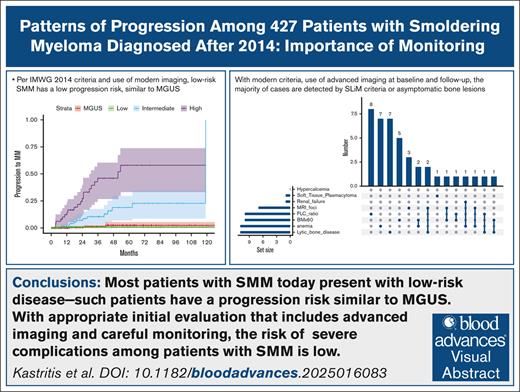
Visual Abstract
TO THE EDITOR:
Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM), may evolve to symptomatic multiple myeloma (MM) with heterogeneous rates and clinical patterns. The 2014 International Myeloma Working Group (IMWG) criteria incorporated biomarkers of malignancy (SLiM criteria) within the definition of symptomatic MM, beyond the historical CRAB criteria (hypercalcemia, renal dysfunction, anemia, and bone lesions)1; thus, patients with SMM may be considered for therapy based on the evolution of biomarkers and not symptomatic complications. Furthermore, availability of advanced imaging improved the identification of early disease,2 potentially altering the natural history of SMM and MGUS by enabling timely therapeutic interventions. The risk of SMM progression is heterogeneous. The IMWG's 20/2/20 model3 provided a simple stratification system based on serum free light chain (FLC) ratio, monoclonal spike, and bone marrow plasma cell infiltration, and was developed from a large cohort that also included patients without access to advanced imaging at diagnosis or during follow-up. We describe the clinical course of SMM in patients diagnosed after 2014, using 2014 IMWG criteria, and managed with contemporary imaging to evaluate how advancements in diagnostics have impacted risk stratification and patterns of disease progression.
We analyzed data of 427 patients with SMM diagnosed between 2014 and 2023 at the Department of Clinical Therapeutics, according to IMWG 2014 criteria, incorporating advanced imaging (low dose whole body computed tomography [ldWBCT], whole body magnetic resonance imaging [WBMRI], or positron emission tomography/computed tomography) at baseline and yearly during follow-up. Per institutional strategy, patients diagnosed with SMM are followed up every 3 months for the first year and every 4 months for the second year, provided there is no evidence of imminent progression or evolving pattern. For patients with high risk SMM we continue to follow-up every 4 months after the completion of 2 years; for low or intermediate risk, we follow them every 6 months. The frequency may increase if an evolving pattern is observed. For comparison, 486 patients with non-immunoglobulin M MGUS diagnosed in the same period were included; baseline imaging was performed in those with at least 1 risk factor (FLC ratio >8, M-spike >1.5 g/dL) but were not routinely followed with yearly imaging. Demographic, clinical, laboratory, and imaging data are prospectively collected, maintained, and followed in our institutional database. Progression to symptomatic MM was defined by SLiM-CRAB criteria. Kaplan-Meier survival analyses were used to compare time-to-progression across risk groups. Analyses were performed using R software.
