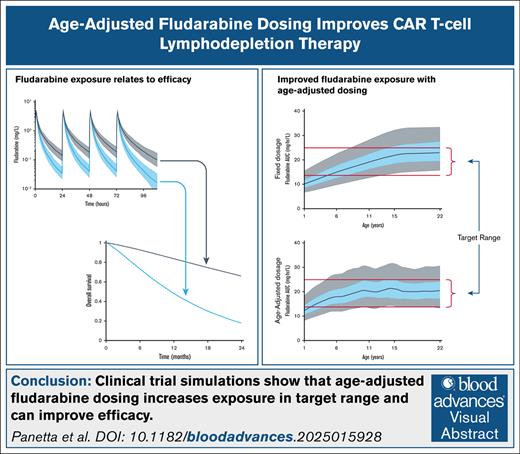
Key Points
This study shows that age-based FLU dosing can increase the number of individuals within a predefined cumulative area under the curve range.
Age-based or TDM-guided FLU dosing has the potential to improve CAR T-cell patient outcomes.
Visual Abstract
Fludarabine (FLU) is used for lymphodepletion and improves the persistence and expansion of chimeric antigen receptor (CAR) T cells in vivo. Higher FLU systemic exposure is associated with lower relapse risk and improved leukemia-free survival in pediatric patients with acute lymphoblastic leukemia treated with CD19 CAR T-cell therapy. FLU pharmacokinetics (PKs) is age dependent, with increased clearance in younger children. Here, we used modeling and simulation, including clinical trial simulations, to define age-adjusted FLU dosage regimens that may maintain effective FLU exposures and improve outcomes. The FLU PK and pharmacodynamic relationships with overall survival (OS) and cumulative incidence of relapse (CIR) were derived from published pediatric populations. Four FLU dosages were considered for the simulations: 75 or 120 mg/m2 cumulative fixed dose, age-adjusted dosing, and doses based on therapeutic drug monitoring (TDM). The target FLU cumulative area under the curve range was defined as 13.8 to 25 mg × h/L. Clinical trial simulations showed that across the pediatric age range, the number of individuals in the target range increased from a median of 22% to 61% with fixed dosages, to 72% with age-adjusted dosing and 94% with TDM. Clinical trial simulations also showed that age-adjusted or TDM dosing could increase the median number of individuals with OS at 24 months by 67% and decrease the median number of individuals with CIR at 12 months by 72%, compared with fixed dosages. In conclusion, these simulation studies support using FLU age-adjusted or TDM dosing to increase the number of individuals achieving exposure within the targeted range and, therefore, improve clinical outcomes.
Introduction
Lymphodepleting chemotherapy administered before the infusion of chimeric antigen receptor (CAR) T cells is critical for their activity in vivo.1-3 It creates an environment conducive to CAR T-cell expansion by eliminating lymphocytes, including inhibitory regulatory T cells and other immune cells, and increasing the availability of proinflammatory cytokines and chemokines, which are normally bound to endogenous immune cells.3-6 Fludarabine (FLU)- and cyclophosphamide (CY)-based lymphodepletion chemotherapy regimens have become the standard before the administration of CAR T cells in pediatric and adult patients.4,7-14
Two publications have evaluated the relationship between FLU exposure and clinical outcomes experienced by pediatric and adolescent, and young adult (AYA) patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL), treated with FLU/CY followed by infusion of CD19 CAR T cells.15,16 One study retrospectively analyzed results from children and young adults (N = 152) with relapsed/refractory B-ALL treated with tisagenlecleucel (Kymriah) infusion after FLU/CY lymphodepletion. They determined that a FLU cumulative systemic area under the curve (AUC) of ≥13.8 mg × h/L was associated with a 2.5-fold lower risk of relapse and twofold lower risk of a composite end point of loss of B-cell aplasia or relapse.15 Separately, another study prospectively analyzed collected FLU pharmacokinetic (PK) data in children and AYA patients (N = 26) with relapsed/refractory B-ALL also receiving treatment with FLU/CY and tisagenlecleucel. They determined that a FLU cumulative AUC of ≥14 mg × h/L correlated with improved leukemia-free survival and lower occurrence of CD19+ leukemic relapse.16 A recent study in adults with B-cell non-Hodgkin lymphoma estimated that a FLU optimal cumulative AUC range of 18 to 20 mg × h/L was associated with improved outcomes.17 Finally, in the context of conditioning therapy for allogeneic hematopoietic cell transplantation (HCT), individuals with a cumulative FLU AUC of >15 mg × h/L had significantly higher disease-free survival (83% vs 53%; P = .04).18
