Key Points
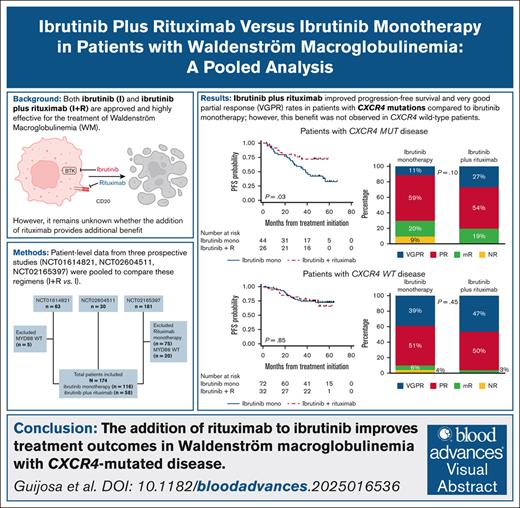
Patients with WM and CXCR4 mutations have improved outcomes with I+R vs I.
In patients with CXCR4 wild-type disease, adding R to I showed no clear additional benefit.
Visual Abstract
Ibrutinib (I) and ibrutinib plus rituximab (I+R) are highly efficacious in treating Waldenström macroglobulinemia (WM). However, the benefit of adding rituximab remains unclear, and a prospective trial to compare these two regimens has not been undertaken. Therefore, we performed a pooled analysis of prospective studies to compare their effectiveness. Patient-level demographic, response, and survival data were pooled from three prospective studies (ClinicalTrials.gov identifiers: NCT01614821, NCT02604511, NCT02165397). We included patients treated with I+R or I, excluding those without MYD88 mutations. Among 174 patients (58 I+R, 116 I), very good partial response (VGPR) rates were comparable across treatment groups (38% I+R vs 28% I; P = .21). The 48-month progression-free survival (PFS) rate was 74% with I+R vs 61% with I (P = .22). The 48-month overall survival rate was 94% vs 89% (P = .45). In patients with CXCR4 mutations, I+R trended toward higher VGPR (27% vs 11%; P = .10), and had a superior 48-month PFS rate (72% vs 43%; P = .03). CXCR4 mutations were associated with inferior VGPR (42% vs 17%; P = .001) and 48-month PFS in the I arm (43% vs 72%; P = .002). In the I+R arm, CXCR4 mutations were associated with numerically inferior VGPR (47% vs 27%; P = .12), but the 48-month PFS rate was not impacted (74% vs 72%; P = .96). I+R significantly improved PFS over I in patients with CXCR4-mutated WM, along with a nonsignificant increase in VGPR in this subgroup. These results support routine CXCR4 testing in patients with WM, and clinical trials of rituximab with covalent or noncovalent Bruton tyrosine kinase inhibitors.
Introduction
Bruton tyrosine kinase (BTK) inhibitors are standard for treating Waldenström macroglobulinemia (WM). Ibrutinib was the first-in-class BTK inhibitor, and is safe and highly effective in WM.1-4 Based on the results of a seminal prospective phase 2 study, the United States Food and Drug Administration approved ibrutinib monotherapy for treating WM in 2015. Somatic mutations in CXCR4 are highly recurrent in WM, detected in 30% to 40% of tested patients,5-8 and are associated with lower rates of very good partial response (VGPR) and inferior progression-free survival (PFS) to ibrutinib in patients who are treatment naïve, and patients with relapsed disease.3,4,9
The INNOVATE study compared ibrutinib plus rituximab with a placebo plus rituximab.10,11 This study showed that the combination was associated with superior outcomes, prompting the United States Food and Drug Administration approval for treating WM in 2018. CXCR4 mutations were associated with lower VGPR rates in patients treated with ibrutinib plus rituximab, but an impact on PFS was less evident.
The National Comprehensive Cancer Network endorses ibrutinib monotherapy, and ibrutinib plus rituximab as preferred treatment options for patients with WM.12 However, it is unclear whether ibrutinib plus rituximab provides additional benefits over ibrutinib monotherapy in WM, and prospective clinical trials to answer this question will unlikely be undertaken.
Therefore, we performed a comparative pooled analysis of prospective studies to evaluate the response and survival outcomes of ibrutinib plus rituximab vs ibrutinib monotherapy in patients with WM. A secondary objective was to assess the impact of CXCR4 mutational status in patients with WM treated with ibrutinib plus rituximab, and ibrutinib monotherapy.
