = .009) groups. (B) Gray matter CBF was not different between VHS groups. (C) OEF was significantly reduced in the VHS = 2 group compared with score = 0 (P < .001) and VHS = 1 (P = .011) groups. (D) CMRO2, calculated as the product of CaO2, CBF, and OEF, was also significantly reduced in the VHS = 2 group compared with VHS = 0 (P < .001) and VHS = 1 (P < .001) groups. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Comparing cerebral hemodynamic measures across VHS subgroups. Box and violin plots are shown for all participants comparing CMRO2 and its various components across 3 subgroups (post hoc Scheffe test; P < .05): VHS = 0 (n = 22), VHS = 1 (n = 56), and VHS = 2 (n = 65). (A) CaO2 was significantly reduced in the VHS = 2 group compared with VHS = 0 (P = .002) and VHS = 1 (P = .009) groups. (B) Gray matter CBF was not different between VHS groups. (C) OEF was significantly reduced in the VHS = 2 group compared with score = 0 (P < .001) and VHS = 1 (P = .011) groups. (D) CMRO2, calculated as the product of CaO2, CBF, and OEF, was also significantly reduced in the VHS = 2 group compared with VHS = 0 (P < .001) and VHS = 1 (P < .001) groups. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Hemodynamic and metabolic imaging
Whole-brain OEF (F(2,137) = 11.160; P < .001) and CMRO2 (F(2,137) = 18.372; P < .001) were significantly different between VHS groups (Figure 4); specifically, both OEF and CMRO2 were reduced in patients with SCD with VHS of 2 compared with their counterparts with VHS of 0 (OEF: Scheffe P < .001; CMRO2: Scheffe P < .001) and VHS = 1 (OEF: Scheffe P = .011; CMRO2: Scheffe P < .001). Mean gray matter CBF (F(1,137) = 19.382, P < .001), whole-brain OEF (F(1,137) = 7.665; P = .006), and whole-brain CMRO2 (F(1,137) = 26.416; P < .001) were significantly different between age groups (Figure 5). All 3 hemometabolic imaging measures were elevated in adult participants compared with pediatric participants (CBF: Scheffe P < .001; OEF: Scheffe P < .001; CMRO2: Scheffe P < .001).
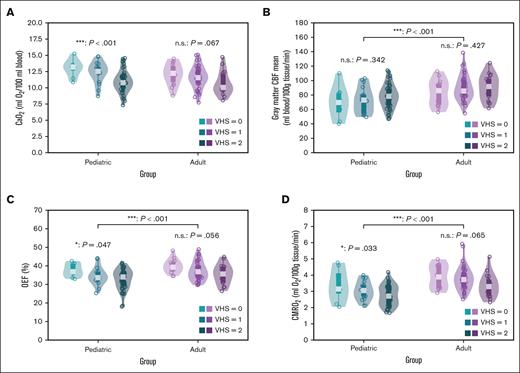
Comparing cerebral hemodynamic measures in pediatric and adult participants separately. Box and violin plots are shown comparing (A) CaO2, (B) gray matter CBF, (C) OEF, and (D) CMRO2 between and within pediatric (n = 73) and adult (n = 70) participants. CaO2, OEF, and CMRO2 was significantly different across hyperintensity score in pediatric participants (CaO2: P < .001; OEF: P = .046; CMRO2: P = .033). Although statistically nonsignificant, we observe similar directionality in the adult participants. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. n.s., not significant.
Comparing cerebral hemodynamic measures in pediatric and adult participants separately. Box and violin plots are shown comparing (A) CaO2, (B) gray matter CBF, (C) OEF, and (D) CMRO2 between and within pediatric (n = 73) and adult (n = 70) participants. CaO2, OEF, and CMRO2 was significantly different across hyperintensity score in pediatric participants (CaO2: P < .001; OEF: P = .046; CMRO2: P = .033). Although statistically nonsignificant, we observe similar directionality in the adult participants. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. n.s., not significant.
