Key Points
Real-world use of the duv/romi combination confirms its efficacy, tolerability, and safety for patients with R/R PTCL.
The duv/romi combination offers a novel strategy to bridge eligible patients with R/R PTCL to allogeneic transplantation.
Visual Abstract
Patients with relapsed or refractory (R/R) peripheral T-cell lymphomas (PTCL) require lineage-specific therapies to bridge to hematopoietic stem cell transplantation (HSCT). A previous phase 1/2 study of duvelisib/romidepsin (duv/romi) reported an overall response rate (ORR) of 58% and a complete response rate (CRR) of 42% with reduced grade 3 to 4 transaminitis (14%). We report real-world duv/romi outcomes in a multicenter, 38-patient R/R PTCL cohort. The median age at diagnosis was 62 years. Histological subtypes included nodal T follicular helper cell (nTFH; n = 17), PTCL-not otherwise specified (n = 14), cutaneous T-cell lymphoma (TCL; n = 3), extranodal natural killer/TCL (n = 1), ALK-negative anaplastic large cell lymphoma (n = 1), adult T-cell leukemia/lymphoma (n = 1), and hepatosplenic TCL (n = 1). The median previous therapy count was 1 (interquartile range [IQR], 1-2); 15 patients relapsed and 23 were refractory to prior treatment, including 8 prior HSCT (5 autologous, 3 allogeneic). After a median of 3 cycles (IQR, 2-4), ORR and CRR were 61% and 47%, respectively, with higher ORR (82% vs 43%) and CRR (71% vs 29%) in nTFH versus non-nTFH. The median progression-free survival and overall survival (HSCT-censored) were 11 and 16 months for nTFH, versus 3.3 and 8.3 months for non-nTFH. The median time to response was 1.9 months (IQR, 1.7-2.6), duration of response was 21 months, and time to next therapy was 17 months. After duv/romi, 11 patients bridged to allo-HSCT. Treatment was well tolerated; the most common grade 3 to 4 toxicities were lymphopenia (n = 15), neutropenia (n = 15), thrombocytopenia (n = 10), and transaminitis (n = 6), seldom leading to discontinuation (n = 4) or death (n = 1). These findings reinforce duv/romi’s efficacy and bridging role to curative HSCT in high-risk R/R PTCL.
Introduction
Among patients with relapsed or refractory (R/R) peripheral T-cell lymphomas (PTCL), overall survival (OS) and progression-free survival (PFS) range from 2.5 to 29.1 months and 3.1 to 9.6 months, respectively, highlighting poor outcomes.1 Thus, there is a critical unmet need for novel treatments that improve response quality and duration, and enable safe bridging to allogeneic hematopoietic stem cell transplantation (allo-HSCT), which can be curative for a subset of patients, ultimately leading to improved survival. Duvelisib (duv) is an oral phosphatidylinositol 3-kinase-δ (PI3K-δ) and PI3K-γ dual inhibitor, which demonstrated overall response rates (ORRs) of 50.0% and 31.6% in patients with PTCL and cutaneous T-cell lymphoma (CTCL), respectively, in a phase 1 trial.2 In the heavily pretreated cohort of the phase 2 PRIMO-EP trial, single-agent (SA) duv demonstrated a robust ORR of 48% and a complete response rate (CRR) of 33%, but adverse events (AEs) necessitated dose interruption in 44.7% of patients.3 Combinatorial strategies with histone deacetylase (HDAC) inhibitors (HDACis) were hypothesized to improve response and survival. In a recent phase 1b/2a trial of R/R T-cell lymphomas (TCL), the addition of the HDACi romidepsin (romi) to duv increased efficacy and attenuation of PI3K inhibitor–driven toxicity.4 This trial demonstrated an ORR of 55% and a CRR of 34%, with event-free survival not reached (NR) among patients who achieved a CR.4,5 Notably, the incidence of grade (gr) 3 to 4 transaminitis (elevated alanine aminotransferase [ALT]/aspartate aminotransferase [AST]) was only 14%.4
Duvelisib and romidepsin (duv/romi) combination therapy is emerging as a guideline-recommended treatment for R/R PTCL and CTCL, although the supporting evidence is currently limited. Confirmation of superior responses and reduction of AEs in a real-world patient population are needed to gain more knowledge about the efficacy and toxicity of this combination therapy to facilitate clinical decisions. This in turn would potentially increase access to this combination through expedited insurance and regulatory agency approvals for at least a subset of patients with R/R PTCL and CTCL. It will also support further design and development of several planned phase 2 studies of combinations of PI3K, HDAC, and DNA methyltransferase inhibitors in TCL.
Herein, we report a multicenter descriptive analysis of duv/romi efficacy and toxicity in patients with R/R PTCL and CTCL, which demonstrates that the addition of duv to romi is safe, well tolerated, and induces high response rates, particularly in the nodal T follicular helper cell lymphoma (nTFH) subtype. We also discuss management of AEs and their functional consequences and compare this regimen with other varied double drug combinations of PI3K, HDAC, and DNA methyltransferase inhibitors, and immunomodulatory drugs.
Methods
Study design and patient eligibility
This multi-institution retrospective and prospective observational study was approved by the Dana-Farber Cancer Institute (DFCI) Institutional Review Board (DFCI protocol number 22-355). It included patients who received care at the Massachusetts General Hospital Cancer Center, Massachusetts General Hospital–affiliated community practices, and DFCI. This study was conducted in accordance with the Declaration of Helsinki.
Eligible patients had pathologically confirmed PTCL and/or CTCL that had relapsed or progressed after at least 1 systemic therapy per the 2016 (fourth edition) or 2022 (fifth edition) World Health Organization classification of lymphoid neoplasms. Patients should have received combination duv/romi between January 2016 and November 2024. Most patients were treated as per the previously reported phase 1b/2a maximum tolerated dose and schedule, which included romi at 10 mg/m2 administered intravenously on days 1, 8, and 15 of a 28-day cycle. Relapsed status was defined as disease recurrence after achieving CR to previous therapy, whereas refractory status was defined as failure to achieve CR by the end of previous therapy. Patients who received duv/romi as part of a clinical trial were excluded. Patients were screened and enrolled in this study both prospectively and retrospectively. Data were collected from electronic medical records through 15 January 2025. Responses were assessed on the basis of the 2014 Consensus of the International Conference on Malignant Lymphomas Imaging Working Group and their 5-point Deauville scale for positron emission tomography/computed tomography interpretation, which was performed at baseline and as clinically indicated. Patients achieving remission were evaluated every 6 months for 2 years or until disease progression. Patients were followed long-term to capture treatment courses, response, and survival status, regardless of treatment response or eligibility for allo-HSCT. Electronic chart review of AEs was continuous throughout treatment, and AEs were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Only gr 3 and 4 AEs and their management were reported. Time to AE onset was defined from treatment start to the first of gr 3 or 4 AE. The maximum AE gr per patient was reported irrespective of causality and its attribution to the duv/romi combination. Preexisting abnormalities (clinical or laboratory) that significantly worsened during duv/romi treatment were considered AEs, whereas neutrophilic leukocytosis in the setting of granulocyte colony-stimulating factor was not recorded as an AE. Primary objectives included assessment of ORR including CRR and partial response rate (PRR) and gr 3/4 hematologic or nonhematologic AEs. The Kaplan-Meier method was used to estimate PFS, OS, duration of response (DoR), time to next therapy, time to response, and time to AE onset, while the reverse Kaplan-Meier method was used to measure time of follow-up.6 Further methodological details are included in the supplemental Data.
