TO THE EDITOR:
Inhibition of the antiapoptotic protein B-cell lymphoma 2 (BCL-2) with venetoclax is central to several non–DNA-damaging treatment regimens for chronic lymphocytic leukemia (CLL) and acute myeloid leukemia. We previously described deleterious BAX variants outside the tumor compartment in patients with CLL on venetoclax as an adaptive response of normal hematopoiesis to sustained BCL-2 inhibition.1 Moreover, BAX variants have also recently emerged as a rare driver in age-related clonal hematopoiesis.2
In the first clinical study of venetoclax in solid tumors, we evaluated BCL-2 inhibition in estrogen receptor–positive metastatic breast cancer (mBEP [ACTRN12615000702516]),3 in which BCL-2 is overexpressed in 85% of patient tumors.4 This unique cohort allowed us to explore the emerging phenomenon of BAX-mutated clonal hematopoiesis during venetoclax therapy in patients without hematologic neoplasms. Seventeen of 41 patients were included based on available peripheral blood mononuclear cell samples; baseline trial cohort characteristics are in supplemental Table 1. Patients received venetoclax (800 mg [n = 16] or 400 mg daily [n = 1]) with tamoxifen until disease progression. An age-matched control cohort with metastatic estrogen receptor-positive breast cancer without prior venetoclax exposure was selected from a local trial cohort; characteristics are summarized in supplemental Table 2. The mBEP cohort had more prior therapies, but prior cytotoxic therapy exposure and the types of cytotoxic agents were similar between cohorts. Error-corrected targeted next-generation sequencing (NGS) panel (QIAseq targeted DNA) was performed with 0.5% variant allele frequency (VAF) sensitivity, as described.5 This study followed the Declaration of Helsinki with institutional ethics committee approval (Peter MacCallum Cancer Centre HREC 15/72; 17/133; 03/09; Melbourne Health HREC/14/MH/332).
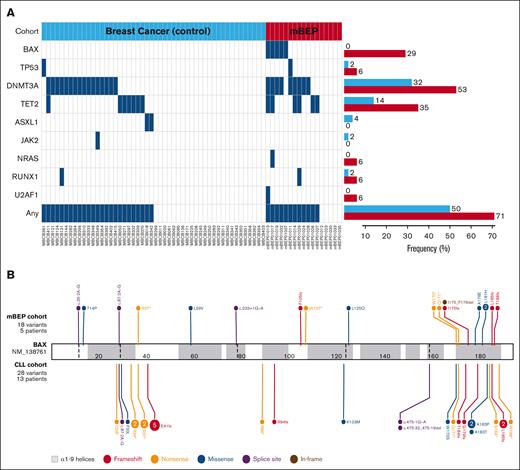
After venetoclax therapy, 18 BAX mutations were detected in 5 of 17 patients (29%) in blood samples, whereas no BAX mutations were found in the control cohort (P < .001 [Fisher exact test]; supplemental Figure 1). In contrast, other clonal hematopoiesis gene mutations were common in both the mBEP and control cohorts (12 [71%] and 25 patients [50%], respectively), most commonly in DNMT3A (53% vs 32%) and TET2 (29% vs 14%); none were statistically significant (Figure 1A).
Comparative mutational landscape. (A) Mutational profile of control (blue; n = 50) and mBEP (red; n = 17) breast cancer cohorts. Each column represents an individual patient. Blue rectangles indicate the presence of mutations in specific genes. The frequency of each mutation is represented by horizontal bars (right), according to the patient cohorts. (B) Lollipop plot of 18 BAX variants (NM_138761.3) detected in 5 patients with breast cancer after venetoclax treatment (mBEP cohort; top), and the previously described 28 BAX variants in 13 patients with CLL cohort (bottom). Created with ProteinPaint. Zhou X. (2016). https://viz.stjude.cloud/tools/proteinpaint.
Comparative mutational landscape. (A) Mutational profile of control (blue; n = 50) and mBEP (red; n = 17) breast cancer cohorts. Each column represents an individual patient. Blue rectangles indicate the presence of mutations in specific genes. The frequency of each mutation is represented by horizontal bars (right), according to the patient cohorts. (B) Lollipop plot of 18 BAX variants (NM_138761.3) detected in 5 patients with breast cancer after venetoclax treatment (mBEP cohort; top), and the previously described 28 BAX variants in 13 patients with CLL cohort (bottom). Created with ProteinPaint. Zhou X. (2016). https://viz.stjude.cloud/tools/proteinpaint.