To gain additional information on the possibility of spatial vascular shunting in the presence of VHSs, we evaluated standard metrics of regional cortical variations in the CBF signal (supplemental Figure 1). There were significant effects of both degree of VHS (F(2,137) = 4.400; P = .014) and age group (F(1,137) = 12.162; P < .001) on gray matter CBF sCoV; specifically, gray matter sCoV was reduced in participants with VHS of 2 compared with those with VHS of 0 only (Scheffe P = .024), indicating reduced spatial heterogeneity of CBF in individuals with imaging evidence suggestive of arteriovenous shunting. With respect to age group, pediatric participants had significantly lower gray matter sCoV than their adult counterparts (Scheffe P < .001). We also observed significant main effects of both degree of VHS (F(2,137) = 3.629; P = .029) and age group (F(1,137) = 52.356; P < .001) on standard deviation gray matter CBF. With respect to VHS, no direct pairwise comparisons were significant after multiple comparison correction. With respect to age group, pediatric participants had significantly lower standard deviation of gray matter CBF than their adult counterparts (Scheffe P < .001). Together, the spatial vascular shunting analyses indicate that differences in gray matter CBF sCoV with respect to degree of VHS are not purely driven by spatial heterogeneity in CBF but rather by a combination of elevated mean CBF and reduced spatial variance.
In a post hoc exploratory analysis, we assessed the effect of degree of VHS on clinical and imaging parameters in pediatric and adult participants separately. Results from 1-way ANOVA and post hoc direct pairwise comparisons are summarized in supplemental Table 3 and Figure 5.
Discussion
This study investigated multiple hematologic and cerebral hemometabolic imaging measures with respect to the degree of venous signal intensity on CBF-weighted ASL MRI of the brain in 143 pediatric and adult participants with SCD. It was observed that total Hb level, arterial blood oxygen content, whole-brain OEF, and whole-brain CMRO2 were significantly reduced in participants with SCD presenting with greater imaging evidence of accelerated vascular transit. Despite the mild reduction in OEF and CMRO2, these values remained in approximately a normal range in most patients.
Physiological mechanisms
Venous hyperintense signal on ASL MRI in patients with SCD has been shown to relate directly to accelerated blood flow velocities, as a result of correspondence with independent phase contrast measures of macrovascular flow velocity12 and corresponding changes after blood transfusion.21 Under the assumption that this macrovascular flow effect manifests at the capillary level as well, the shortened residency times within the capillaries may reduce blood-tissue water exchange resulting in a greater proportion of labeled blood water spins remaining in the blood compartment. This causes a greater signal difference between the control and label conditions in the capillaries and veins. This imaging phenomenon has been shown to be more prevalent in persons with SCD,12 which is marked by elevated arterial blood flow velocities, compared with in healthy persons. Previous studies have suggested, in smaller adult cohorts, that VHS on ASL MRI is associated with elevations in CBF,12,13 reductions in OEF,13 reductions in CaO2,15 and reductions in CMRO214 in persons with SCD. Here, we report that persons with SCD presenting with VHSs of 2, the greatest degree of VHS, had significantly, albeit only mildly, lower global OEF and CMRO2 than those with VHSs of 0 or 1, extending prior observations by demonstrating that these effects in a larger cohort of both pediatric and adult participants with SCD.
It remains unclear whether accelerated capillary transit is a cause, or a consequence, of any metabolic oxygen aberrations. Specifically, rapid capillary transit time is not necessarily a cause of altered oxygen extraction since red cells with oxygenated Hb are continuously entering into the capillary bed, which differs from an imaging experiment in which only a finite bolus of tracer is assessed. As such, although oxygen extraction per mL of blood over time may be reduced, given the continuous supply of oxygen substrate under ongoing physiological conditions, it is unclear whether this rapid capillary transit contributes directly to the observed mild reduction in OEF and CMRO2 observed. As such, this study provides evidence that accelerated blood transit is associated with reduced Hb, blood oxygen content, OEF, and CMRO2, yet does not necessarily inform causality.