Results
Baseline and treatment characteristics
We prospectively and retrospectively identified 38 patients who were treated with duv/romi, including 17 patients with nTFH and 21 patients with non-nTFH lymphomas, including 14 with PTCL-not otherwise specified (NOS), 3 with CTCL (1 mycosis fungoides, 1 Sézary syndrome, and 1 primary cutaneous gamma-delta), 1 with anaplastic lymphoma kinase–negative anaplastic large cell lymphoma, 1 with extranodal natural killer/T-cell lymphoma, 1 with hepatosplenic T-cell lymphoma, and 1 with adult T-cell leukemia/lymphoma (Table 1). Their clinical characteristics are presented in Table 1. Notably, the median age was 62 years, with an equal sex distribution (male, n = 17/38 [50%]) and a predominantly White cohort (n = 28/38 [74%]). The most common histologies were angioimmunoblastic T-cell lymphoma (nTFH subtype; n = 13/17 [76%]) and PTCL-NOS (non-nTFH subtype; n = 14/21 [67%]). For most patients, International Prognostic Index and Prognostic Index for T-cell lymphoma (PIT) were intermediate to high-risk and comparable between nTFH and non-nTFH subtypes. However, patients with nTFH had lower PIRT (Prognostic Index for Relapsed/Refractory mature T-cell and natural killer cell lymphomas) scores compared with patients with non-nTFH (P = .009).7 Of 38 patients, 29 (76%) were initially treated with an anthracycline-based therapy, and 8 (21%) received an HSCT, autologous (n = 6) or allogeneic (n = 2), before study inclusion. Of 38 patients, 15 (39%) had relapsed disease, whereas 23 (61%) had primary refractory lymphoma. The median number of previous therapies was 1, with no statistical difference between the 2 major (nTFH vs non-nTFH) subgroups. The most common salvage therapies in second- and third-line treatment before duv/romi included chemotherapy and HDACis such as romi.
Baseline demographic and clinical characteristics of the multicenter R/R cohort treated with combination duv/romi
| Characteristic . | All∗ (N = 38) . | nTFH (n = 17) . | Non-nTFH (n = 21) . | P value† . |
|---|---|---|---|---|
| Age at diagnosis, median (IQR), y | 62 (53-72) | 65 (61-71) | 56 (50-72) | .4 |
| Biological sex, n (%) | ||||
| Female | 19 (50) | 9 (53) | 10 (48) | >.99 |
| Male | 19 (50) | 8 (47) | 11 (52) | |
| Race, self-identified, n (%) | ||||
| White | 28 (74) | 13 (76) | 15 (71) | .7 |
| African American or Black | 1 (3) | 1 (6) | 0 | |
| Asian | 3 (8) | 2 (12) | 1 (5) | |
| Other | 1 (3) | 0 | 1 (5) | |
| Unknown | 3 (8) | 01 (6) | 34 (19) | |
| Ethnicity, self-identified, n (%) | ||||
| Hispanic | 4 (11) | 3 (18) | 1 (5) | .2 |
| Non-Hispanic | 32 (84) | 13 (76) | 19 (90) | |
| Other | 1 (3) | 0 | 1 (5) | |
| Unknown | 1 (3) | 1 (6) | 0 | |
| Histological subtype, n (%) | ||||
| nTFH | 17 (48) | 17 (100) | 0 | — |
| AITL | 13 (34) | 13 (76) | 0 | |
| ALK– ALCL | 1 (3) | 0 | 1 (5) | |
| ATLL | 1 (3) | 0 | 1 (5) | |
| CTCL | 3 (8) | 0 | 3 (14) | |
| MF | 1 (3) | 0 | 1 (6) | |
| SS | 1 (3) | 0 | 1 (5) | |
| GD | 1 (3) | 0 | 1 (5) | |
| ENKTCL | 1 (3) | 0 | 1 (5) | |
| HSTCL | 1 (3) | 0 | 1 (5) | |
| PTCL-NOS | 14 (37) | 0 | 14 (67) | |
| IPI at diagnosis, n (%) | ||||
| 0 | 0 | 0 | 0 | .2 |
| 1 | 5 (13) | 1 (6) | 4 (19) | |
| 2 | 14 (37) | 9 (53) | 5 (24) | |
| 3 | 10 (26) | 3 (18) | 7 (33) | |
| 4 | 4 (11) | 2 (12) | 2 (10) | |
| 5 | 0 | 0 | 0 | |
| NA | 5 (13) | 2 (12) | 3 (14) | |
| PIT at diagnosis, n (%) | ||||
| 0 | 1 (3) | 0 | 1 (5) | .6 |
| 1 | 14 (37) | 6 (35) | 8 (38) | |
| 2 | 14 (37) | 8 (47) | 6 (29) | |
| 3 | 4 (11) | 1 (6) | 3 (14) | |
| 4 | 0 | 0 | 0 | |
| NA | 5 (13) | 2 (12) | 3 (14) | |
| PIRT at diagnosis, n (%) | ||||
| Low (0-1) | 1 (3) | 1 (6) | 0 | .009 |
| Intermediate (2-3) | 16 (42) | 11 (65) | 5 (24) | |
| High (4-6) | 6 (16) | 0 | 6 (29) | |
| NA | 15 (39) | 5 (29) | 10 (48) | |
| Previous lines of therapy, median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | .1 |
| Prior therapies received, frontline, n (%) | 38 (100) | 17/17 (100) | 21/21 (100) | |
| CHOP | 12 (32) | 7 (41) | 5 (24) | |
| Mini-CHOP | 2 (5) | 1 (6) | 1 (65) | |
| CHOEP | 9 (24) | 3 (17) | 6 (29) | |
| CEOP‡ | 1 (3) | 1 (6) | 0 | |
| CHOP + azacitidine | 2 (5) | 2 (12) | 0 | |
| BV-CHP | 3 (8) | 0 | 3 (14) | |
| Alemtuzumab | 1 (3) | 0 | 1 (5) | |
| Azacitidine + romi | 2 (5) | 2 (12) | 0 | |
| Extracorporeal photopheresis + bexarotene | 1 (3) | 0 | 1 (5) | |
| Methotrexate + prednisolone | 2 (5) | 0 | 2 (10) | |
| ICE | 2 (5) | 0 | 2 (10) | |
| Pola-R-CHP§ | 2 (5) | 2 (12) | 0 | |
| CHP‖ | 1 (3) | 1 (6) | 0 | |
| Previous therapies received, second line, n (%) | 10/38 (26) | 4/17 (24) | 6/21 (29) | |
| BV-CHP | 2 (5) | 0 | 2 (10) | — |
| Alemtuzumab | 1 (3) | 0 | 1 (5) | |
| Azacitidine | 1 (3) | 1 (6) | 0 | |
| Romi | 2 (5) | 1 (6) | 1 (5) | |
| Azacitidine + romi | 2 (5) | 2 (12) | 0 | |
| Gemcitabine + oxaliplatin | 1 (3) | 0 | 1 (5) | |
| Pegasparaginase | 1 (3) | 0 | 1 (5) | |
| Previous therapies received, third line and onward, n (%) | 5/38 (13) | 0 | 5/21 (24) | — |
| Romi | 2 (5) | 2 (10) | ||
| Pralatrexate | 1 (3) | 1 (5) | ||
| Alemtuzumab | 1 (3) | 1 (5) | ||
| Pembrolizumab | 1 (3) | 1 (5) | ||
| Ruxolitinib | 1 (3) | 1 (5) | ||
| IVAC | 1 (3) | 1 (5) | ||
| DHAP + cytarabine | 2 (5) | 2 (10) | ||