Toxicities have also been observed in recipients of allogeneic HCT with higher FLU AUC. These include data demonstrating a 3.4-fold increased risk of nonrelapse mortality (FLU AUC of >25 mg × h/L),19 a 4.6-fold increase in risk of therapy-related mortality (FLU AUC of >26 mg × h/L),20 a 2.7-fold increase in nonrelapse mortality (FLU AUC of >24 mg × h/L),21 and increased rates of immune effector cell–associated neurotoxicity syndrome (ICANS; FLU AUC of >24 mg × h/L).18 Furthermore, in the context of CAR T-cell lymphodepletion therapy, high FLU exposure (FLU AUC of >20 mg × h/L) was associated with an increased risk of ICANS (grade ≥1) but not cytokine release syndrome in adult patients treated with axicabtagene ciloleucel.17 However, in pediatric patients treated with tisagenlecleucel, optimal vs suboptimal FLU exposure was not associated with increased cytokine release syndrome (grade ≥3) or ICANS (grade ≥1 or ≥3).15
Notably, although these data collectively suggest that an optimal FLU exposure range of 13.8 to 25 mg × h/L is necessary to improve patient outcomes, FLU PKs are known to be highly variable, with exposures after fixed doses varying as much as sixfold.16,18,22 This high variability translates to a large percentage of FLU exposures outside the suggested therapeutic range of 13.8 to 25 mg × h/L. In addition, FLU clearance is higher in lower-weight/younger individuals, making that subpopulation more likely to be underexposed.18,22
In this study, we retrospectively simulated FLU PKs for a cohort of pediatric and AYA patients with relapsed/refractory B-ALL treated on an investigator-initiated CD19 CAR T-cell therapy trial (SJCAR19; ClinicalTrials.gov identifier: NCT03573700), in which patients received FLU/CY lymphodepletion followed by an infusion of an autologous CD19.41BBζ-CAR T-cell product.9 Our primary goal was to use modeling and simulation to investigate the feasibility of prospectively adjusting FLU dosages based on patient age and, therefore, anticipated FLU clearance. Specifically, we evaluated whether age-adjusted or therapeutic drug monitoring (TDM) vs fixed dosages of FLU would lead to a higher proportion of patients in the target FLU cumulative AUC range of 13.8 to 25 mg × h/L. In addition, based on the historically observed relationships between FLU cumulative exposure and outcomes, our secondary goal was to determine the cumulative incidence of relapse (CIR) and overall survival (OS) among patient groups.
Methods
PK simulation methods
The PK approach used simulations based on population FLU PKs from a published study in a pediatric HCT conditioning regimen.22 The FLU cumulative AUC was used to define the target range of exposure. Based on published results relating FLU AUC to efficacy and toxicity outcomes, we defined the optimal therapeutic window for FLU AUC to be between 13.8 and 25 mg × h/L.15-17,19-21 Three different FLU fixed-dosing schedules were considered for the simulations (Table 1). They included: (1) the dosing schedule used in our clinical study (25 mg/m2 per day for 3 days; cumulative 75 mg/m2),9 (2) the dosing schedule used in previously published studies of tisagenlecleucel (30 mg/m2 per day for 4 days; cumulative 120 mg/m2),15,16 and (3) a newly developed age-adjusted dosing schedule based on the observed relationship between age and FLU PKs.22 In this age-adjusted model, age was divided into 4 categories, with FLU dosages ranging between 26.25 and 37 mg/m2 per day for 4 days. In addition, a TDM dosing approach was considered, such that FLU dosage was adjusted in real time based on an individual’s FLU PKs. Specifically, each daily dose on days 2 to 4 was adjusted based on the individual’s PKs from the previous day to target a cumulative FLU AUC of 19.4 mg × h/L, the middle of the therapeutic window. The maximum single FLU dosage was set to 40 mg/m2 because of toxicity risks of higher dosages. Therefore, the largest cumulative FLU dosage available for the TDM approach was 160 mg/m2.