Methods
Patients
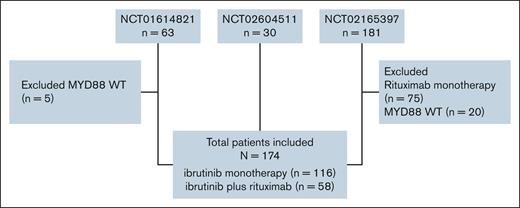
We collected demographic, response, and survival patient-level data from three prospective studies evaluating ibrutinib monotherapy or in combination with rituximab in patients with WM. NCT01614821 was a single-arm study that included 63 patients with relapsed or refractory (RR) WM treated with ibrutinib monotherapy.1,3 NCT02604511 was a single-arm study that included 30 patients with treatment-naïve (TN) WM treated with ibrutinib monotherapy.2,4 NCT02165397 included three arms: arm A included 75 patients with RR and TN WM treated with ibrutinib plus rituximab, arm B included 75 patients with RR and TN WM treated with rituximab, and arm C included 31 patients with RR, rituximab-refractory WM treated with ibrutinib monotherapy.10,11,13 Responses in all 3 trials were assessed using the modified consensus criteria established by the 6th International Workshop on Waldenström Macroglobulinemia.14 Data from NCT02165397 were obtained through the Yale University Open Data Access project (ID #2022-4928).
For this analysis, we included patients treated with ibrutinib plus rituximab, or ibrutinib monotherapy. We excluded patients without MYD88 mutations (n = 25), and those treated with rituximab monotherapy (n = 75). Figure 1 shows the CONSORT diagram.
Statistical design
Clinicopathological data were categorized as follows: age (>65 and ≤65 years), sex (male and female), hemoglobin level (<115 and ≥115 g/L), platelet count (<100 and ≥100 103/μL), serum immunoglobulin M (IgM) level (>70 and ≤70 g/L), serum beta-2-microglobulin level (>3.5 and ≤3.5 mg/L), treatment history (previously treated and TN), and CXCR4 mutational status (mutated and wild-type). The International Prognostic Scoring for WM (IPSSWM) was calculated as previously described.15 Data on hemoglobin level (n = 6; 3.4%), platelet count (n = 3; 1.7%), and IPSSWM (n = 5; 2.9%) were missing. Categorical responses were assessed based on the modified 6th International Workshop on Waldenström Macroglobulinemia,14 and divided into VGPR or better, partial response, minor response, and no response.
Categorical variables were reported as frequencies and proportions. Continuous variables were analyzed in two ways: (1) they were dichotomized based on clinically relevant thresholds (as described above), and reported as frequencies and proportions, and (2) they were also analyzed in their continuous form, and summarized using medians and interquartile ranges, given the relatively small sample size. Differences between groups were assessed using Fisher’s exact or χ2 tests for categorical variables, including dichotomized continuous variables. For continuous variables analyzed in their original form, differences between groups were assessed using the Wilcoxon rank-sum test.
Univariate and multivariate logistic regression models were fitted to evaluate the association between treatment arms and VGPR attainment. The outcome measure for the logistic regression was the odds ratio (OR) with a 95% confidence interval (CI). PFS was defined as the time between treatment initiation and disease progression, death, or last follow-up. Overall survival (OS) was defined as the time between treatment initiation and death, or last follow-up. PFS and OS curves were estimated using the Kaplan-Meier method for incomplete observations, and compared using the log-rank test. Univariate and multivariate proportional-hazard Cox regression models were fitted to assess the prognostic value of the treatment arms for PFS and OS. The outcome measure for the proportional-hazard Cox regression was the hazard ratio (HR) with a 95% CI. Multivariate analyses included age, sex, hemoglobin, platelet count, serum beta-2-microglobulin (or the composite IPSSWM score for PFS to avoid overadjustment), treatment status, and CXCR4 mutational status, based on their established prognostic relevance in large WM cohorts.15-17 Lactate dehydrogenase and albumin (components of the Modified Staging System for WM; MSS-WM) were not available in the pooled trials.16
P values <.05 were considered significant. Correction for multiple testing was performed using the Benjamini-Hochberg false discovery rate method: across seven subgroups for the ibrutinib plus rituximab vs ibrutinib monotherapy comparison (CXCR4-mutated, CXCR4-wild-type, TN, previously treated, low, intermediate, and high IPSSWM), and across two subgroups in the CXCR4 mutation analysis (ibrutinib monotherapy vs ibrutinib plus rituximab). All calculations and graphics were obtained using STATA version 17 (College Station, TX).
Results
Patients’ characteristics
The final analytical cohort included 174 patients: 58 patients treated with ibrutinib plus rituximab, and 116 with ibrutinib monotherapy. Baseline characteristics according to treatment are shown in Table 1. There was a higher proportion of men (75% vs 59%; P = .03) and previously treated patients (72% vs 47%; P = .001) in the ibrutinib monotherapy group than in the ibrutinib plus rituximab group. The median beta-2-microglobulin level was higher in the ibrutinib monotherapy group (5.2 vs 3.8 mg/L; P = .001). The proportion of patients with serum IgM ≥70 g/dL was higher in the ibrutinib monotherapy than in the ibrutinib plus rituximab group (7% vs 0%; P = .04). There were no differences between groups in the other variables (P > .05 for all remaining variables). Baseline differences by CXCR4 mutational status were also evaluated, and are shown in Table 2. Patients with CXCR4 mutations had a higher frequency of platelet count <100 103/μL (17% vs 4%), and a higher median serum IgM level (43.1 vs 33.6 g/L; P = .01). No other significant differences were observed (P > .05).