The genomic characteristics of the BAX (NM_138761.3) mutations are summarized in Figure 1B and supplemental Table 3, alongside previously reported BAX mutations in patients with CLL1 for comparison. The median VAF was 0.95% (range, 0.5-16.0), with only 2 variants having >2% VAF. In our prior CLL study, the median VAF was 1.55%, and 12 of 28 variants (43%) had >2% VAF. Three patients had a single detectable BAX mutation, 1 patient had 2 mutations, and 1 had a remarkable 13 different mutations. Seven mutations (39%) were missense (n = 6) or in-frame (n = 1), and 11 (61%) were truncating (including nonsense [n = 4], frameshift [n = 4], or splice site [n = 3]). Three missense mutations (50%) were clustered in the critical α9 helix, which targets BAX to the mitochondrial outer membrane to initiate the apoptotic cascade, as reported previously.1,6 Although truncating variants (n = 6) upstream in the gene are predicted to result in nonsense-mediated decay and no functional protein, it is interesting to note that 3 missense variants (Thr14Pro, Leu59Val, and Leu125Gln) were also observed upstream. Mutations affecting the Leu125 codon have been observed in 2 patients (Leu125Arg and Leu125Gln) outside of this study cohort,7 suggesting that this amino acid residue may be relevant to BAX protein function, possibly by disrupting the α5-α6 hinge region required for “unlatching” of the BAX protein.8
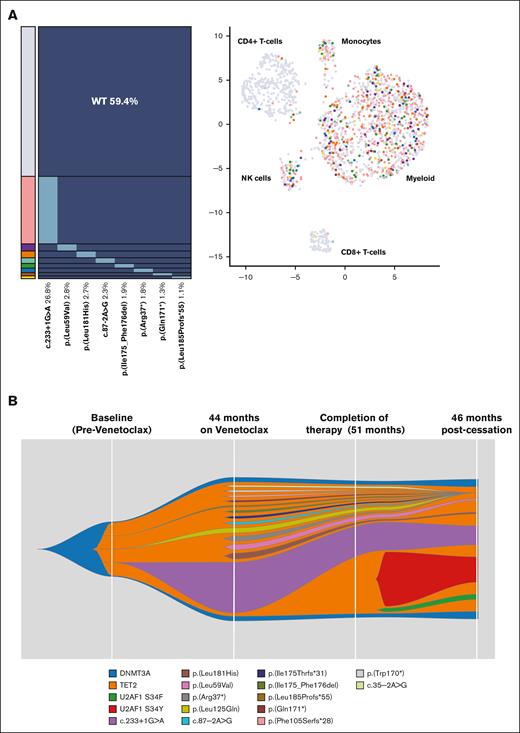
Patient mBEP_01-013 had 13 BAX mutations detected by the NGS panel after 44 months of venetoclax therapy, with no detectable BAX mutations at study entry. To investigate clonal composition, single-cell sequencing was performed using a custom DNA and protein Tapestri panel (supplemental Methods; supplemental Tables 4-7; supplemental Figures 2 and 3), with 8 BAX mutations detected (1725 cells analyzed; supplemental Table 8). These mutations were present in multiple independent heterozygous clones and demonstrated a myeloid-biased lineage distribution but were also detected in the natural killer cell compartment (Figure 2A; supplemental Figure 4); the DNMT3A and TET2 mutations detected by the targeted NGS panel were not targeted in the single-cell experiment. This finding is similar to the preferential involvement of monocytes, granulocytes, and natural killer cells by mutations commonly observed in clonal hematopoiesis.9
Mutation profiling on a patient (mBEP_01-013) with breast cancer. (A) Single-cell analysis using a combined DNA and protein Tapestri panel on a peripheral blood sample after venetoclax therapy for 44 months. Eight BAX variants were detected on the Tapestri panel (1725 cells) in multiple independent heterozygous clones with myeloid-biased lineage distribution. (B) Fish plot representing the inferred clonal dynamic changes from bulk sequencing data (targeted NGS panel) from multiple time points: baseline before venetoclax; 44 and 51 months on venetoclax therapy; and 46 months after cessation of venetoclax. BAX-specific deep sequencing (duplex-UMI panel) on the baseline sample revealed 3 mutations: c.233+1G>A (VAF, 0.013%), Leu125Gln (VAF, 0.04%), and Leu185Profs∗55 (VAF, 0.009%). UMI, unique molecular identifier.