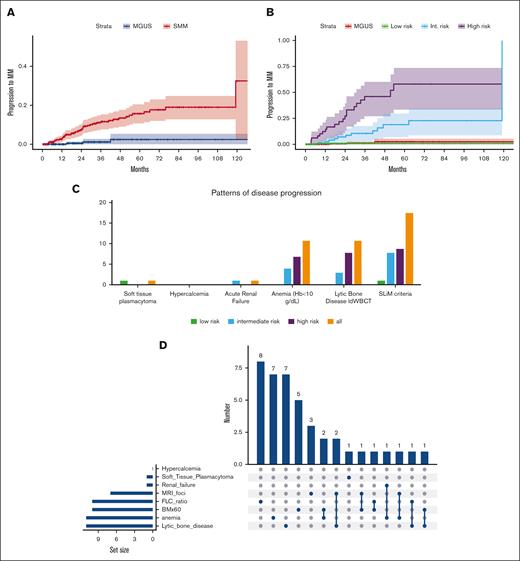
The median age of patients with SMM was 65 years; 6% had estimated glomerular filtration rate <40 mL/min per 1.73 m2 but renal dysfunction was unrelated to monoclonal gammopathy after thorough investigation. Per the 20/2/20 model,3 52% of patients with SMM were classified as low risk (score = 0), 31% as intermediate risk (score = 1), and 17% as high risk (score = 2-3), a distribution similar to the 1 observed in the large screening iStopMM trial for SMM.4 Full data for cytogenetics were available for 119 (28%) patients with SMM; accordingly, per IMWG scoring tool, 49% were classified as low risk, 34% as low-intermediate risk, 12% as intermediate risk, and 4% as high risk.3Table 1 shows detailed characteristics. After median follow-up of 36 months (interquartile range, 17-64), 42 (10%) patients with SMM progressed to symptomatic MM; at the same period 3 (0.6%) non-immunoglobulin M MGUS patients progressed to symptomatic MM (Figure 1A). Cumulative progression rates at 1-, 2- and 3-years, were 3%, 8%, and 12%, respectively (vs 1% at 3 years for MGUS patients). The 1-, 2-, and 3-years progression rates for low risk per 20/2/20 group were 0%, 1%, and 1%, respectively; for intermediate risk were 1%, 8%, and 13%, respectively; and for high risk were 13%, 26%, and 43%, respectively (P < .001) (Figure 1B). Based on cytogenetics, those with any abnormality (including +1q21 and del17p) had a 1-, 2-, and 3-year progression rates of 4%, 19%, and 25% vs 8%, 8%, and 23% for those without, respectively (P = .832). Per the IMWG scoring tool with cytogenetics, 1-, 2-, and 3- year progression rate was 0%, 0%, and 9%, respectively, for low risk; 14%, 14%, and 40%, respectively, for low-intermediate risk; and 23%, 37%, and 66%, respectively, for intermediate/high risk (pooled together due to small numbers) (P = .055).
Characteristics and patterns of progression among 427 patients with SMM diagnosed between 2014 and 2023
| Patients’ characteristics . | (N = 427) . |
|---|---|
| Median age (y), (range) | 65 (28-88) |
| Median Hb (g/dL), (range) | 12.9 (9-16.8) |
| IgG/IgA/IgD/no heavy chain/biclonal, (%) | 71/24/0.3/3/1.5 |
| Light chain kappa/lambda/biclonal, (%) | 57/42/1 |
| Median M-spike (g/dL), (range) | 1.31 (0-4.4) |
| Serum creatinine >2 mg/dL | 2.3%∗ |
| eGFR <40 mL/min per 1.73 m2 | 6%∗ (unrelated to MG) |
| Albumin (g/dL), (range) | 4.4 (3.1-5.3) |
| B2 microglobulin (mg/L), (range) | 2.1 (0.7-20) |
| Proteinuria (grams per day), (range) | 0.005 (0-2) |
| Any FISH abnormality (n = 119) | 30% |
| Risk per 20/2/20 model | |
| Low risk (0 points) | 52% |
| Intermediate risk (1 point) | 31% |
| High risk (2-3 point) | 17% |
| Risk per IMWG scoring tool† | |
| Low risk | 49% |
| Low-intermediate | 34% |
| Intermediate | 12% |
| High | 4% |
| Patterns of disease progression | |
| All progression events | 42 (100%) |
| Soft tissue plasmacytoma | 2% (1) |
| Hypercalcemia | 0 |
| ARF | 2% (1) |
| Anemia (Hb < 10 g/dL) | 24% (11) |
| Lytic bone disease in ldWBCT | 24% (11) |
| SLiM criteria | 43% (18) |
| Patients’ characteristics . | (N = 427) . |
|---|---|
| Median age (y), (range) | 65 (28-88) |
| Median Hb (g/dL), (range) | 12.9 (9-16.8) |
| IgG/IgA/IgD/no heavy chain/biclonal, (%) | 71/24/0.3/3/1.5 |
| Light chain kappa/lambda/biclonal, (%) | 57/42/1 |
| Median M-spike (g/dL), (range) | 1.31 (0-4.4) |
| Serum creatinine >2 mg/dL | 2.3%∗ |
| eGFR <40 mL/min per 1.73 m2 | 6%∗ (unrelated to MG) |
| Albumin (g/dL), (range) | 4.4 (3.1-5.3) |
| B2 microglobulin (mg/L), (range) | 2.1 (0.7-20) |
| Proteinuria (grams per day), (range) | 0.005 (0-2) |
| Any FISH abnormality (n = 119) | 30% |
| Risk per 20/2/20 model | |
| Low risk (0 points) | 52% |
| Intermediate risk (1 point) | 31% |
| High risk (2-3 point) | 17% |
| Risk per IMWG scoring tool† | |
| Low risk | 49% |
| Low-intermediate | 34% |
| Intermediate | 12% |
| High | 4% |
| Patterns of disease progression | |
| All progression events | 42 (100%) |
| Soft tissue plasmacytoma | 2% (1) |
| Hypercalcemia | 0 |
| ARF | 2% (1) |
| Anemia (Hb < 10 g/dL) | 24% (11) |
| Lytic bone disease in ldWBCT | 24% (11) |
| SLiM criteria | 43% (18) |
eGFR, estimated glomerular filtration rate; FISH, fluorescence in situ hybridization; Hb, hemoglobin; Ig, immunoglobulin.