FLU dosing regimens
| Age group, y . | Fixed dosing: SJCAR19 . | Fixed dosing: tisagenlecleucel . | Age-adjusted dosing . |
|---|---|---|---|
| <6 | 25 mg/m2 per d × 3 d | 30 mg/m2 per d × 4 d | 37 mg/m2 per d × 4 d |
| ≥6 and <11 | 33.75 mg/m2 per d × 4 d | ||
| ≥11 and <15 | 30 mg/m2 per d × 4 d | ||
| ≥15 | 26.25 mg/m2 per d × 4 d |
| Age group, y . | Fixed dosing: SJCAR19 . | Fixed dosing: tisagenlecleucel . | Age-adjusted dosing . |
|---|---|---|---|
| <6 | 25 mg/m2 per d × 3 d | 30 mg/m2 per d × 4 d | 37 mg/m2 per d × 4 d |
| ≥6 and <11 | 33.75 mg/m2 per d × 4 d | ||
| ≥11 and <15 | 30 mg/m2 per d × 4 d | ||
| ≥15 | 26.25 mg/m2 per d × 4 d |
For each dosing schedule, 1000 randomly sampled PK studies with individuals uniformly distributed over the age range of 1 to 22 years were simulated. The Centers for Disease Control and Prevention growth charts23 were used to estimate the median weight for the age and sex of each individual. Furthermore, it was assumed that individuals had normal renal function (ie, estimated glomerular filtration rate of 140 mL/min per 1.73 m2). Based on these simulated PK studies, the cumulative FLU AUC was calculated for each dosing schedule, and the percentage of studies in the target range was determined.
Clinical trial simulation methods
Clinical trial simulations were based on a study size of 18 participants. Resampling with replacement, accounting for the expected age distribution of a pediatric and AYA clinical study, was used from the aforementioned simulated PK studies to generate a series of simulated clinical trials (N = 1000). For each simulated clinical trial, the number of individuals of 18 with a cumulative FLU AUC in the target range was evaluated, and the median and 95% prediction interval (95% PI) of all the simulated clinical trials were reported.