Mutation profiling on a patient (mBEP_01-013) with breast cancer. (A) Single-cell analysis using a combined DNA and protein Tapestri panel on a peripheral blood sample after venetoclax therapy for 44 months. Eight BAX variants were detected on the Tapestri panel (1725 cells) in multiple independent heterozygous clones with myeloid-biased lineage distribution. (B) Fish plot representing the inferred clonal dynamic changes from bulk sequencing data (targeted NGS panel) from multiple time points: baseline before venetoclax; 44 and 51 months on venetoclax therapy; and 46 months after cessation of venetoclax. BAX-specific deep sequencing (duplex-UMI panel) on the baseline sample revealed 3 mutations: c.233+1G>A (VAF, 0.013%), Leu125Gln (VAF, 0.04%), and Leu185Profs∗55 (VAF, 0.009%). UMI, unique molecular identifier.
No patients developed myeloid neoplasm after a median follow-up of 6 years (range, 4.9-6.7) from the start of venetoclax therapy. There were no significant differences in cytopenias between patients with and without BAX mutations (supplemental Table 9). Breast cancer–specific progression-free survival was similar among patients (supplemental Figure 5). Samples from patient mBEP_01013 showed only 3 of 13 variants (c.233+1G>A, Leu181His, and Leu59Val) detectable at 46 months after cessation of venetoclax (while on letrozole, palbociclib, and denosumab, followed by exemestane and everolimus) were detectable at 5.9%, 0.51%, and 0.48% VAFs, respectively, decreasing from 15.7%, 1.8%, and 1.4% at cessation. Additionally, 2 U2AF1 variants (Ser34Phe and Ser34Tyr) emerged during this period (supplemental Table 8; Figure 2B). This patient had stable breast cancer and no overt myeloid neoplasm at the last follow-up, 46 months after venetoclax cessation.
We evaluated changes in other mutations in 9 patients to understand the effect of venetoclax on clonal hematopoiesis outside of BAX mutations. There was no significant change in the number of mutations per patient (median of 2 mutations before and after venetoclax): 5 patients gained 8 mutations, whereas 10 mutations in 4 patients became undetectable (supplemental Figure 6). VAF changes were minimal (median VAF, 0.8 vs 1.2%; P = .06 [by paired Wilcoxon test]).
Therapy-related clonal hematopoiesis is a well-recognized phenomenon after cytotoxic therapy, often characterized by the enrichment of mutations in the DNA damage response pathway, such as TP53 and PPM1D, which typically exist at very low levels before treatment and are undetectable by conventional sequencing.10-12 BAX, a proapoptotic BCL-2 family member, is crucial in apoptosis regulation. Recent studies have identified BAX mutations as a rare driver of clonal hematopoiesis, with 24 BAX mutations observed in a cohort of 12 315 patients with solid cancers (prevalence 0.2%)13 and in only 0.004% of 200 618 individuals in the UK Biobank.2 Deep sequencing (mean 10 530×; range, 7574-11 816) was performed on baseline pre-venetoclax samples from 2 patients (mBEP_01013 and mBEP_01019), using a duplex-UMI (unique molecular identifier) hybridization targeted DNA panel (Twist Bioscience). Of the 13 mutations observed in patient mBEP_01013, 3 were detected: c.233+1G>A (VAF, 0.013%), Leu125Gln (VAF, 0.04%), and Leu185Profs∗55 (VAF, 0.009%). Of the 2 mutations observed in patient mBEP_01019, Ala178Glu (0.025%) and Leu181His (0.052%) were both detected. These data support preexisting BAX mutations at low levels being selected during treatment with venetoclax.