Not related to monoclonal gammopathy after extensive investigation.
Only in those with available cytogenetics.
Time-to-disease progression and myeloma progression events in the cohort of 427 patients with SMM diagnosed between 2014 and 2023. (A) Time-to-progression to symptomatic myeloma in patients with SMM and non-immunoglobulin M (IgM) MGUS diagnosed over the same time period. (B) Time-to-progression to symptomatic myeloma in patients with SMM according to 20/2/20 risk category and non-IgM MGUS diagnosed over the same time period. (C) Patterns of disease progression according to risk category per 20/2/20 model. (D) UpSet plot of disease progression events. Hb, hemoglobin; int., intermediate.
Time-to-disease progression and myeloma progression events in the cohort of 427 patients with SMM diagnosed between 2014 and 2023. (A) Time-to-progression to symptomatic myeloma in patients with SMM and non-immunoglobulin M (IgM) MGUS diagnosed over the same time period. (B) Time-to-progression to symptomatic myeloma in patients with SMM according to 20/2/20 risk category and non-IgM MGUS diagnosed over the same time period. (C) Patterns of disease progression according to risk category per 20/2/20 model. (D) UpSet plot of disease progression events. Hb, hemoglobin; int., intermediate.
We further analyzed the patterns of progression in this cohort which was followed by modern imaging and for which SLiM criteria were also applicable. Among the 42 patients with SMM who progressed, 1 (∼2%) developed a soft tissue plasmacytoma, 1 (∼2%) developed acute renal dysfunction, 24% progressed with anemia (defined as hemoglobin <10 g/dL), 24% developed lytic bone disease detected by ldWBCT imaging, and 43% progressed based on SLiM criteria. The patterns of progression per risk group are shown in Table 1, supplemental Table 1, and Figure 1C. Among the 11 patients with lytic bone disease, 10 were asymptomatic (no pain reported, lytic lesions identified on routine imaging, in all with ldWBCT), 1 had multiple small lytic lesions (>5 mm but <10 mm), and 1 patient had a lytic lesion with mass at T8 and fracture).
Among those with SLiM-based progression events, 12 developed FLC ratio >100, 6 developed >1 focal lesion in magnetic resonance imaging (4 patients the ldWBCT was negative, 1 had anemia, and 1 had bone marrow [BM] infiltration >60%) and in 6 patients a repeat biopsy showed a BM infiltration >60% (6 patients had >1 SLiM). The patient who developed a soft tissue plasmacytoma, 55 months after initial diagnosis, had been initially rated as low risk. The patient who developed acute renal failure (ARF) was at intermediate risk at diagnosis, had not been followed due to other major comorbidities, and developed ARF after 17 months.
Our data from a modern cohort of patients with SMM highlights the relatively low risks of complications when modern criteria for diagnosis and risk stratification are used. Advanced imaging mitigates this risk, reclassifying some SMM as symptomatic MM at diagnosis (and likely contributed to the observed lower progression rates among high-risk SMM compared to historical cohorts, which may have overestimated the risk of progression), while being able to identify patients with early asymptomatic lytic bone disease. Similar to modern clinical trials, like AQUILA,5 using IWMG 2014 criteria and advanced imaging at trial entry and follow-up, the vast majority of patients are detected by SLiM criteria; asymptomatic bone lesions, detected during routine follow-up imaging; or manageable anemia. Our group has published that ∼10% of patients with SMM could be identified as progressors with ldWBCT alone.2 In the AQUILA study 65% of patients in the observation arm progressed by SLiM criteria or on serial imaging without cases of renal failure.5 Our results differ from recently published data from United States and Germany,6,7 in which a significant proportion of patients progressed with symptomatic bone lesions and some with ARF; however, these cohorts included patients diagnosed even before 2014, did not mandate advanced imaging at diagnosis and yearly follow-up and were performed in tertiary centers, which may introduce selection bias.