Patients’ baseline characteristics according to treatment group
| Variable . | Ibrutinib monotherapy (n = 116) . | Ibrutinib + rituximab (n = 58) . | P value . |
|---|---|---|---|
| Median age (range), y | 66 (56-71) | 66 (55-77) | .74 |
| Age >65 y, n (%) | 61 (53) | 36 (62) | .24 |
| Male sex, n (%) | 87 (75) | 34 (59) | .03 |
| Median hemoglobin (range), g/L | 105 (96-119) | 103 (92-116) | .50 |
| Hemoglobin <115 g/L, n (%) | 81 (70) | 41 (73) | .71 |
| Median platelet count (range), 103/μL | 215 (150-282) | 242 (166-340) | .15 |
| Platelet count <100 103/μL, n (%) | 11 (10) | 4 (8) | .67 |
| Median beta-2-microglobulin (range), mg/L | 5.2 (3.3-7.5) | 3.8 (3.0-5.0) | .001 |
| Beta-2-microglobulin >3.5 mg/L, n (%) | 96 (83) | 43 (74) | .18 |
| Median serum IgM (range), g/L | 38.4 (27.4-55.6) | 35.8 (18.2-51.4) | .17 |
| Serum IgM >70 g/L, n (%) | 8 (7) | 0 (0) | .04 |
| Previously treated, n (%) | 84 (72) | 27 (47) | .001 |
| CXCR4 mutations, n (%) | 44 (38) | 26 (45) | .38 |
| Low IPSSWM, n (%) | 21 (18) | 7 (13) | .67 |
| Intermediate IPSSWM, n (%) | 48 (42) | 25 (46) | |
| High IPSSWM, n (%) | 46 (40) | 22 (41) |
| Variable . | Ibrutinib monotherapy (n = 116) . | Ibrutinib + rituximab (n = 58) . | P value . |
|---|---|---|---|
| Median age (range), y | 66 (56-71) | 66 (55-77) | .74 |
| Age >65 y, n (%) | 61 (53) | 36 (62) | .24 |
| Male sex, n (%) | 87 (75) | 34 (59) | .03 |
| Median hemoglobin (range), g/L | 105 (96-119) | 103 (92-116) | .50 |
| Hemoglobin <115 g/L, n (%) | 81 (70) | 41 (73) | .71 |
| Median platelet count (range), 103/μL | 215 (150-282) | 242 (166-340) | .15 |
| Platelet count <100 103/μL, n (%) | 11 (10) | 4 (8) | .67 |
| Median beta-2-microglobulin (range), mg/L | 5.2 (3.3-7.5) | 3.8 (3.0-5.0) | .001 |
| Beta-2-microglobulin >3.5 mg/L, n (%) | 96 (83) | 43 (74) | .18 |
| Median serum IgM (range), g/L | 38.4 (27.4-55.6) | 35.8 (18.2-51.4) | .17 |
| Serum IgM >70 g/L, n (%) | 8 (7) | 0 (0) | .04 |
| Previously treated, n (%) | 84 (72) | 27 (47) | .001 |
| CXCR4 mutations, n (%) | 44 (38) | 26 (45) | .38 |
| Low IPSSWM, n (%) | 21 (18) | 7 (13) | .67 |
| Intermediate IPSSWM, n (%) | 48 (42) | 25 (46) | |
| High IPSSWM, n (%) | 46 (40) | 22 (41) |
Patients’ baseline characteristics according to CXCR4 mutational status
| Variable . | CXCR4 WT (n = 104) . | CXCR4 MUT (n = 70) . | P value . |
|---|---|---|---|
| Median age (range), y | 66 (55-71) | 66 (56-71) | .48 |
| Age >65 y, n (%) | 56 (54) | 41 (59) | .54 |
| Male sex, n (%) | 75 (72) | 46 (66) | .37 |
| Median hemoglobin (range), g/L | 102 (94-116) | 108 (99-120) | .10 |
| Hemoglobin <115 g/L, n (%) | 76 (73.8) | 46 (67.6) | .38 |
| Median platelet count (range), 103/μL | 252 (174-329) | 184 (121-247) | .001 |
| Platelet count <100 103/μL, n (%) | 4 (4) | 11 (17) | .004 |
| Median beta-2-microglobulin (range), mg/L | 5.0 (3.3-7.2) | 4.0 (3.0-7.0) | .082 |
| Beta-2-microglobulin >3.5 mg/L, n (%) | 88 (85) | 51 (73) | .058 |
| Median serum IgM (range), g/L | 33.6 (20.8-51.3) | 43.1 (32.5-58.7) | .011 |
| Serum IgM >70 g/L, n (%) | 5 (5) | 3 (4) | .87 |
| Previously treated, n (%) | 70 (67) | 41 (59) | .24 |
| Ibrutinib + rituximab, n (%) | 32 (31) | 26 (37) | .38 |
| Low IPSSWM, n (%) | 19 (18) | 9 (14) | .63 |
| Intermediate IPSSWM, n (%) | 42 (41) | 31 (47) | |
| High IPSSWM, n (%) | 42 (41) | 26 (39) |
| Variable . | CXCR4 WT (n = 104) . | CXCR4 MUT (n = 70) . | P value . |
|---|---|---|---|
| Median age (range), y | 66 (55-71) | 66 (56-71) | .48 |
| Age >65 y, n (%) | 56 (54) | 41 (59) | .54 |
| Male sex, n (%) | 75 (72) | 46 (66) | .37 |
| Median hemoglobin (range), g/L | 102 (94-116) | 108 (99-120) | .10 |
| Hemoglobin <115 g/L, n (%) | 76 (73.8) | 46 (67.6) | .38 |
| Median platelet count (range), 103/μL | 252 (174-329) | 184 (121-247) | .001 |