Additionally, hemorheological characteristics in SCD may further contribute to changes in capillary flow and oxygen metabolism. Blood viscosity (ie, resistance to deformation at a given flow rate) is an important factor of fluid flow, in which reduced viscosity generally yields higher flow velocities. Important contributors to higher viscosity is higher Hb, red blood cell (RBC) deformability, and RBC aggregation.28 Anemia will, in principle, reduce blood viscosity under oxygenated conditions compared with blood with higher, normal Hb levels.29 However, under deoxygenated conditions, HbS polymerization can impair RBC deformability, effectively overwhelming the effect of anemia alone and increasing overall blood viscosity.30 It is logical that changes in viscosity also contribute to changes in capillary residency times. Although total Hb is 1 of the most important contributors to blood viscosity, it is difficult to disambiguate these effects from those of other pathophysiological factors in SCD. Of note, the participants in the present study had an overall HbS fraction of 66.33% ± 19.56%. Interestingly, adults with VHS of 2, who had a greater proportion on transfusion therapy, had a notably lower HbS fraction (53.24% ± 26.04%) than the other subgroups but similar total Hb level. Together, this may suggest that this specific subgroup had lower blood viscosity than their counterparts thus contributing to higher flow velocities and vascular shunting. Current methods to measure blood viscosity are limited, and future studies may specifically assess the interplay between HbS percentage, hematocrit, and blood viscosity to elucidate the contribution of each measure to blood flow velocity and capillary residency times.
Importantly, metabolic (eg, OEF and CMRO2) imaging measures remained within approximately normal physiological ranges from literature (31%-44%; and 2.9-3.7 mL O2/100 g tissue per minute, respectively).31 Compensatory hemodynamic mechanisms in SCD may provide sufficient oxygen offloading and metabolism for normal tissue function; however, for individuals with SCD and severe capillary shunting, the delicate equipoise of cerebral hemodynamics may be at greater risk of disruption after any further stressor on the hemorheological or cerebrovascular system.
Age effects
Prior studies evaluating imaging evidence of accelerated arteriovenous transit have focused on adult cohorts with SCD; thus, leaving a gap in the literature on the relevance of venous hyperintense signal on ASL in children with SCD with respect to oxygen metabolism. Here, we enrolled 143 adult and pediatric participants to evaluate additional hypothesized relationships with age and possible spatial vascular shunting. When assessing the effect of degree of VHS on OEF and CMRO2 in pediatric and adult participants separately, we observed significant, albeit mild, reductions in OEF and CMRO2 in pediatric participants. Additionally, we observed significant reductions in total Hb level and CaO2, indicative of severe chronic anemia, with greater VHS in pediatric participants and similar findings in the adult participants. These findings suggest the possible utility of VHS on ASL as a potential biomarker for altered hemodynamics in patients with SCD. Importantly, there are currently no available screening methods for stroke risk in adults with SCD. Although transcranial doppler ultrasound to measure macrovascular blood flow velocity is used clinically in children with SCD for screening,32 age-related changes in blood flow velocities33 complicate the use of transcranial doppler in adults. Thus, development of potential biomarkers such as VHS on ASL imaging may provide key methods to identify patients with SCD at high risk for stroke.
Vascular compartment vs spatial shunting
We also observed significant differences of gray matter CBF sCoV and standard deviation with degree of VHS. However, further investigation of these changes indicates a small effect of these measures with only reductions of sCoV in those with VHS of 2 compared with those with VHS of 0 surviving post hoc multiple comparisons. Together with null findings of direct comparisons of standard deviation between VHS groups, this finding is likely driven by increases in CBF as opposed to increased spatial heterogeneity of CBF with greater VHS. No clear evidence of spatial shunting (eg, blood being rerouted spatially in the setting of hyperemic states) was observed and rather data here suggest that shunting is primarily a vascular compartment shunting (eg, accelerated transit through capillaries).