Although functional studies have demonstrated a survival advantage of tumor cell models with biallelic loss of BAX under the selective pressure of venetoclax therapy,1,6 the functional impact of monoallelic or heterozygous BAX mutations is poorly understood; BAX single-mutant animal models are being explored. From our current study, most of the observed BAX mutations were of low VAF. Notably, in at least 1 patient with single-cell data, these heterozygous BAX mutations were present in multiple independent clones. Together, these data are consistent with loss of a single BAX allele conferring a fitness advantage to myeloid lineage cells in the context of venetoclax exposure. With prolonged follow-up, regression of these BAX-mutated clones was observed in this patient after venetoclax cessation. There are several limitations to this study. Larger studies are necessary to explore the impact of venetoclax on other clonal hematopoiesis mutations and whether these mutations promote the acquisition of BAX mutations or contribute to possible future hematologic complications. Additionally, we did not determine whether the breast tumor also acquired BAX mutations. The impact of these “off-target” BAX mutations outside the tumor compartment on treatment response or resistance to therapy remains unknown.
In conclusion, we observed BAX-mutated clonal hematopoiesis occurring in patients treated with venetoclax outside the context of hematologic malignancy. These data further strengthen the role of BAX as a driver gene of clonal hematopoiesis and warrant further studies to understand the mechanisms driving this selection process and their implications on treatment efficacy and normal blood homeostasis.
Acknowledgments: The authors thank Luxi Lal, Elliot Surgenor, and the Victorian Cancer Biobank for sample coordination and technical assistance.
This work was supported by grants from the Snowdome Foundation, Wilson Centre for Blood Cancer Genomics, Australia and the National Health and Medical Research Council (GNT2011139 [P.B.]; grants 1174902, 2011139, and 2034294 [A.W.R.]); and investigator-initiated study support from AbbVie and Genentech (Roche) for the mBEP study (ACTRN12615000702516). G.J.L. is supported by the National Health and Medical Research Council (1175960) and the US Breast Cancer Research Foundation.
Contribution: I.S.T., G.J.L., and P.B. contributed to conception and design; D.G., S.-J.D., and G.J.L. contributed to provision of study materials or patients; I.S.T., C.E.T., C.M., and S.F. collected and assembled data; I.S.T., T.N., C.C.C., J.F.S., A.W.R., G.J.L., and P.B. analyzed and interpreted data; I.S.T. and P.B. wrote the manuscript; and all authors provided final approval of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: I.S.T. reports speaker’s bureau fees from Pfizer and Novartis; and honoraria from Jazz. C.C.C. reports advisory board fees from AbbVie, Pfizer, and Sumitomo Pharma Oncology; and speaker’s bureau fees from Otsuka, Bristol Myers Squibb, AstraZeneca, and AbbVie. C.E.T. is a recipient of a share in venetoclax-related royalty payments and rights received by employer, the Walter and Eliza Hall Institue of Medical Research. J.F.S. reports advisory board fees from AbbVie, Celgene, F. Hoffmann-La Roche Ltd, Janssen, Acerta, Genentech Inc, Gilead, and Takeda; speaker’s bureau fees from AbbVie, Celgene, and F. Hoffmann-La Roche Ltd; research funding from AbbVie, Celgene, F. Hoffmann-La Roche Ltd, and Janssen; and expert testimony fees from Celgene and F. Hoffmann-La Roche Ltd. D.G. is a recipient of a share in venetoclax-related royalty payments and rights received by employer, the Walter and Eliza Hall Institute of Medical Research. A.W.R. reports research funding from AbbVie; is an inventor on a patent related to venetoclax; and is a recipient of a share in venetoclax-related royalty payments and rights received by employer, the Walter and Eliza Hall Institute of Medical Research. S.-J.D. reports advisory board fees from Adela Bio. G.J.L. reports advisory board fees from AbbVie and Pfizer; research funding (to institute) from Amgen, AbbVie, Genentech (Roche), Pfizer, and Servier; and royalty payments (to institute) from AbbVie and Genentech. P.B. reports advisory board fees from Adaptive Biotechnologies, Roche Diagnostics, and AbbVie; and speaker’s bureau fees from AstraZeneca and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Piers Blombery, Department of Pathology, Peter MacCallum Cancer Centre, 305 Grattan St, Melbourne, VIC 3000, Australia; email: piers.blombery@petermac.org.
References
Author notes
G.J.L. and P.B. are joint senior authors.
Original data are available on request from the corresponding author, Piers Blombery (piers.blombery@petermac.org).
The full-text version of this article contains a data supplement.