| Platelet count <100 103/μL, n (%) | 4 (4) | 11 (17) | .004 |
| Median beta-2-microglobulin (range), mg/L | 5.0 (3.3-7.2) | 4.0 (3.0-7.0) | .082 |
| Beta-2-microglobulin >3.5 mg/L, n (%) | 88 (85) | 51 (73) | .058 |
| Median serum IgM (range), g/L | 33.6 (20.8-51.3) | 43.1 (32.5-58.7) | .011 |
| Serum IgM >70 g/L, n (%) | 5 (5) | 3 (4) | .87 |
| Previously treated, n (%) | 70 (67) | 41 (59) | .24 |
| Ibrutinib + rituximab, n (%) | 32 (31) | 26 (37) | .38 |
| Low IPSSWM, n (%) | 19 (18) | 9 (14) | .63 |
| Intermediate IPSSWM, n (%) | 42 (41) | 31 (47) | |
| High IPSSWM, n (%) | 42 (41) | 26 (39) |
MUT, mutated; WT, wild-type.
Response to therapy
Figure 2A shows categorical responses for ibrutinib plus rituximab, and ibrutinib monotherapy. The VGPR rate for ibrutinib plus rituximab was 38% vs 28% for ibrutinib monotherapy, with an OR of VGPR of 1.54 (95% CI, 0.79-2.99; P = .21) favoring ibrutinib plus rituximab. In a multivariate model adjusting for categorical groups of age, sex, hemoglobin level, platelet count, serum beta-2-microglobulin level, treatment status, and CXCR4 mutations, ibrutinib plus rituximab was associated with an OR of VGPR of 1.54 (95% CI, 0.71-3.35; P = .27) compared with ibrutinib monotherapy. In this model, CXCR4 mutations were independently associated with lower odds of VGPR (OR, 0.31; 95% CI, 0.14-0.70; P = .005).
Categorical response analyses. Categorical responses to ibrutinib plus rituximab (I+R) and ibrutinib monotherapy (I) in the entire cohort (I, 118; I+R, 58) (A); patients with CXCR4 mutations (I, 44; I+R, 26), showing numerical improvements in VGPR (B); and patients with high IPSSWM scores (I, 46; I+R, 22) (C). Categorical responses according to CXCR4 mutational status in the entire cohort (WT, 104; mutated [MUT], 70) (D); in patients treated with I+R (WT, 32; MUT, 26) (E); and in patients treated with I alone (WT, 72; MUT, 44) (F). mR, minor response; NR, no response; PR, partial response.
Categorical response analyses. Categorical responses to ibrutinib plus rituximab (I+R) and ibrutinib monotherapy (I) in the entire cohort (I, 118; I+R, 58) (A); patients with CXCR4 mutations (I, 44; I+R, 26), showing numerical improvements in VGPR (B); and patients with high IPSSWM scores (I, 46; I+R, 22) (C). Categorical responses according to CXCR4 mutational status in the entire cohort (WT, 104; mutated [MUT], 70) (D); in patients treated with I+R (WT, 32; MUT, 26) (E); and in patients treated with I alone (WT, 72; MUT, 44) (F). mR, minor response; NR, no response; PR, partial response.
There were numerically higher odds of VGPR for ibrutinib plus rituximab over ibrutinib monotherapy in patients with CXCR4 mutations (27% vs 11%; Figure 2B), with an OR of 2.87 (95% CI, 0.81-10.3; P = .10). In a multivariate model adjusting for categorical groups of age, sex, hemoglobin level, platelet count, serum beta-2-microglobulin level, and treatment status, ibrutinib plus rituximab had numerically higher odds of VGPR with an OR of 3.61 (95% CI, 0.83-15.7; P = .09) than ibrutinib monotherapy.
There were numerically higher odds of VGPR with ibrutinib plus rituximab over ibrutinib monotherapy in patients with high IPSSWM scores (55% vs 30%; Figure 2C), with an OR of VGPR of 2.74 (95% CI, 0.96-7.82; P = .06) compared with ibrutinib monotherapy. In a multivariate model adjusting for treatment status and CXCR4 mutations, ibrutinib plus rituximab was associated with an OR of VGPR of 2.75 (95% CI, 0.87-8.76; P = .09) compared with ibrutinib monotherapy.
There were no significant differences in VGPR rates between ibrutinib plus rituximab and ibrutinib monotherapy in patients without CXCR4 mutations, patients who were TN, previously treated patients, and patients with low or intermediate IPSSWM scores (P > .05 for all subgroups).