Limitations
Findings should be considered in the context of the following limitations. First, the VHS was categorically assessed as an ordinal score upon subjective assessment.12,13 In contrast to previous studies,12-14 we found no significant differences in mean gray matter CBF with respect to degree of VHS; however, we did observe small increases in CBF in those with evidence of VHS compared with those without. The heterogeneity of those presenting with VHS of 1 may diminish the statistical power to identify changes in CBF as autoregulatory increases in CBF are hypothesized to be one of the earliest compensatory responses of the cerebrovascular system. Future studies are incorporating multidelay ASL to quantitatively measure transit times in the arterial, capillary, and venous systems. Additionally, we found that gray matter CBF was significantly elevated in adults vs children with SCD; a finding contrary to previous reports.14 Most pediatric participants were imaged using a pCASL variant with a longer labeling duration (LD) of 1500 to 1650 milliseconds vs adult participants imaged with a shorter LD (1000 milliseconds). However, the total time since labeling (LD + post-labeling delay [PLD]) were similar between sequences. Our study population was representative of a typical SCD population and included a range of HbS fraction and the 2 most common treatments (oral hydroxyurea and blood transfusion). SCD blood demonstrates a right-shifted oxyhemoglobin dissociation curve, indicative of decreased oxygen affinity, that is partially dependent on HbS and HbF levels.34,35 In this study, we did not observe significant differences in HbS fraction across the VHS subgroups, and the majority of participants were on hydroxyurea treatment, which increases HbF production and causes a leftward shift of the oxyhemoglobin dissociation curve. Nonetheless, as with most SCD studies, oxygen affinity was not directly measured and could be the topic of future investigation. TRUST MRI was used to measure global OEF measures, which were then used for global CMRO2 calculations. There is some inconsistency between Yv-T2-Hb calibration models for susceptibility-weighted oxygenation measures in patients with SCD, although we observed similar trends across all calibration models used in this study (supplemental Table 2). Finally, we demonstrated that these changes in oxygen hemodynamics with VHS on ASL imaging persist in the presence of prior infarcts. A prospective, longitudinal study is required to understand the possible role of capillary shunting on cerebral infarct development.
Conclusion
We assessed relationships between VHS on CBF-weighted ASL MRI and whole-brain–level physiology in persons with SCD, both children and adults. Across the 3 VHS groups, we observed that CaO2, whole-brain OEF, and whole-brain CMRO2 were reduced in those with the highest VHS; although OEF and CMRO2 values remained, on average, within healthy ranges. These data provide evidence that the venous hyperintensity observed on ASL MRI may be a surrogate marker of accelerated arteriovenous transit, and mildly reduced oxygen metabolism. Further validation of this imaging phenomenon with multidelay ASL imaging or longitudinal studies may provide a useful tool for identifying patients at high risk for future infarction and assist with triaging patients to risk-appropriate therapies, and this work is ongoing.
Acknowledgments
The authors thank Chuck Nockowski, Ryan Robison, and Niral Patel for experimental support.
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (grants 3R01NS123281-S1, 5R01NS123281, and 5R01NS096127) and NIH/National Heart, Lung, and Blood Institute (grant 5R01HL155207).
Authorship
Contribution: M.J.D. and L.C.J. were responsible for study conceptualization and design; S.D., L.M., and C.C. were responsible for study recruitment; S.L.W., R.S.J., and M.J.D. were responsible for image collection; L.T.D., S.P., D.M., L.C.J., and M.J.D. were responsible for image reading; A.K.S., S.L.W., and R.S.J. were responsible for data analyses; A.K.S., W.T.R., M.A.A., L.C.J., and M.J.D. were responsible for interpretation of the results; A.K.S. was responsible for writing the manuscript and making figures; and all authors revised and provided feedback on the manuscript and read and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.J.D. receives research-related support from the National Institutes of Health (National Institute of Neurological Disorders and Stroke, National Cancer Institute, National Institute on Aging, National Center for Complementary and Investigative Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute), Philips Healthcare, and Pfizer Inc; is a paid consultant for Graphite Bio, Pfizer Inc, Global Blood Therapeutics, Woolsey Pharmaceuticals, Alterity Pharmaceuticals, and LymphaTouch; is a paid advisory board member for Pfizer Inc, Novartis, and bluebird bio; and is the chief executive officer of Biosight Inc, which operates as a clinical research organization. These agreements have been approved by Vanderbilt University Medical Center in accordance with its conflict-of-interest policy. The remaining authors declare no competing financial interests.
Correspondence: Manus J. Donahue, Behavioral and Cognitive Neurology, Vanderbilt University Medical Center, 1500 21st Ave South Village at Vanderbilt, Suite 2600, Nashville, TN 37212; email: m.donahue@vumc.org.
References
Author notes
Data are available on request from the corresponding author, Manus J. Donahue (m.donahue@vumc.org).
The full-text version of this article contains a data supplement.