| Gemcitabine + oxaliplatin | 2 (5) | 2 (10) | ||
| Nivolumab | 1 (3) | 1 (5) | ||
| Brentuximab vedotin | 1 (3) | 1 (5) | ||
| Brentuximab vedotin + gemcitabine | 1 (3) | 1 (5) | ||
| Denileukin diftitox (NCT01871727) | 1 (3) | 1 (5) | ||
| Received HSCT before duv/romi, n (%) | 8/38 (21) | 5/17 (29) | 3/21 (14) | — |
| Autologous | 5 (13) | 4 (24) | (5) | |
| Allogeneic | 3 (8) | 1 (6) | 2 (10) | |
| Response to therapy preceding duv/romi,¶n (%) | ||||
| Relapsed | 15 (39) | 6 (35) | 9 (43) | .9 |
| Primary refractory | 23 (61) | 11 (65) | 12 (57) |
| Characteristic . | All∗ (N = 38) . | nTFH (n = 17) . | Non-nTFH (n = 21) . | P value† . |
|---|---|---|---|---|
| Age at diagnosis, median (IQR), y | 62 (53-72) | 65 (61-71) | 56 (50-72) | .4 |
| Biological sex, n (%) | ||||
| Female | 19 (50) | 9 (53) | 10 (48) | >.99 |
| Male | 19 (50) | 8 (47) | 11 (52) | |
| Race, self-identified, n (%) | ||||
| White | 28 (74) | 13 (76) | 15 (71) | .7 |
| African American or Black | 1 (3) | 1 (6) | 0 | |
| Asian | 3 (8) | 2 (12) | 1 (5) | |
| Other | 1 (3) | 0 | 1 (5) | |
| Unknown | 3 (8) | 01 (6) | 34 (19) | |
| Ethnicity, self-identified, n (%) | ||||
| Hispanic | 4 (11) | 3 (18) | 1 (5) | .2 |
| Non-Hispanic | 32 (84) | 13 (76) | 19 (90) | |
| Other | 1 (3) | 0 | 1 (5) | |
| Unknown | 1 (3) | 1 (6) | 0 | |
| Histological subtype, n (%) | ||||
| nTFH | 17 (48) | 17 (100) | 0 | — |
| AITL | 13 (34) | 13 (76) | 0 | |
| ALK– ALCL | 1 (3) | 0 | 1 (5) | |
| ATLL | 1 (3) | 0 | 1 (5) | |
| CTCL | 3 (8) | 0 | 3 (14) | |
| MF | 1 (3) | 0 | 1 (6) | |
| SS | 1 (3) | 0 | 1 (5) | |
| GD | 1 (3) | 0 | 1 (5) | |
| ENKTCL | 1 (3) | 0 | 1 (5) | |
| HSTCL | 1 (3) | 0 | 1 (5) | |
| PTCL-NOS | 14 (37) | 0 | 14 (67) | |
| IPI at diagnosis, n (%) | ||||
| 0 | 0 | 0 | 0 | .2 |
| 1 | 5 (13) | 1 (6) | 4 (19) | |
| 2 | 14 (37) | 9 (53) | 5 (24) | |
| 3 | 10 (26) | 3 (18) | 7 (33) | |
| 4 | 4 (11) | 2 (12) | 2 (10) | |
| 5 | 0 | 0 | 0 | |
| NA | 5 (13) | 2 (12) | 3 (14) | |
| PIT at diagnosis, n (%) | ||||
| 0 | 1 (3) | 0 | 1 (5) | .6 |
| 1 | 14 (37) | 6 (35) | 8 (38) | |
| 2 | 14 (37) | 8 (47) | 6 (29) | |
| 3 | 4 (11) | 1 (6) | 3 (14) | |
| 4 | 0 | 0 | 0 | |
| NA | 5 (13) | 2 (12) | 3 (14) | |
| PIRT at diagnosis, n (%) | ||||
| Low (0-1) | 1 (3) | 1 (6) | 0 | .009 |
| Intermediate (2-3) | 16 (42) | 11 (65) | 5 (24) | |
| High (4-6) | 6 (16) | 0 | 6 (29) | |
| NA | 15 (39) | 5 (29) | 10 (48) | |
| Previous lines of therapy, median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | .1 |
| Prior therapies received, frontline, n (%) | 38 (100) | 17/17 (100) | 21/21 (100) | |
| CHOP | 12 (32) | 7 (41) | 5 (24) | |
| Mini-CHOP | 2 (5) | 1 (6) | 1 (65) | |
| CHOEP | 9 (24) | 3 (17) | 6 (29) | |
| CEOP‡ | 1 (3) | 1 (6) | 0 | |
| CHOP + azacitidine | 2 (5) | 2 (12) | 0 | |
| BV-CHP | 3 (8) | 0 | 3 (14) | |
| Alemtuzumab | 1 (3) | 0 | 1 (5) | |
| Azacitidine + romi | 2 (5) | 2 (12) | 0 | |
| Extracorporeal photopheresis + bexarotene | 1 (3) | 0 | 1 (5) | |
| Methotrexate + prednisolone | 2 (5) | 0 | 2 (10) | |
| ICE | 2 (5) | 0 | 2 (10) | |
| Pola-R-CHP§ | 2 (5) | 2 (12) | 0 | |
| CHP‖ | 1 (3) | 1 (6) | 0 | |
| Previous therapies received, second line, n (%) | 10/38 (26) | 4/17 (24) | 6/21 (29) | |
| BV-CHP | 2 (5) | 0 | 2 (10) | — |
| Alemtuzumab | 1 (3) | 0 | 1 (5) | |
| Azacitidine | 1 (3) | 1 (6) | 0 | |
| Romi | 2 (5) | 1 (6) | 1 (5) | |
| Azacitidine + romi | 2 (5) | 2 (12) | 0 | |
| Gemcitabine + oxaliplatin | 1 (3) | 0 | 1 (5) | |
| Pegasparaginase | 1 (3) | 0 | 1 (5) | |
| Previous therapies received, third line and onward, n (%) | 5/38 (13) | 0 | 5/21 (24) | — |
| Romi | 2 (5) | 2 (10) | ||
| Pralatrexate | 1 (3) | 1 (5) | ||
| Alemtuzumab | 1 (3) | 1 (5) | ||
| Pembrolizumab | 1 (3) | 1 (5) | ||
| Ruxolitinib | 1 (3) | 1 (5) | ||
| IVAC | 1 (3) | 1 (5) | ||
| DHAP + cytarabine | 2 (5) | 2 (10) | ||
| Gemcitabine + oxaliplatin | 2 (5) | 2 (10) | ||
| Nivolumab | 1 (3) | 1 (5) | ||
| Brentuximab vedotin | 1 (3) | 1 (5) | ||
| Brentuximab vedotin + gemcitabine | 1 (3) | 1 (5) | ||
| Denileukin diftitox (NCT01871727) | 1 (3) | 1 (5) | ||
| Received HSCT before duv/romi, n (%) | 8/38 (21) | 5/17 (29) | 3/21 (14) | — |
| Autologous | 5 (13) | 4 (24) | (5) | |
| Allogeneic | 3 (8) | 1 (6) | 2 (10) | |
| Response to therapy preceding duv/romi,¶n (%) | ||||
| Relapsed | 15 (39) | 6 (35) | 9 (43) | .9 |
| Primary refractory | 23 (61) | 11 (65) | 12 (57) |
AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK–, anaplastic lymphoma kinase–negative; ATLL, adult T-cell leukemia/lymphoma; BV-CHP, brentuximab vedotin, cyclophosphamide, doxorubicin, and prednisone; CEOP, cyclophosphamide, etoposide, vincristine, and prednisone; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHP, cyclophosphamide, doxorubicin, and prednisone; DHAP, dexamethasone, doxorubicin, and cisplatin; ENKTCL, extranodal natural killer/T-cell lymphoma; GD, gamma-delta; HSTCL, hepatosplenic T-cell lymphoma; ICE, ifosfamide, carboplatin, and etoposide; IPI, International Prognostic Index; IVAC, ifosfamide, vinblastine, and cytarabine; MF, mycosis fungoides; mini-CHOP, CHOP administered at abbreviated doses; NA, not available; Pola-R-CHP, polatuzumab, rituximab, cyclophosphamide, doxorubicin, and prednisone; SS, Sézary syndrome.