Next, clinical trial simulations were used to generate simulated clinical outcomes after FLU/CY/CAR T-cell treatment. This included the expected number of individuals who would experience disease relapse or death after treatment, given each dosing regimen. These simulations assumed similar efficacy of CD19 CAR T-cell products across FLU dosing regimens (OS at 24 months, 56.5% [95% confidence interval, 41.8-71.2]15; CIR at 12 months, 52.7% [95% confidence interval, 10.9-94.5]).16 A Weibull hazard function was used to fit the AUC-based outcomes data reported by Fabrizio et al and Dekker et al (supplemental Figure 1).15,16 These models were then used to quantify the probability of either CIR at 12 months or OS at 24 months after CAR T-cell treatment, relative to the simulated FLU cumulative AUC. The median and 95% PI of individuals with CIR and OS were reported. Finally, a successful simulated clinical trial was defined as achieving a FLU cumulative AUC in the target range in ≥67% of participants, OS at 24 months of ≥55%, or CIR at 12 months of ≤40%. This survival threshold was chosen because it is higher than the median number of individuals aged <10 years expected to survive at least 24 months, given the fixed dosage of 30 mg/m2 per day for 4 days (50%).15 The relapse threshold was chosen because it is lower than the median number of individuals aged <10 years expected to relapse within 12 months, given the fixed dosage of 30 mg/m2 per day for 4 days (50%).16
Results
FLU PK simulations support age-adjusted dosing
Based on previous population PK studies,18,22 it is anticipated that FLU clearance will be significantly higher in lower-weight and younger individuals. Simulation of the expected FLU PK exposure in a 5-year-old vs 15-year-old shows that the median clearance is expected to be 65% higher in the younger individual (Figure 1A). To assess the impact of FLU clearance differences on cumulative AUC across the age range, PK simulations were used to generate expected AUC for patients treated with each of the 3 fixed-dosage regimens (Figure 1B-C). Based on PK simulations, the expected variability in FLU clearance results in a low percentage of individuals with FLU AUC inside the target range (13.8-25 mg × h/L) for both fixed-dosage regimens (25 mg/m2 per day × 3 days: 29%, Figure 1B; 30 mg/m2 per day × 4 days: 60%, Figure 1C). These simulations also demonstrated differences in FLU clearance across the pediatric age range. Using a FLU regimen of 25 mg/m2 per day for 3 days, it was predicted that ≥75% of individuals aged <11 years would have FLU exposure below the lower AUC threshold of 13.8 mg × h/L (Figure 1B). With an increase of the FLU dosage to 30 mg/m2 per day for 4 days, the predicted percent of individuals aged <11 years in the target exposure range improved; however, there were still ≥50% of individuals aged ≤6 years with predicted exposures below the therapeutic window (Figure 1C). In addition, in this cohort, individuals aged ≥15 years had a higher percentage of predicted exposures above the upper threshold of 25 mg × h/L (25%; Figure 1C). Next, we evaluated an age-adjusted FLU dosing schema (Table 1). As hypothesized, the age-adjusted FLU dosing model improved the percentage of individuals falling within the FLU AUC target range to 75% (Figure 1D). Finally, we evaluated the TDM approach. The percentage of individuals across the age range within the FLU AUC target range was 94%. However, most of the individuals who were not in the target range were aged <5 years. Specifically, 99% of the individuals aged >5 years were in the target range, whereas only 73% of the individuals aged ≤5 years were within target range. These younger individuals were under the target range because of the estimated higher FLU clearance and the limitation on the maximum FLU dosage.
Simulated FLU AUC vs age. (A) FLU concentration vs time given 30 mg/m2 per day for 4 days based on the population PKs from Langenhorst et al.22 Curve and blue shading: median and quartile range for a person aged 5 years; curve and gray shading: median and quartile range for a person aged 15 years. (B-D) Cumulative FLU AUC vs age for different FLU dosing regimens. (B) FLU 25 mg/m2 per day for 3 days. (C) FLU 30 mg/m2 per day for 4 days. (D) Age-adjusted FLU dose (37 mg/m2 per day × 4 days for age <6 years; 33.75 mg/m2 per day × 4 days for age ≥6 but <11 years; 30 mg/m2 per day × 4 days for age ≥11 but <15 years; and 26.25 mg/m2 per day × 4 days for age ≥15 years). Panels B-D: median (black curve), 25th to 75th percentiles (blue shaded region), and 5th to 95th percentiles (gray shaded region).
Simulated FLU AUC vs age. (A) FLU concentration vs time given 30 mg/m2 per day for 4 days based on the population PKs from Langenhorst et al.22 Curve and blue shading: median and quartile range for a person aged 5 years; curve and gray shading: median and quartile range for a person aged 15 years. (B-D) Cumulative FLU AUC vs age for different FLU dosing regimens. (B) FLU 25 mg/m2 per day for 3 days. (C) FLU 30 mg/m2 per day for 4 days. (D) Age-adjusted FLU dose (37 mg/m2 per day × 4 days for age <6 years; 33.75 mg/m2 per day × 4 days for age ≥6 but <11 years; 30 mg/m2 per day × 4 days for age ≥11 but <15 years; and 26.25 mg/m2 per day × 4 days for age ≥15 years). Panels B-D: median (black curve), 25th to 75th percentiles (blue shaded region), and 5th to 95th percentiles (gray shaded region).