Patients with CXCR4 mutations had lower odds of VGPR than patients without CXCR4 mutations (17% vs 42%; OR, 0.29; 95% CI, 0.14-0.61; P = .001; Figure 2D). Patients with CXCR4 mutations had numerically lower odds of VGPR in the ibrutinib plus rituximab arm (27% vs 47%; OR, 0.42; 95% CI, 0.14-1.27; P = .12; Figure 2E), and lower odds of VGPR than patients without CXCR4 mutations in the ibrutinib monotherapy group (11% vs 39%; OR, 0.20; 95% CI, 0.07-0.57; P = .003; Figure 2F). In multivariate models adjusting for categorical groups of age, sex, hemoglobin level, platelet count, serum beta-2-microglobulin level, and treatment status, CXCR4 mutations were independently associated with lower odds of VGPR in the entire cohort (OR, 0.31; 95% CI, 0.14-0.70; P = .005) and in patients treated with ibrutinib monotherapy (OR, 0.20; 95% CI, 0.07-0.63; P = .007), and had numerically lower odds of VGPR in patients treated with ibrutinib plus rituximab (OR, 0.43; 95% CI, 0.11-1.72; P = .24).
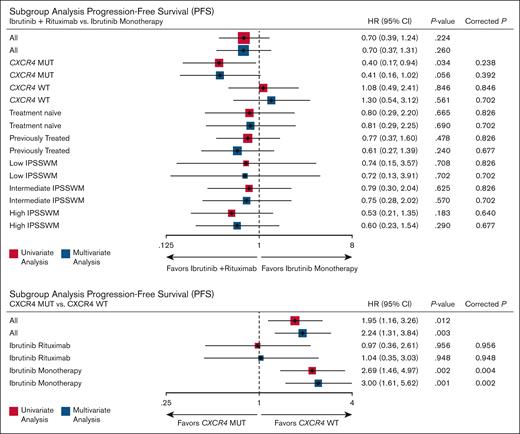
Figure 3 presents the forest plot of all subgroup analyses assessing the effect of ibrutinib plus rituximab on VGPR. No subgroups demonstrated a statistically significant benefit after adjustment for multiple testing. The only significant association after correction was the adverse impact of CXCR4 mutations on VGPR in patients receiving ibrutinib monotherapy (adjusted and corrected P = .01), an effect not observed with ibrutinib plus rituximab.
Subgroup analyses of VGPR rates comparing ibrutinib plus rituximab vs ibrutinib monotherapy (above), and the impact of CXCR4 mutations on VGPR within each treatment arm (below). False discovery rate correction was applied for multiple comparisons: 7 subgroup comparisons (above) and 2 (below).
Subgroup analyses of VGPR rates comparing ibrutinib plus rituximab vs ibrutinib monotherapy (above), and the impact of CXCR4 mutations on VGPR within each treatment arm (below). False discovery rate correction was applied for multiple comparisons: 7 subgroup comparisons (above) and 2 (below).
No significant differences in VGPR rates were observed between previously treated and untreated patients with WM (P = .98).
Survival analysis
The median follow-up time for the ibrutinib monotherapy group was longer at 54.6 months (95% CI, 50.8-57.1) than at 49.7 months (95% CI, 48.1-50.2) for the ibrutinib plus rituximab group (P < .001). There were 58 progression events (42 in the ibrutinib monotherapy, and 16 in the ibrutinib plus rituximab arm), including 15 deaths (11 in the ibrutinib monotherapy, and 4 in the ibrutinib plus rituximab arm).
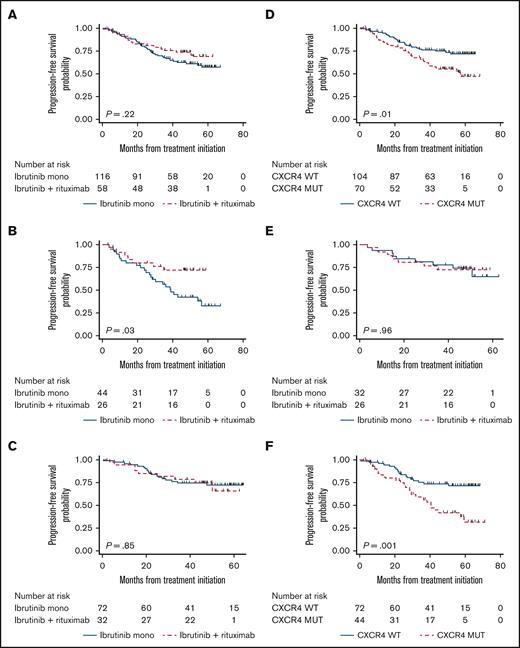
The median PFS was not reached for both treatment arms. The 48-month PFS rate was 61% (95% CI, 51-70) for ibrutinib monotherapy, and 74% (95% CI, 60-83) for ibrutinib plus rituximab for an HR of 0.70 (95% CI, 0.39-1.24; P = .22; Figure 4A). In a multivariate model adjusting for IPSSWM, treatment status, and CXCR4 mutations, ibrutinib plus rituximab had an HR of 0.70 (95% CI, 0.37-1.31; P = .26) when compared with ibrutinib monotherapy. In this model, CXCR4 mutations were associated with an HR of 2.24 (95% CI, 1.31-3.84; P = .003) compared with CXCR4 wild-type status. IPSSWM and treatment status were not statistically associated with PFS (P > .05 for all subgroups).