Two patients received retreatment with duv/romi in discrete lines of therapy, and treatment response was assessed independently for each line of therapy. Hence, 38 lines of therapy were evaluated among 36 patients.
P values for the comparison between patients in different national cohorts were calculated using Kruskal-Wallis and χ2 tests for nonnormally distributed continuous variables and categorical variables, respectively.
One patient received CHOP in the first cycle followed by CEOP in cycles 2 to 6.
One patient was treated with frontline rituximab-CHOP for diffuse large B-cell lymphoma (DLBCL). Upon relapse, they received 4 cycles of Pola-R-CHP, followed by 2 cycles of Pola-R-cyclophosphamide, etoposide phosphate, prednisone (CEP), to which they were primarily refractory, and were subsequently diagnosed with AITL. Another patient received frontline Pola-R-CHP for primary cutaneous DLBCL and second-line duv/romi for nTFH.
One patient received CHOP for 5 weeks, which was complicated by Pneumocystis jirovecii pneumonia, respiratory failure requiring mechanical ventilation, anuric acute kidney injury status after dialysis, Pseudomonas urinary tract infection, and sepsis requiring vasopressors, and was thus switched to CHP chemotherapy after 5 weeks. They received CHP for 2 weeks before switching to second-line azacitidine + romi.
“Relapsed” defined as disease recurrence after achieving CR to previous therapy, and “primary refractory” defined as failure to achieve CR by the end of previous therapy.
Most patients (n = 31/38 [82%]) received prophylaxis against Pneumocystis jirovecii pneumonia and varicella zoster virus (n = 32/38 [84%]), whereas 3% (n = 1) of the patients received antifungal prophylaxis with fluconazole (Table 2). Of the 38 patients, 16 (42%) patients received granulocyte colony-stimulating factor once or more during duv/romi treatment: 9 (24%) with filgrastim and 7 (18%) with pegfilgrastim (Table 2). Twenty-four percent of the patients (n = 9/38) received duv lead-in cycles ranging from 1 to 5. The median number of duv/romi cycles received was 3, with the nTFH subtype receiving a higher number (median of 4 cycles) compared with the most common non-nTFH subtypes such as PTCL-NOS (median of 2 cycles). Four patients received ≤1 cycle of the combination, 12 received 2 cycles, and 22 received >2 cycles.
Treatment characteristics of combination duv/romi in multicenter R/R cohort
| Treatment characteristics . | All∗ (N = 38) . |
|---|---|
| Pneumocystis jirovecii pneumonia prophylaxis, n (%) | 31 (82) |
| Atovaquone | 12 (32) |
| Trimethoprim-sulfamethoxazole | 21 (55) |
| Varicella zoster virus prophylaxis, n (%) | 32 (84) |
| Acyclovir | 25 (66) |
| Valacyclovir | 8 (21) |
| Valganciclovir | 1 (3) |
| Antifungal prophylaxis, fluconazole, n (%) | 1 (3) |
| Granulocyte colony-stimulating factor, n (%) | 16 (42) |
| Filgrastim | 9 (24) |
| Pegfilgrastim | 7 (18) |
| No. of lead-in duv cycles received by subtype, n (%) | 9 (24) |
| AITL | |
| 1 | 2 (5) |
| 2 | 1 (3) |
| 3 | 1 (3) |
| PTCL-NOS | |
| 3 | 2 (6) |
| 5 | 1 (3) |
| CTCL | |
| 1 | 1 (3) |
| ENKTCL | |
| 1 | 1 (3) |
| No. of lead-in romi cycles received by subtype, n (%) | 2 (5) |
| AITL | |
| 4 | 1 (3) |
| PTCL/CTCL-MF | |
| 4 | 1 (3) |
| No. of combination duv/romi cycles received, median (IQR) | 3 (2-4) |
| nTFH including AITL | 4 (3-5) |
| PTCL-NOS | 2 (2-3) |
| CTCL | 2 (2-3) |
| ALK– ALCL | 4 |
| ENKTCL | 4 |
| ATLL | 3 |
| HSTCL | 2 |
| Duration of combination duv/romi treatment at full/abbreviated doses, median (IQR), mo | 2.9 (1.8-4.1) |
| nTFH including AITL | 3.9 (3.1-4.9) |
| PTCL-NOS | 2.0 (1.3-2.8) |
| CTCL | 1.8 (1.7-3.0) |
| ALK– ALCL | 3.4 |
| ENKTCL | 3.7 |
| ATLL | 2.0 |
| HSTCL | 1.4 |
| Treatment characteristics . | All∗ (N = 38) . |
|---|---|
| Pneumocystis jirovecii pneumonia prophylaxis, n (%) | 31 (82) |
| Atovaquone | 12 (32) |
| Trimethoprim-sulfamethoxazole | 21 (55) |
| Varicella zoster virus prophylaxis, n (%) | 32 (84) |
| Acyclovir | 25 (66) |
| Valacyclovir | 8 (21) |
| Valganciclovir | 1 (3) |
| Antifungal prophylaxis, fluconazole, n (%) | 1 (3) |
| Granulocyte colony-stimulating factor, n (%) | 16 (42) |
| Filgrastim | 9 (24) |
| Pegfilgrastim | 7 (18) |
| No. of lead-in duv cycles received by subtype, n (%) | 9 (24) |
| AITL | |
| 1 | 2 (5) |
| 2 | 1 (3) |
| 3 | 1 (3) |
| PTCL-NOS | |
| 3 | 2 (6) |
| 5 | 1 (3) |
| CTCL | |
| 1 | 1 (3) |
| ENKTCL | |
| 1 | 1 (3) |
| No. of lead-in romi cycles received by subtype, n (%) | 2 (5) |
| AITL | |
| 4 | 1 (3) |
| PTCL/CTCL-MF | |
| 4 | 1 (3) |
| No. of combination duv/romi cycles received, median (IQR) | 3 (2-4) |
| nTFH including AITL | 4 (3-5) |
| PTCL-NOS | 2 (2-3) |
| CTCL | 2 (2-3) |
| ALK– ALCL | 4 |
| ENKTCL | 4 |
| ATLL | 3 |
| HSTCL | 2 |
| Duration of combination duv/romi treatment at full/abbreviated doses, median (IQR), mo | 2.9 (1.8-4.1) |
| nTFH including AITL | 3.9 (3.1-4.9) |
| PTCL-NOS | 2.0 (1.3-2.8) |
| CTCL | 1.8 (1.7-3.0) |
| ALK– ALCL | 3.4 |
| ENKTCL | 3.7 |
| ATLL | 2.0 |
| HSTCL | 1.4 |
Lead-in duv, cycles of combination duv/romi, and duration of treatment were reported in all lines of therapy (N = 38).