Clinical trial simulations: age-adjusted dosing increases the number of individuals in the FLU target exposure range
Given the relatively small sample size expected in a single-institution pediatric and AYA CAR T-cell clinical trial, we used clinical trial simulations (N = 1000 trials; n = 18 participants per trial) to determine the feasibility of successfully observing an increase in the number of individuals within the target FLU exposure range using different FLU dosage schemas. For both fixed-dosage regimens, clinical trial simulations estimated a low median percentage of individuals falling within the target FLU AUC range (25 mg/m2 per day × 3 days, 22% [95% PI, 6-39]; 30 mg/m2 per day × 4 days, 61% [95% PI, 39-83]; Table 2; Figure 2). However, with age-adjusted and TDM dosing, the median percentage of individuals within the target FLU AUC range increased to 72% (95% PI, 50-89) and 94% (95% PI, 78-100), respectively.
Percentage of individuals in the FLU cumulative AUC target range based on clinical trial simulations
| Dosing regimen . | Individuals in target AUC range, median (25th-75th percentile),∗ % . |
|---|---|
| 25 mg/m2 per d × 3 d | 22 (17-28) |
| 30 mg/m2 per d × 4 d | 61 (56-67) |
| Age-adjusted dosing | 72 (67-78) |
| TDM dosing | 94 (89-100) |
| Dosing regimen . | Individuals in target AUC range, median (25th-75th percentile),∗ % . |
|---|---|
| 25 mg/m2 per d × 3 d | 22 (17-28) |
| 30 mg/m2 per d × 4 d | 61 (56-67) |
| Age-adjusted dosing | 72 (67-78) |
| TDM dosing | 94 (89-100) |
The percentage is based on a study sample size of 18.
Clinical trial simulation of the percentage of individuals with a FLU cumulative AUC in the target range with different dosing schemas. Simulated percentage of individuals (of a study sample size of 18) with a cumulative FLU AUC in the target range of AUC of ≥13.8 to ≤25 mg × h/L using 4 different FLU dosing regimens. R1, 25 mg/m2 per day for 3 days; R2, 30 mg/m2 per day for 4 days; R3, age-adjusted dosing; or R4, TDM-guided dosing. Horizontal line, median; box, 25th to 75th percentile; whiskers, quartile range.
Clinical trial simulation of the percentage of individuals with a FLU cumulative AUC in the target range with different dosing schemas. Simulated percentage of individuals (of a study sample size of 18) with a cumulative FLU AUC in the target range of AUC of ≥13.8 to ≤25 mg × h/L using 4 different FLU dosing regimens. R1, 25 mg/m2 per day for 3 days; R2, 30 mg/m2 per day for 4 days; R3, age-adjusted dosing; or R4, TDM-guided dosing. Horizontal line, median; box, 25th to 75th percentile; whiskers, quartile range.
Next, the clinical trial simulations were used to determine the probability of successfully targeting FLU in at least 12 of 18 patients (67%). This threshold was chosen because it is higher than the median number of individuals expected to be in target given the fixed dosage of 30 mg/m2 per day for 4 days (61%). Both age-adjusted and TDM-guided FLU dosing showed >80% probability of observing a successful clinical trial. Specifically, 82% and 100% of simulated clinical trials were successfully targeted for age-based and TDM-guided dosing, respectively. In contrast, only 42% and 0% of simulated clinical trials were successfully targeted when given the fixed FLU dosages of 30 mg/m2 per dose for 4 days and 25 mg/m2 per day for 3 days, respectively.