Another key observation is that low-risk SMM has a low progression risk, similar to MGUS. This is re-assuring for patients who are diagnosed with “myeloma” because of >10% plasma cells without any other risk factor. Our cohort is enriched for low-risk patients, probably reflecting earlier diagnosis, similar to screening studies.4 For high-risk SMM the risk of progression remains significant, similar across real-world cohorts6,7 but lower than older cohorts.3 New biomarkers8-12 and dynamic models13 may further refine prognostication.
Our cohort has significant differences compared to patients enrolled in older trials evaluating interventions in SMM, such as the QUIREDEX study.14 The GEM-Pethema study used also older diagnostic and high-risk stratification criteria with 31% of patients fulfilling SLiM criteria at enrollment; notably, most failures at screening were due to identification of lytic lesions by advanced imaging,15 emphasizing the need for meticulous evaluation of SMM at diagnosis. In the E3A06 study,16 ∼10% of patients had FLC ratio >100, and 3% had BM infiltration ≥60%; whereas ∼48% had abnormal magnetic resonance imaging findings. The 3-year progression-free survival rate in the observation arm for low-risk patients per 20/2/20 model (n = 25) was ∼20%, rather high compared to the IMWG3 and our cohort. The AQUILA study,5 included patients based on IMWG 2014 criteria, with median time-to-progression in the observation arm for high risk of 22.1 months, shorter than in our cohort.
Our data, along with others,4-7 depict the profile of SMM today and combined with recent trial data5 emphasize the importance of appropriate monitoring for SMM management. The results of older intervention studies in SMM14-16 may not be applicable anymore. For low-risk patients the risk of major complications is minimal and observation should remain the reasonable approach; but for intermediate-risk patients, carefully designed trials should be considered.
All patients gave written informed consent for access and analysis of their data and an approval for analysis and publication was given from the Ethics Committee/Scientific Counsel of Alexandra Hospital.
Acknowledgment: This study was supported by HORIZON-MISS-2021-Cancer grant 101097094 (ELMUMY).
Contribution: E.K. designed the study, collected data, performed the analysis, and wrote the first and final draft of the manuscript; I.S., P.M., F.T., I.N.-S., N.K., D.F., M.M., E.E.-P., V.S., N.K., A.P., S.G., M.G., and E.T. collected data and critically reviewed the manuscript; and M.A.D. designed the study and critically reviewed the manuscript.
Conflict-of-interest disclosure: E.K. reports honoraria from Pfizer, GlaxoSmithKline (GSK), Janssen, and Prothena; and research funding from Pfizer, GSK, and Janssen. I.N.-S. reports honoraria from Janssen and research funding from Cellectar Biosciences and AstraZeneca. F.T. reports honoraria from Sanofi and Janssen. M.M. reports honoraria from Janssen and GSK. M.G. reports honoraria from GENESIS Pharma, BeiGene, Janssen, and Bristol Myers Squibb (BMS); and research funding from AbbVie, Integris, Swixx BioPharma, Cellectar Biosciences, and Amgen. E.L. reports honoraria from Amgen, BMS, GSK, Janssen, Pfizer, Sanofi, Takeda, Novartis, Swixx BioPharma, and Antengene Corp; and research funding from BMS, GSK, Janssen, Sanofi, Takeda, and Antengene Corp. M.A.D. reports honoraria from Regeneron, Takeda, GSK, BMS, Janssen, BeiGene, Swixx BioPharma, AstraZeneca, Sanofi, and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Efstathios Kastritis, Department of Clinical Therapeutics, School of Medicine, Alexandra General Hospital, National and Kapodistrian University of Athens, 80 Vas. Sofias Ave, 11528 Athens, Greece; email: ekastritis@med.uoa.gr.
References
Author notes
Anonymized data are available upon reasonable request from the corresponding author, Efstathios Kastritis (ekastritis@med.uoa.gr and ekastritis@gmail.com) and after institutional review board approval.
The full-text version of this article contains a data supplement.