Efficacy
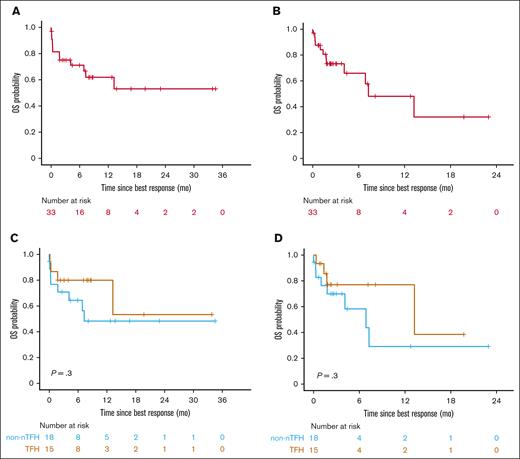
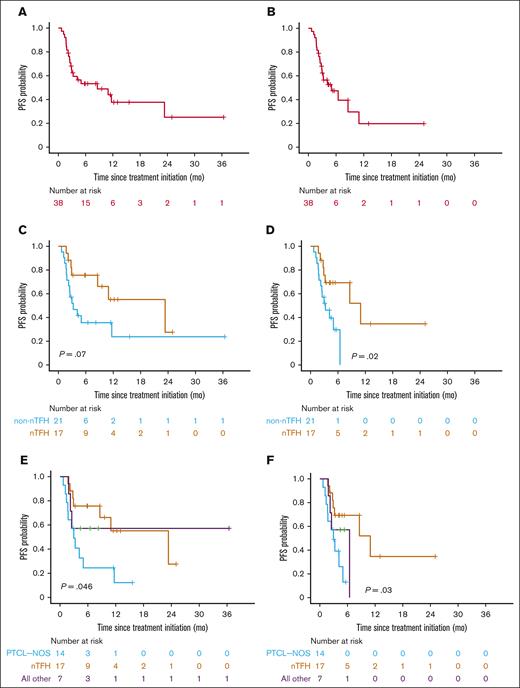
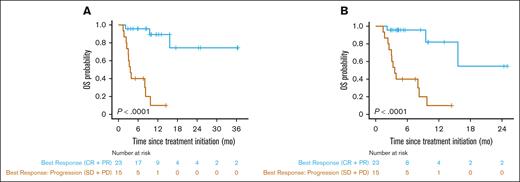
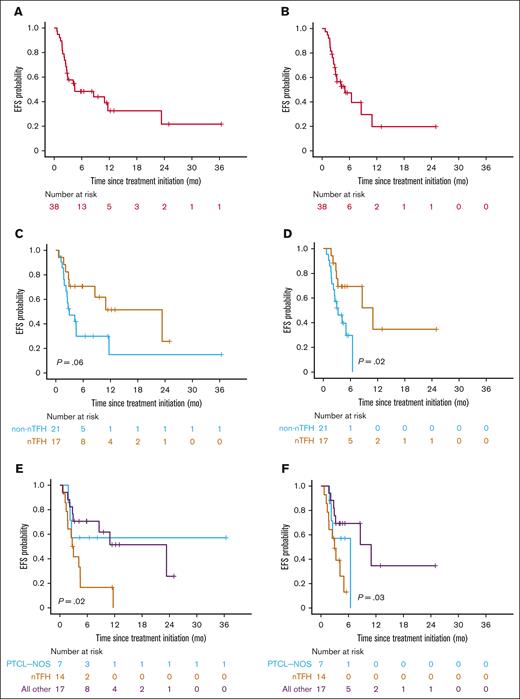
Duv/romi showed varied efficacy across disease subtypes, with an ORR of 61% (47% CRR, 13% PRR) with or without duv lead-in. Patients with nTFH subtype exhibited a significantly higher ORR of 82% (71% CRR, 12% PRR; P = .02 by Fisher exact test) compared with patients with non-nTFH subtypes such as PTCL-NOS (36% ORR, 29% CRR, and 7% PRR), with a median follow-up of 11 months (95% confidence interval [CI], 9.5-17; Table 3). Of note, 11 of the 13 (85%) patients with angioimmunoblastic T-cell lymphoma under the nTFH category demonstrated a CR. The median OS since treatment initiation was 16 months in the cohort (95% CI, 9.5 to NR) upon standard censoring, as opposed to 9.7 months (95% CI, 8.3 to NR) after censoring patients who underwent allo-HSCT (Figure 1A-B; Table 3). The median OS was 16 months for the nTFH subtype (95% CI, 9.5 to NR), as opposed to NR (95% CI, 8.0 to NR) for the non-nTFH subtype (Figure 1C). When censored by allo-HSCT, the median OS for patients with nTFH (16 months; 95% CI, 9.5 to NR) was longer than that of the non-nTFH subtype (8.3 months; 95% CI, 8.0 to NR), but the difference was not statistically significant (Figure 1D). The median PFS of patients with nTFH subtype (23 months; 95% CI, 8.6 to NR) was comparable to that of patients with non-nTFH subtype (3.3 months; 95% CI, 2.2 to NR) with standard censoring, but was significantly higher with allo-HSCT censoring (11 months; 95% CI, 8.6 to NR vs 3.3 months; 95% CI, 2.2 to NR; P = .03; respectively; Figure 2; Tables 3 and 4). When stratified by response type, patients with ORR (CRR + PRR) demonstrated significantly longer OS (P < .0001) than those who demonstrated progression of disease (stable disease + progressive disease [PD]) regardless of censoring (Figure 3; Table 4). The median event-free survival showed a very similar pattern to the median PFS in the overall cohort, and histological subgroup comparisons demonstrated significant differences between nTFH and non-nTFH groups only under allo-HSCT censoring (Figure 4; Table 4).
Efficacy of combination duv/romi in multicenter R/R cohort
| Outcome measure . | All (N = 38) . | Lead-in duv (n = 10) . | No lead-in duv (n = 28) . | nTFH subtype (including AITL) (n = 17) . | Non-nTFH subtype (n = 21) . | AITL (n = 13) . | PTCL-NOS (n = 14) . | CTCL (n = 3) . | ALK– ALCL (n = 1) . | ENKTCL (n = 1) . | ATLL (n = 1) . | HSTCL (n = 1) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment response, n (%) | ||||||||||||
| OR∗ | 23 (61) | 5 (50) | 18 (64) | 14 (82) | 9 (43) | 11 (85) | 5 (36) | 2 (67) | 1 (100) | 1 (100) | 0 | 0 |
| CR | 18 (47) | 4 (40) | 14 (50) | 12 (71) | 6 (29) | 11 (85) | 4 (29) | 0 | 1 (100) | 1 (100) | 0 | 0 |
| PR | 5 (13) | 1 (10) | 4 (14) | 2 (12) | 3 (14) | 0 | 1 (7) | 2 (67) | 0 | 0 | 0 | 0 |
| SD | 2 (5) | 1 (10) | 1 (4) | 0 | 2 (10) | 0 | 2 (14) | 0 | 0 | 0 | 0 | 0 |
| PD | 13 (34) | 4 (40) | 9 (32) | 3 (18) | 10 (48) | 2 (15) | 7 (50) | 1 (33) | 0 | 0 | 1 (100) | 1 (100) |
| Survival probability, mo | ||||||||||||
| T = 0: best response date | ||||||||||||
| OS | All other subtypes: | |||||||||||
| Median (95% CI) | 13 (6.9 to NR) | 13 (3.2 to NR) | 7.3 (4.1 to NR) | 13 (3.2 to NR) | NR (1.6 to NR) | 6.9 (1.8 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.7 (0.5-0.8) | 0.7 (0.5-1.0) | 0.6 (0.5-0.9) | 0.7 (0.5-1.0) | 0.6 (0.4-0.9) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.6 (0.4-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.9) | 0.4 (0.1-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.5 (0.3-0.8) | 0.5 (0.2-1.0) | 0.5 (0.3-0.8) | 0.5 (0.2-1.0) | - | 0.4 (0.1-1.0) | ||||||
| OS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 7.3 (4.1 to NR) | 13 (3.2 to NR) | 6.9 (4.1 to NR) | 13 (3.2 to NR) | 7.3 (1.6 to NR) | 6.9 (1.8 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.6 (0.4-0.8) | 0.6 (0.3-1.0) | 0.6 (0.4-0.9) | 0.6 (0.3-1.0) | 0.5 (0.3-1.0) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.4 (0.3-0.8) | 0.6 (0.3-1.0) | 0.3 (0.1-0.9) | 0.6 (0.3-1.0) | 0.4 (0.1-1.0) | — | ||||||
| PFS | ||||||||||||
| Median (95% CI) | 2.8 (1.1 to NR) | 21 (2.3 to NR) | 1.7 (0 to NR) | 21 (8.6 to NR) | 0.9 (0 to NR) | NR (0 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.4-0.7) | 0.7 (0.5-1.0) | 0.3 (0.2-0.6) | 0.8 (0.6-1.0) | 0.3 (0.1-0.7) | 0.5 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.3 (0.2-0.6) | 0.6 (0.3-1.0) | 0.2 (0.1-0.6) | 0.6 (0.4-1.0) | 0.1 (0.02-0.7) | 0.5 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.04-0.8) | — | 0.2 (0.1-0.6) | — | — | 0.5 (0.3-1.0) | ||||||
| PFS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 1.8 (1.1 to NR) | 8.6 (1.3 to NR) | 1.1 (0 to NR) | 8.6 (2.3 to NR) | 0.9 (0 to NR) | 1.1 (0 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.4 (0.2-0.6) | — | — | — | — | — | ||||||
| S(t) at t = 12 (95% CI) | 0.2 (0.04-0.8) | — | — | — | — | — | ||||||
| T = 0: treatment start date | ||||||||||||
| OS | ||||||||||||