Clinical trial simulations: age-adjusted dosing increases the probability of OS and decreases the probability of CIR
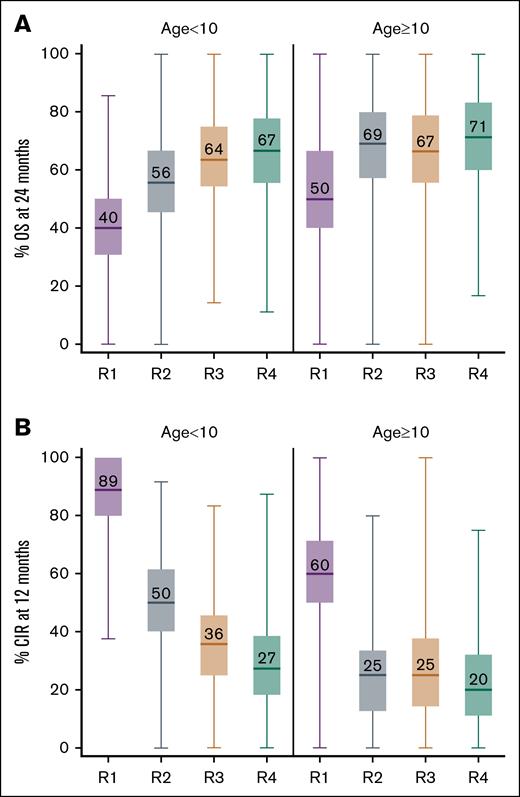
Clinical trial simulations predicted a higher OS for individuals who received age-adjusted or TDM FLU dosing compared with the other fixed-dose schedules (Figure 3A). This difference was most prominent for individuals aged <10 years (median OS: 40% [95% PI, 13-67], 56% [95% PI, 27-85], 64% [95% PI, 33-91], and 67% [95% PI, 33-100] for FLU dosing of 25 mg/m2 per day × 3 days, 30 mg/m2 per day × 4 days, age-adjusted, and TDM dosing, respectively; Figure 3A). Similarly, the clinical trial simulations predicted a decrease in CIR at 12 months among individuals who received age-adjusted dosing compared with either fixed-dosage schedule (Figure 3B). Again, this difference was largest for individuals aged <10 years (median CIR: 89% [95% PI, 64-100], 50% [95% PI, 20-82], 36% [95% PI, 9-67], and 27% [95% PI, 0-60] for FLU dosing of 25 mg/m2 per day × 3 days, 30 mg/m2 per day × 4 days, age-adjusted, and TDM dosing, respectively; Figure 3B). These differences in clinical outcomes for younger individuals (aged <10 years) correlate with anticipated lower cumulative FLU AUC when using a fixed-dose regimen (Figure 1). In addition, for patients aged ≥10 years, the simulations predicted a similar OS and CIR in patients receiving either a fixed dosage of 30 mg/m2 per day for 4 days or age-adjusted dosing (Figure 3B).
Clinical trial simulation of the percentage of individuals with either OS at 24 months or CIR at 12 months. Simulated percentage of individuals (of a study sample size of 18) with OS at 24 months (A) and CIR at 12 months (B) using 4 different FLU dosing regimens. R1, 25 mg/m2 per day for 3 days; R2, 30 mg/m2 per day for 4 days; R3, age-adjusted dosing; or R4, TDM-guided dosing. Horizontal line, median; box, 25th to 75th percentile; whiskers, quartile range.
Clinical trial simulation of the percentage of individuals with either OS at 24 months or CIR at 12 months. Simulated percentage of individuals (of a study sample size of 18) with OS at 24 months (A) and CIR at 12 months (B) using 4 different FLU dosing regimens. R1, 25 mg/m2 per day for 3 days; R2, 30 mg/m2 per day for 4 days; R3, age-adjusted dosing; or R4, TDM-guided dosing. Horizontal line, median; box, 25th to 75th percentile; whiskers, quartile range.