Survival analyses. Kaplan-Meier survival estimates comparing PFS of ibrutinib plus rituximab vs ibrutinib monotherapy for the whole cohort (A); patients with CXCR4 mutations (B); and patients without CXCR4 mutations (C). PFS according to CXCR4 mutational status for the whole cohort (D); patients treated with I+R (E); and patients treated with I alone (F).
Survival analyses. Kaplan-Meier survival estimates comparing PFS of ibrutinib plus rituximab vs ibrutinib monotherapy for the whole cohort (A); patients with CXCR4 mutations (B); and patients without CXCR4 mutations (C). PFS according to CXCR4 mutational status for the whole cohort (D); patients treated with I+R (E); and patients treated with I alone (F).
In subgroup analyses, ibrutinib plus rituximab was associated with a superior PFS in patients with CXCR4 mutations, with a 48-month PFS rate of 72% (95% CI, 51-86) vs 43% (95% CI, 27-58) for ibrutinib monotherapy, with an HR of 0.40 (95% CI, 0.17-0.94; P = .03; Figure 4B). In a multivariate model adjusting for treatment status and IPSSWM scores, ibrutinib plus rituximab was associated with an HR of 0.41 (95% CI, 0.16-1.02; P = .06) when compared with ibrutinib monotherapy in patients with CXCR4 mutations.
There were no statistical differences in PFS between arms in patients without CXCR4 mutations (Figure 4C), patients who were TN or previously treated, and patients with low IPSSWM, intermediate IPSSWM, or high IPSSWM scores (P > .05 for all subgroups).
CXCR4 mutations were associated with an inferior PFS in the entire cohort, with a 48-month PFS rate of 54% vs 73% (HR, 1.94; 95% CI, 1.16-3.26; P = .01; Figure 4D). CXCR4 mutations did not impact PFS in patients treated with ibrutinib plus rituximab with a 48-month PFS rate of 72% vs 74% (HR, 0.97; 95% CI, 0.36-2.61; P = .96; Figure 4E), and were associated with inferior PFS in patients treated with ibrutinib monotherapy with a 48-month PFS rate of 43% vs 72% (HR, 2.69; 95% CI, 1.46-4.97; P = .002; Figure 4F). In multivariate models adjusting for treatment status and IPSSWM scores, CXCR4 mutations were associated with an inferior PFS in the entire cohort (HR, 2.24; 95% CI, 1.31-3.84; P = .003), not associated with an inferior PFS in patients treated with ibrutinib plus rituximab (HR, 1.04; 95% CI, 0.35-3.03; P = .95), and associated with an inferior PFS in patients treated with ibrutinib monotherapy (HR, 3.00; 95% CI, 1.60-5.62; P = .001).
Figure 5 presents the forest plot of all subgroup analyses assessing the effect of ibrutinib plus rituximab on PFS. No subgroups demonstrated a statistically significant benefit after adjustment for multiple testing. The only significant association after correction was the adverse impact of CXCR4 mutations on PFS in patients receiving ibrutinib monotherapy (adjusted and corrected P = .002), an effect not observed with ibrutinib plus rituximab.
Subgroup analyses of PFS comparing ibrutinib plus rituximab vs ibrutinib monotherapy (above), and the impact of CXCR4 mutations on VGPR within each treatment arm (below). False discovery rate correction was applied for multiple comparisons: 7 subgroup comparisons (above) and 2 (below).
Subgroup analyses of PFS comparing ibrutinib plus rituximab vs ibrutinib monotherapy (above), and the impact of CXCR4 mutations on VGPR within each treatment arm (below). False discovery rate correction was applied for multiple comparisons: 7 subgroup comparisons (above) and 2 (below).
The median OS was not reached for both treatment arms. The 48-month OS rate was 89% (95% CI, 80-94) for ibrutinib monotherapy, and 94% (95% CI, 82-98) for ibrutinib plus rituximab for an HR of 0.64 (95% CI, 0.20-2.02; P = .45). There were no detectable differences in OS between arms in patients with or without CXCR4 mutations, patients who were TN or previously treated, and patients with low IPSSWM, intermediate IPSSWM, or high IPSSWM scores (P > .05 for all subgroups).
No differences were seen in PFS (P = .08) or OS (P = .18) across patients with previously treated and untreated WM.