| Median (95% CI) | 16 (9.5 to NR) | 16 (9.5 to NR) | NR (8.0 to NR) | 16 (9.5 to NR) | NR (8.3 to NR) | NR (3.6 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.7 (0.6-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-1.0) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.6 (0.4-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.9) | 0.5 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.5 (0.3-0.8) | 0.5 (0.2-1.0) | 0.5 (0.3-0.8) | 0.4 (0.2-1.0) | 0.5 (0.3-0.9) | 0.5 (0.3-1.0) | ||||||
| OS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 9.7 (8.3 to NR) | 16 (9.5 to NR) | 8.3 (8.0 to NR) | 16 (9.5 to NR) | 9.7 (3.3 to NR) | 8.0 (3.6 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.7 (0.6-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-1.0) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.5 (0.3-0.8) | 0.6 (0.4-1.0) | 0.4 (0.2-0.8) | 0.6 (0.3-1.0) | 0.3 (0.1-1.0) | — | ||||||
| S(t) at t = 24 (95% CI) | 0.3 (0.1-0.8) | 0.3 (0.1-1.0) | 0.4 (0.2-0.8) | 0.3 (0.1-1.0) | 0.3 (0.1-1.0) | — | ||||||
| PFS | ||||||||||||
| Median (95% CI) | 8.6 (3.0 to NR) | 23 (8.6 to NR) | 3.3 (2.2 to NR) | 23 (11 to NR) | 3.0 (1.7 to NR) | NR (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.4-0.7) | 0.8 (0.6-1.0) | 0.4 (0.2-0.6) | 0.8 (0.7-1.0) | 0.2 (0.1-0.6) | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.4 (0.2-0.6) | 0.6 (0.3-0.9) | 0.2 (0.1-0.6) | 0.6 (0.4-1.0) | 0.1 (0.02-0.7) | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.3 (0.1-0.6) | 0.3 (0.1-1.0) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.6 (0.3-1.0) | ||||||
| PFS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 5.0 (3.0 to NR) | 11 (8.6 to NR) | 3.3 (2.2 to NR) | 11 (8.6 to NR) | 3.0 (1.7 to NR) | 6.5 (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.3-0.7) | 0.7 (0.5-1.0) | 0.3 (0.1-0.7) | 0.8 (0.7-1.0) | — | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| EFS | ||||||||||||
| Median (95% CI) | 4.5 (2.8 to NR) | 23 (8.6 to NR) | 3.0 (2.2 to NR) | 23 (8.6 to NR) | 2.8 (1.7 to NR) | NR (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.3-0.7) | 0.7 (0.5-1.0) | 0.3 (0.1-0.6) | 0.8 (0.6-1.0) | 0.2 (0.04-0.6) | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.3 (0.2-0.6) | 0.5 (0.3-0.9) | 0.2 (0.03-0.7) | 0.6 (0.3-1.0) | — | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | 0.2 (0.03-0.7) | 0.3 (0.1-1.0) | — | 0.6 (0.3-1.0) | ||||||
| EFS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 5.0 (3.0 to NR) | 11 (8.6 to NR) | 3.3 (2.2 to NR) | 11 (8.6 to NR) | 3.0 (1.7 to NR) | 6.5 (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.3-0.7) | 0.7 (0.5-1.0) | 0.3 (0.1-0.1) | 0.8 (0.7-1.0) | — | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| Duration, mo | ||||||||||||
| TTNT,† median (95% CI) | 17 (6.4 to NR) | 11 (11 to NR) | 17 (3.7 to NR) | 25 (11 to NR) | 6.4 (2.5 to NR) | 25 (25 to NR) | 6.4 (3.5 to NR) | 6.5 (2.3 to NR) | ||||
| Time of follow-up,‡ median (95% CI) | 11 (9.5-17) | 18 (10 to NR) | 11 (8.5 to 17) | 11 (8.5 to NR) | 11 (8.2 to NR) | 12 (9.5 to NR) | 14 (7.5 to NR) | 10 (8.2 to NR) | ||||
| (n = 23) | (n = 5) | (n = 18) | (n = 14) | (n = 9) | (n = 11) | (n = 5) | (n = 4) | |||||
| TTR,§ median (IQR) | 1.9 (1.7-2.6) | 2.4 (2.3-5.6) | 1.8 (1.7-2.2) | 2.0 (1.8-2.8) | 1.8 (1.5-2.3) | 2.1 (1.7-2.6) | 1.5 (1.4-1.8) | 2.1 (1.8-3.1) | ||||
| DoR,|| median (95% CI) | 21 (11 to NR) | 8.6 (2.3 to NR) | 21 (11 to NR) | 21 (8.6 to NR) | 11 (11 to NR) | 21 (8.6 to NR) | 11 (1.8 to NR) | NR (0.9 to NR) | ||||
| Allo-HSCT consolidation after duv/romi, n (%) | ||||||||||||
| Immediately following (including bridging therapy) | 11 (29) | 1 (10) | 10 (36) | 6 (35) | 5 (24) | 5 (38) | 3 (21) | 2 (29) | ||||
| Any time following (including future therapy lines) | 14 (37) | 2 (20) | 12 (43) | 7 (41) | 7 (33) | 5 (38) | 4 (29) | 3 (43) | ||||
| Outcome measure . | All (N = 38) . | Lead-in duv (n = 10) . | No lead-in duv (n = 28) . | nTFH subtype (including AITL) (n = 17) . | Non-nTFH subtype (n = 21) . | AITL (n = 13) . | PTCL-NOS (n = 14) . | CTCL (n = 3) . | ALK– ALCL (n = 1) . | ENKTCL (n = 1) . | ATLL (n = 1) . | HSTCL (n = 1) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment response, n (%) | ||||||||||||
| OR∗ | 23 (61) | 5 (50) | 18 (64) | 14 (82) | 9 (43) | 11 (85) | 5 (36) | 2 (67) | 1 (100) | 1 (100) | 0 | 0 |
| CR | 18 (47) | 4 (40) | 14 (50) | 12 (71) | 6 (29) | 11 (85) | 4 (29) | 0 | 1 (100) | 1 (100) | 0 | 0 |
| PR | 5 (13) | 1 (10) | 4 (14) | 2 (12) | 3 (14) | 0 | 1 (7) | 2 (67) | 0 | 0 | 0 | 0 |
| SD | 2 (5) | 1 (10) | 1 (4) | 0 | 2 (10) | 0 | 2 (14) | 0 | 0 | 0 | 0 | 0 |
| PD | 13 (34) | 4 (40) | 9 (32) | 3 (18) | 10 (48) | 2 (15) | 7 (50) | 1 (33) | 0 | 0 | 1 (100) | 1 (100) |
| Survival probability, mo | ||||||||||||
| T = 0: best response date | ||||||||||||
| OS | All other subtypes: | |||||||||||
| Median (95% CI) | 13 (6.9 to NR) | 13 (3.2 to NR) | 7.3 (4.1 to NR) | 13 (3.2 to NR) | NR (1.6 to NR) | 6.9 (1.8 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.7 (0.5-0.8) | 0.7 (0.5-1.0) | 0.6 (0.5-0.9) | 0.7 (0.5-1.0) | 0.6 (0.4-0.9) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.6 (0.4-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.9) | 0.4 (0.1-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.5 (0.3-0.8) | 0.5 (0.2-1.0) | 0.5 (0.3-0.8) | 0.5 (0.2-1.0) | - | 0.4 (0.1-1.0) | ||||||
| OS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 7.3 (4.1 to NR) | 13 (3.2 to NR) | 6.9 (4.1 to NR) | 13 (3.2 to NR) | 7.3 (1.6 to NR) | 6.9 (1.8 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.6 (0.4-0.8) | 0.6 (0.3-1.0) | 0.6 (0.4-0.9) | 0.6 (0.3-1.0) | 0.5 (0.3-1.0) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.4 (0.3-0.8) | 0.6 (0.3-1.0) | 0.3 (0.1-0.9) | 0.6 (0.3-1.0) | 0.4 (0.1-1.0) | — | ||||||