Next, clinical trial simulations were used to determine the probability of observing an OS of 55% at 24 months, and a CIR of 40% relapse at 12 months. Both age-adjusted and TDM-guided FLU dosing showed >80% probability of observing a successful clinical trial for either outcome. Specifically, for OS, 85% and 90% of simulated clinical trials were successful using age-adjusted and TDM-guided dosing, respectively, compared with 77% and 26% of simulated clinical trials with fixed dosages of 30 mg/m2 per dose for 4 days and 25 mg/m2 per day for 3 days, respectively. Similarly, for CIR, 82% and 92% of simulated clinical trials were successful using age-adjusted and TDM-guided dosing, respectively. This compared with only 58% and 0% of simulated clinical trials being successful when using a fixed FLU dosage of 30 mg/m2 per dose for 4 days or 25 mg/m2 per day for 3 days, respectively.
Discussion
The necessity of lymphodepletion before CAR T-cell therapy is well established, and there is increasing evidence that FLU exposure affects clinical outcomes in pediatric and AYA patients with B-ALL, treated with CD19 CAR T-cell therapy.15,16 In this study, we conducted clinical trial simulations to test whether dose adjustments to FLU could improve the number of individuals achieving a desired cumulative FLU AUC (target range, 13.8-25 mg × h/L), and correlate FLU AUC to clinical outcomes (OS and CIR). We found that using an age-adjusted FLU dosing schema (Table 1), in which the FLU dosage is increased in younger individuals with higher clearance and decreased in older individuals with lower clearance, increased the percentage of patients in the target exposure range to 75%. This compared with only 29% to 60% when using current fixed-dose FLU regimens (either 25 mg/m2 per day × 3 days or 30 mg/m2 per dose × 4 days, respectively). Furthermore, these simulations showed improvements in both OS and CIR among those receiving age-adjusted dosing. This highlights the potential of using more precise FLU dosing to improve clinical outcomes in pediatric and AYA patients treated with CAR T-cell therapy.
Considering the challenges of conducting large prospective pediatric CAR T-cell trials, we used clinical trial simulations to investigate whether age-adjusted FLU dosing would (1) increase the number of individuals within the target AUC range, and (2) would improve OS and decrease CIR. This approach partially addressed the issue of limited participants and lack of statistical power to evaluate outcomes in prospective clinical trials. These simulations assessed the feasibility of conducting a successful trial, defined as maintaining 12 of 18 individuals in the FLU cumulative exposure target range, 10 of 18 individuals with OS at 24 months, and 7 of 18 individuals with CIR at 12 months. In all cases, the simulations demonstrated that age-adjusted dosing provided >80% power to detect each of these measures vs <80% power using currently used FLU fixed-dosage schedules.
In addition, we sought to determine how TDM-based FLU dosing compared with the proposed age-adjusted dosing regimen. The TDM-based dosing simulations predicted that 94% of patients would be within the target AUC range compared with the predicted 75% targeting success rate using age-adjusted dosing. This exercise showed an improvement in the power to detect at least 12 of 18 individuals within the FLU exposure target range using TDM-based dosing relative to age-adjusted dosing (100% vs 82%). Notably, relative to age-based dosing, simulations with TDM-based dosing showed only nominal improvements in OS and CIR. In addition, TDM would primarily benefit those aged <6 years, who are expected to have the highest FLU clearance and thus require the highest FLU dosages. However, simulations show that some younger patients would require FLU dosages higher than are typically acceptable because of toxicity risk thus limiting the likelihood of achieving optimal FLU exposure in this subgroup of individuals. Overall, TDM dosing is resource-intensive, may not be accessible to all institutions/treating providers, and requires additional blood draws and more time in the clinic for patients. Therefore, we propose that any added benefit to using TDM in this clinical setting must be assessed in conjunction with the increased burden required of patients, providers, and institutions. The proposed age-adjusted FLU dosing may be more feasible to implement widely while still improving the percentage of studies within the target FLU AUC range and, therefore, enhance patient outcomes when compared with currently used fixed-dose FLU regimens. In cases in which TDM is feasible, using age-based dosing may improve the likelihood of achieving the optimal FLU exposure quicker.