Discussion
Without prospective data evaluating the effect of adding rituximab to ibrutinib in patients with WM, we compared the efficacy of rituximab plus ibrutinib vs ibrutinib monotherapy in WM using individual patient-level data from three prospective studies. Given the incidence of rituximab-related infusion reactions and flare, which can transiently worsen symptoms and limit its use in patients with serum IgM levels >40 g/L despite concomitant ibrutinib, as well as the logistical challenges of rituximab infusion vs single-agent oral ibrutinib, its routine addition to ibrutinib warrants thoughtful consideration.18,19
Notably, in the subgroup of patients with CXCR4 mutations, adding rituximab to ibrutinib demonstrated a significant PFS benefit. Preclinically, CXCR4 mutations have been shown to confer ibrutinib resistance through AkT and ERK activation despite effective BTK inhibition,20 with demonstrated clinical relevance.1,2 The near twofold increase in the 48-month PFS rate observed with rituximab and ibrutinib compared with ibrutinib alone in this study suggests a synergistic effect, supporting a potential role of rituximab in mitigating or delaying resistance in this subset of patients. Recent studies in chronic lymphocytic leukemia show that BTK inhibitors downregulate immunosuppressive molecules transcriptionally (eg, CTLA-4, CD200), enhancing T-cell cytotoxicity when combined with bispecific antibodies. These immune-modulatory effects of BTK inhibitor therapy may complement rituximab’s antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity.21,22
However, the numerically lower VGPR rates of ibrutinib plus rituximab in patients with CXCR4 mutations compared with their wild-type counterparts, along with the independent association of CXCR4 mutations with lower odds of VGPR and inferior PFS in our pooled analysis, underscore a potential unmet therapeutic need. While ibrutinib plus rituximab outperforms ibrutinib monotherapy in this subgroup, its role relative to alternative regimens in CXCR4-mutated disease remains unclear.
The phase 3 ASPEN trial demonstrated a numerically improved efficacy of zanubrutinib monotherapy over ibrutinib monotherapy in patients with WM.23,24 In patients with CXCR4 mutations, the VGPR rates for zanubrutinib and ibrutinib were 21% vs 10%, respectively (P = .35). However, patients with CXCR4 mutations had numerically lower VGPR rates than patients with CXCR4 wild-type disease treated with zanubrutinib at 21% vs 45%, respectively (P value not provided). In a recent biomarker study from ASPEN,25CXCR4 mutations were also associated with a 50% higher risk of disease progression or death in patients treated with zanubrutinib with an HR of 1.5 (95% CI, 0.8-2.0; P = .20).
The 21% VGPR rate with zanubrutinib in patients with CXCR4 mutations was only marginally <27% observed with ibrutinib plus rituximab. In comparison, the 48-month PFS rate on zanubrutinib in patients with CXCR4 mutations, though not reported, appears lower than the 74% on ibrutinib plus rituximab reported here. The advantage of the lower rates of arrhythmia and other adverse events with zanubrutinib over ibrutinib, and the absence of rituximab should be considered when counseling patients.
Acalabrutinib, tirabrutinib, and orelabrutinib are covalent BTK inhibitors, and pirtobrutinib is a noncovalent BTK inhibitor used to treat patients with WM.26-30 However, data on the impact of CXCR4 mutations on the outcomes of patients treated with these agents are unavailable.
Other highly active WM treatments, such as bendamustine plus rituximab and proteasome inhibitor-based therapies, are independent of CXCR4 status in their efficacy.31,32 However, bendamustine plus rituximab has the drawback of stem cell toxicity associated with a small risk of secondary myeloid neoplasms, while proteasome inhibitors exhibit lower overall efficacy in retrospective studies with an increased incidence of peripheral neuropathy.33,34
Our results show that rituximab provides additional benefits in patients with WM and CXCR4 mutations. Adding rituximab to zanubrutinib could also improve outcomes in this patient subgroup. Preclinical lymphoma models suggest a synergistic advantage of newer-generation BTK inhibitors in enhancing rituximab-induced antibody-dependent cellular cytotoxicity.35 Thus, the combination of BTK inhibitors plus rituximab is a particularly interesting approach for patients with CXCR4-mutated disease, and it should be evaluated in prospective clinical trials. Ibrutinib plus rituximab remains a viable option for CXCR4-mutated disease alongside other standard treatments, such as zanubrutinib and bendamustine plus rituximab. However, the benefits of adding rituximab in patients without CXCR4 mutations were not evident, and the added toxicity and logistics associated with rituximab would make this approach less preferred in this patient subgroup. However, the possibility of a modest benefit in patients with wild-type CXCR4 cannot be excluded given the limited sample size in this subgroup, although any such effect is likely to be less pronounced than in CXCR4-mutated disease.