| PFS | ||||||||||||
| Median (95% CI) | 2.8 (1.1 to NR) | 21 (2.3 to NR) | 1.7 (0 to NR) | 21 (8.6 to NR) | 0.9 (0 to NR) | NR (0 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.4-0.7) | 0.7 (0.5-1.0) | 0.3 (0.2-0.6) | 0.8 (0.6-1.0) | 0.3 (0.1-0.7) | 0.5 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.3 (0.2-0.6) | 0.6 (0.3-1.0) | 0.2 (0.1-0.6) | 0.6 (0.4-1.0) | 0.1 (0.02-0.7) | 0.5 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.04-0.8) | — | 0.2 (0.1-0.6) | — | — | 0.5 (0.3-1.0) | ||||||
| PFS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 1.8 (1.1 to NR) | 8.6 (1.3 to NR) | 1.1 (0 to NR) | 8.6 (2.3 to NR) | 0.9 (0 to NR) | 1.1 (0 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.4 (0.2-0.6) | — | — | — | — | — | ||||||
| S(t) at t = 12 (95% CI) | 0.2 (0.04-0.8) | — | — | — | — | — | ||||||
| T = 0: treatment start date | ||||||||||||
| OS | ||||||||||||
| Median (95% CI) | 16 (9.5 to NR) | 16 (9.5 to NR) | NR (8.0 to NR) | 16 (9.5 to NR) | NR (8.3 to NR) | NR (3.6 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.7 (0.6-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-1.0) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.6 (0.4-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.8) | 0.7 (0.5-1.0) | 0.5 (0.3-0.9) | 0.5 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.5 (0.3-0.8) | 0.5 (0.2-1.0) | 0.5 (0.3-0.8) | 0.4 (0.2-1.0) | 0.5 (0.3-0.9) | 0.5 (0.3-1.0) | ||||||
| OS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 9.7 (8.3 to NR) | 16 (9.5 to NR) | 8.3 (8.0 to NR) | 16 (9.5 to NR) | 9.7 (3.3 to NR) | 8.0 (3.6 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.7 (0.6-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-0.9) | 0.8 (0.6-1.0) | 0.7 (0.5-1.0) | 0.7 (0.4-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.5 (0.3-0.8) | 0.6 (0.4-1.0) | 0.4 (0.2-0.8) | 0.6 (0.3-1.0) | 0.3 (0.1-1.0) | — | ||||||
| S(t) at t = 24 (95% CI) | 0.3 (0.1-0.8) | 0.3 (0.1-1.0) | 0.4 (0.2-0.8) | 0.3 (0.1-1.0) | 0.3 (0.1-1.0) | — | ||||||
| PFS | ||||||||||||
| Median (95% CI) | 8.6 (3.0 to NR) | 23 (8.6 to NR) | 3.3 (2.2 to NR) | 23 (11 to NR) | 3.0 (1.7 to NR) | NR (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.4-0.7) | 0.8 (0.6-1.0) | 0.4 (0.2-0.6) | 0.8 (0.7-1.0) | 0.2 (0.1-0.6) | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.4 (0.2-0.6) | 0.6 (0.3-0.9) | 0.2 (0.1-0.6) | 0.6 (0.4-1.0) | 0.1 (0.02-0.7) | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.3 (0.1-0.6) | 0.3 (0.1-1.0) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.6 (0.3-1.0) | ||||||
| PFS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 5.0 (3.0 to NR) | 11 (8.6 to NR) | 3.3 (2.2 to NR) | 11 (8.6 to NR) | 3.0 (1.7 to NR) | 6.5 (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.3-0.7) | 0.7 (0.5-1.0) | 0.3 (0.1-0.7) | 0.8 (0.7-1.0) | — | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| EFS | ||||||||||||
| Median (95% CI) | 4.5 (2.8 to NR) | 23 (8.6 to NR) | 3.0 (2.2 to NR) | 23 (8.6 to NR) | 2.8 (1.7 to NR) | NR (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.3-0.7) | 0.7 (0.5-1.0) | 0.3 (0.1-0.6) | 0.8 (0.6-1.0) | 0.2 (0.04-0.6) | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.3 (0.2-0.6) | 0.5 (0.3-0.9) | 0.2 (0.03-0.7) | 0.6 (0.3-1.0) | — | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | 0.2 (0.03-0.7) | 0.3 (0.1-1.0) | — | 0.6 (0.3-1.0) | ||||||
| EFS, allo-HSCT–censored | ||||||||||||
| Median (95% CI) | 5.0 (3.0 to NR) | 11 (8.6 to NR) | 3.3 (2.2 to NR) | 11 (8.6 to NR) | 3.0 (1.7 to NR) | 6.5 (2.2 to NR) | ||||||
| S(t) at t = 6 (95% CI) | 0.5 (0.3-0.7) | 0.7 (0.5-1.0) | 0.3 (0.1-0.1) | 0.8 (0.7-1.0) | — | 0.6 (0.3-1.0) | ||||||
| S(t) at t = 12 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| S(t) at t = 24 (95% CI) | 0.2 (0.1-0.6) | 0.3 (0.1-1.0) | — | 0.4 (0.2-1.0) | — | — | ||||||
| Duration, mo | ||||||||||||
| TTNT,† median (95% CI) | 17 (6.4 to NR) | 11 (11 to NR) | 17 (3.7 to NR) | 25 (11 to NR) | 6.4 (2.5 to NR) | 25 (25 to NR) | 6.4 (3.5 to NR) | 6.5 (2.3 to NR) | ||||
| Time of follow-up,‡ median (95% CI) | 11 (9.5-17) | 18 (10 to NR) | 11 (8.5 to 17) | 11 (8.5 to NR) | 11 (8.2 to NR) | 12 (9.5 to NR) | 14 (7.5 to NR) | 10 (8.2 to NR) | ||||
| (n = 23) | (n = 5) | (n = 18) | (n = 14) | (n = 9) | (n = 11) | (n = 5) | (n = 4) | |||||
| TTR,§ median (IQR) | 1.9 (1.7-2.6) | 2.4 (2.3-5.6) | 1.8 (1.7-2.2) | 2.0 (1.8-2.8) | 1.8 (1.5-2.3) | 2.1 (1.7-2.6) | 1.5 (1.4-1.8) | 2.1 (1.8-3.1) | ||||
| DoR,|| median (95% CI) | 21 (11 to NR) | 8.6 (2.3 to NR) | 21 (11 to NR) | 21 (8.6 to NR) | 11 (11 to NR) | 21 (8.6 to NR) | 11 (1.8 to NR) | NR (0.9 to NR) | ||||
| Allo-HSCT consolidation after duv/romi, n (%) | ||||||||||||
| Immediately following (including bridging therapy) | 11 (29) | 1 (10) | 10 (36) | 6 (35) | 5 (24) | 5 (38) | 3 (21) | 2 (29) | ||||
| Any time following (including future therapy lines) | 14 (37) | 2 (20) | 12 (43) | 7 (41) | 7 (33) | 5 (38) | 4 (29) | 3 (43) | ||||
EFS, event-free survival; OR, overall response; PET, positron emission tomography; PR, partial response; S(t), survival probability; SD, stable disease; TTNT, time to next therapy; TTR, time to response.