There are a few limitations in this simulation study. First, the population FLU PK model used for the simulations was based on a HCT study, because there were no available data from FLU PKs in CAR T-cell studies.22 Therefore, we assumed that FLU PKs are similar between these 2 clinical indications and patient populations. This is a reasonable assumption based on the recent relationships identified between FLU exposure and CAR T-cell therapy outcomes.15,16 These studies independently determined the cumulative threshold AUC to be 13.8 and 14 mg × h/L, with 1 group using prospective PK sampling and the other using simulations based on a previously published population PK model18 to arrive at those estimates. This suggests that simulations on a population scale can be highly accurate in this context. Another limitation relates to the CAR T-cell product. The previously reported relationship between FLU exposure and outcome was based on OS and CIR from individuals treated with tisagenlecleucel.15,16 Therefore, it is possible that when using a different CAR T-cell product, these estimations will change, and results may not be widely generalizable. However, in an adult patient population with lymphoma treated with a different CAR T-cell product, similar relationships were seen.17 This suggests that FLU PKs may be important across patient populations and products. However, to help address these limitations, prospective testing of FLU PKs is necessary, including in different patient populations treated with different CAR T-cell products. Furthermore, the clinical trial simulations in this study suggest a high probability of validating the effectiveness of age-adjusted dosing in a modest-size study with 18 individuals. Finally, CY exposure was not considered in this study because it has not been studied in the context of lymphodepletion therapy for CAR T-cell treatment. Given the variability of CY and its active metabolites,24-26 evaluating its PKs may also be informative in quantifying lymphodepletion and CAR T-cell outcomes. Therefore, work at our institution is underway to conduct both FLU and CY PK studies in the context of CAR T-cell therapies. Ultimately, the pharmacodynamic effects of lymphodepletion therapy on host cytokine and chemokines must be considered to define optimal conditioning before CAR T-cell administration.
In summary, exposure to FLU is variable in pediatric and AYA patients treated with CAR T-cell therapy and critically affects survival. Our modeling and clinical trial simulation approaches provide evidence that age-adjusted or TDM-guided FLU dosing in pediatric CAR T-cell studies improves the proportion of patients achieving optimal FLU exposure and increases survival outcomes. Therefore, we suggest incorporating PK monitoring in clinical trials to further understand this effect, and we are currently embarking on prospective clinical trials to validate our findings.
Acknowledgments
This research included work conducted by the Center for Translational Pharmacology, which is supported, in part, by the American Lebanese Syrian Associated Charities of St. Jude Children's Research Hospital and the National Institutes of Health (NIH)/National Cancer Institute (NCI) grant (P30 CA021765 [M.L.]). This work was also supported by the NIH/NCI grant 1K08CA279927-01A1 (A.C.T.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: J.C.P. and M.L. wrote the manuscript; J.C.P., M.L., S.N., A.C.T., and S.G. designed the research; J.C.P., S.N., and A.C.T. performed the research; J.C.P., S.N., and A.C.T. analyzed the data; and all authors reviewed and approved the final draft of the manuscript.
Conflict-of-interest disclosure: S.G. is a member of the data safety monitoring board of Immatics; serves on the scientific advisory board of Be Biopharma; served as a consultant for Cargo Therapeutics within the last 12 months; and has patents and patent applications in the fields of T cell and/or gene therapy for cancer. The remaining authors declare no competing financial interests.
Correspondence: Markos Leggas, Department of Pharmacy and Pharmaceutical Sciences, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Mail Stop 313, Memphis, TN 38105-2794; email: mark.leggas@stjude.org.
References
Author notes
Original data are available on request from the corresponding author, Markos Leggas (mark.leggas@stjude.org).
The full-text version of this article contains a data supplement.