Our findings advocate for CXCR4 mutational testing in WM, especially when considering therapy with covalent BTK inhibitors. Bone marrow aspirate is the preferred source of malignant cells for DNA isolation for CXCR4 mutational testing purposes. Multiple next-generation sequencing platforms are commercially available for CXCR4 mutation detection. Our study did not evaluate CXCR4 mutation subgroups (ie, nonsense and frameshift), as these data were unavailable in the INNOVATE study. Nonsense CXCR4 mutations were associated with worse outcomes than frameshift mutations in patients with WM treated with ibrutinib.9 Furthermore, patients with a higher mutational burden of CXCR4 S338X treated with ibrutinib appeared to have worse outcomes than patients with WM with lower mutational burden.36
This study has inherent limitations due to its pooled design. These include significant variation in follow-up durations across studies, which is important given the potential for response deepening over time; a lack of formal randomization, which increases the risk of selection bias and may have contributed to imbalances in baseline characteristics such as prior treatment status; and differences in MYD88 and CXCR4 testing methodologies (eg, polymerase chain reaction vs next-generation sequencing). In addition, limitations in the available pooled data prevented adjustment for key prognostic markers such as lactate dehydrogenase and albumin, and did not allow for a detailed analysis of causes of death. This limited the ability to assess cause-specific survival, which is particularly relevant as most patients with WM die from non-WM–related causes.37 Despite these limitations, the baseline characteristics of the pooled cohort were consistent with those of typical WM populations, and enabled a meaningful comparison that is unlikely to be achieved through a prospective clinical trial.
Our findings hint at the potential benefit of BTK inhibitors plus chemoimmunotherapy combinations, which are under investigation and have shown deep responses thus far. However, the specific patient subsets most likely to benefit remain unclear. The ongoing BRAWM study, evaluating 12 months of acalabrutinib plus 6 cycles of bendamustine and rituximab, recently reported a 64% VGPR-or-better rate.38 A phase 2 trial of zanubrutinib plus bendamustine and rituximab showed a 71% VGPR-or-better rate.39 The ZEBRA trial (ClinicalTrials.gov identifier: NCT06561347), evaluating 4 cycles of bendamustine and rituximab combined with 15 months of zanubrutinib to reduce toxicity while maintaining efficacy, is ongoing.
Conclusion
Based on this pooled analysis of patient-level data from three prospective studies, adding rituximab to ibrutinib was associated with numerical improvements in VGPR and 48-month PFS rates compared with ibrutinib monotherapy in patients with CXCR4 mutations. Our findings support CXCR4 mutational testing in WM, and the development of clinical trials adding rituximab to regimens with covalent and noncovalent BTK inhibitors.
Acknowledgments
The authors thank the WMR Fund and the Bayer Family Research Fund for their support.
Authorship
Contribution: J.J.C. designed the study; A.G. and J.J.C. analyzed the data and wrote the initial manuscript; and all the authors critically reviewed the manuscript and accepted the final manuscript.
Conflict-of-interest disclosure: S.S. received research funds or honoraria from ADC Therapeutics, BeiGene, and Cellectar. A.R.B. received research funds and/or honoraria from Adaptive, BeiGene, CSL Behring, Genzyme, Karyopharm, Pharmacyclics, and Sanofi. G.v.K. received research funds or honoraria from AbbVie, Eli Lilly, and Pharmacyclics. S.P.T. received research funding and/or consulting fees from AbbVie/Pharmacyclics, Janssen, BeiGene Inc, Eli Lilly, Bristol Myers Squibb, and Ono Pharmaceuticals; and is a named inventor of MYD88 and CXCR4 testing for Waldenström macroglobulinemia, and has assigned all interests to their institution. J.J.C. received research funds or honoraria from AbbVie, AstraZeneca, BeiGene, Cellectar, Johnson & Johnson, Loxo, Mustang Bio, Nurix, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Jorge J. Castillo, Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 221, Boston, MA 02215; email: jorgej_castillo@dfci.harvard.edu.
References
Author notes
Deidentified data are available on request from the corresponding author, Jorge J. Castillo (jorgej_castillo@dfci.harvard.edu).

![Categorical response analyses. Categorical responses to ibrutinib plus rituximab (I+R) and ibrutinib monotherapy (I) in the entire cohort (I, 118; I+R, 58) (A); patients with CXCR4 mutations (I, 44; I+R, 26), showing numerical improvements in VGPR (B); and patients with high IPSSWM scores (I, 46; I+R, 22) (C). Categorical responses according to CXCR4 mutational status in the entire cohort (WT, 104; mutated [MUT], 70) (D); in patients treated with I+R (WT, 32; MUT, 26) (E); and in patients treated with I alone (WT, 72; MUT, 44) (F). mR, minor response; NR, no response; PR, partial response.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/18/10.1182_bloodadvances.2025016536/2/m_blooda_adv-2025-016536-gr2.jpeg?Expires=1769080097&Signature=PBam9HmKnc-lE9SCHiYT49vNsbni-8HkPmZvaILbqCda5S~WopMuZL53lYQ64ENADSh-jJw9kHYnVfytKdGRNbXx3t7uRklaYLzRNazQvieO8Dg65EevuJRRUtzRKr5onA3TfxvvnFrtyb4hoU7pkGfOqjifUgfcXrOHyGqeLm2-YAzNp4Kj3~CqoNiCyj0anDkWId0uEa3DOS1M~UVEm77YI1Z0urDQ7cAdHTxES2h5CD6bDrgHZ3-2rBg1~t4XsIUBS2IRWJJ~ePmi-Ry-nXwBj-6vUd7xF-WKUvV9AmnQ2sm9wfx5gHC~CEkbPz6ZN5JnbEg~G-l3tSwy0NQKAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)