Response rates were measured at best PET response to duv/romi.
TTNT was measured from treatment start date to initiation of next line of therapy after duv/romi, only in patients who received a subsequent line of therapy after duv/romi. Patients who subsequently underwent stem cell transplantation were censored at that event date.
Time of follow-up was measured from treatment initiation date to date of latest clinical update.
TTR was reported from treatment start date to date of first response, only in patients who achieved CR/PR to duv/romi.
DoR was measured from date of first evaluable PET response to date of progression or last follow-up, only in patients who achieved CR/PR to duv/romi.
OS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show OS estimates since duv/romi treatment initiation. (A) Overall cohort with standard censoring. (B) Overall cohort with allo-HSCT after duv/romi as censoring events. (C) Comparison by histological subtype (nTFH vs non-nTFH) with standard censoring. (D) Comparison by histological subtype (nTFH vs non-nTFH) and allo-HSCT censored. P values calculated by log-rank test.
OS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show OS estimates since duv/romi treatment initiation. (A) Overall cohort with standard censoring. (B) Overall cohort with allo-HSCT after duv/romi as censoring events. (C) Comparison by histological subtype (nTFH vs non-nTFH) with standard censoring. (D) Comparison by histological subtype (nTFH vs non-nTFH) and allo-HSCT censored. P values calculated by log-rank test.
PFS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show PFS estimates since duv/romi treatment initiation. (A) Overall cohort with standard censoring. (B) Overall cohort with allo-HSCT after duv/romi as censoring events. (C) Comparison by histological subtype (nTFH vs non-nTFH) with standard censoring. (D) Comparison by histological subtype (nTFH vs non-nTFH) and HSCT censored. (E) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) with standard censoring. (F) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) and allo-HSCT censored. P values calculated by log-rank test.
PFS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show PFS estimates since duv/romi treatment initiation. (A) Overall cohort with standard censoring. (B) Overall cohort with allo-HSCT after duv/romi as censoring events. (C) Comparison by histological subtype (nTFH vs non-nTFH) with standard censoring. (D) Comparison by histological subtype (nTFH vs non-nTFH) and HSCT censored. (E) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) with standard censoring. (F) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) and allo-HSCT censored. P values calculated by log-rank test.
Survival estimates and hazard ratios of combination duv/romi in multicenter R/R cohort
| . | HR [95% CI] (P value) . | ||
|---|---|---|---|
| nTFH vs non-nTFH (ref) . | nTFH vs PTCL-NOS vs all other (ref) . | CRR/PRR (ref) vs progression . | |
| OS∗ | |||
| Standard censoring | 0.72 [0.26-2.02] (.5) | nTFH: 0.74 [0.18-3.0] (.7); PTCL-NOS: 1.0 [0.26-4.2] (.96) | 17.9 [3.9-82] (.0002) |
| Allo-HSCT–censored | 0.66 [0.23-1.87] (.4) | nTFH: 0.66 [0.16-2.7] (.6); PTCL-NOS: 0.99 [0.25-4.0] (.99) | 13.0 [2.9-58] (.0008) |
| PFS∗ | |||
| Standard censoring | 0.44 [0.18-1.09] (.07) | nTFH: 0.82 [0.21-3.2] (.8) PTCL-NOS: 2.50 [0.68-9.2] (.2) | — |
| Allo-HSCT–censored | 0.31 [0.11-0.87] (.03) | nTFH: 0.45 [0.12-1.7] (.2) PTCL-NOS: 1.84 [0.57-6.0] (.3) | — |
| EFS∗ | |||
| Standard censoring | 0.45 [0.19-1.07] (.07) | nTFH: 0.93 [0.25-3.5] (.9) PTCL-NOS: 2.9 [0.80-11] (.1) | |
| Allo-HSCT–censored | 0.31 [0.11-0.87] (.03) | nTFH: 0.45 [0.12-1.7] (.2) PTCL-NOS: 1.84 [0.57-6.0] (.3) | |
| Treatment response and duration | |||
| ORR | P† = .02 | ||
| TTNT | P‡ = .2 | ||
| Time of follow-up | P‡ = .9 | ||
| TTR | P§ = .3 | ||
| DoR | P‡ = .8 | ||
| . | HR [95% CI] (P value) . | ||
|---|---|---|---|
| nTFH vs non-nTFH (ref) . | nTFH vs PTCL-NOS vs all other (ref) . | CRR/PRR (ref) vs progression . | |
| OS∗ | |||
| Standard censoring | 0.72 [0.26-2.02] (.5) | nTFH: 0.74 [0.18-3.0] (.7); PTCL-NOS: 1.0 [0.26-4.2] (.96) | 17.9 [3.9-82] (.0002) |
| Allo-HSCT–censored | 0.66 [0.23-1.87] (.4) | nTFH: 0.66 [0.16-2.7] (.6); PTCL-NOS: 0.99 [0.25-4.0] (.99) | 13.0 [2.9-58] (.0008) |
| PFS∗ | |||
| Standard censoring | 0.44 [0.18-1.09] (.07) | nTFH: 0.82 [0.21-3.2] (.8) PTCL-NOS: 2.50 [0.68-9.2] (.2) | — |
| Allo-HSCT–censored | 0.31 [0.11-0.87] (.03) | nTFH: 0.45 [0.12-1.7] (.2) PTCL-NOS: 1.84 [0.57-6.0] (.3) | — |
| EFS∗ | |||
| Standard censoring | 0.45 [0.19-1.07] (.07) | nTFH: 0.93 [0.25-3.5] (.9) PTCL-NOS: 2.9 [0.80-11] (.1) | |
| Allo-HSCT–censored | 0.31 [0.11-0.87] (.03) | nTFH: 0.45 [0.12-1.7] (.2) PTCL-NOS: 1.84 [0.57-6.0] (.3) | |
| Treatment response and duration | |||
| ORR | P† = .02 | ||
| TTNT | P‡ = .2 | ||
| Time of follow-up | P‡ = .9 | ||
| TTR | P§ = .3 | ||
| DoR | P‡ = .8 | ||
HR, hazard ratio; ref, reference.
OS, PFS, and EFS calculated since treatment initiation.
P value calculated by Fisher exact test.
P value calculated by log-rank test.
P value calculated by Kruskal-Wallis test.
OS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show OS estimates since duv/romi treatment initiation. (A) Comparison by best response (CR + PR) vs best response: progression (PD + SD) on duv/romi with standard censoring. (B) Comparison by best response (CR + PR) vs best response: progression (PD + SD) on duv/romi and allo-HSCT censored. P values calculated by log-rank test. PR, partial response; SD, stable disease.
OS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show OS estimates since duv/romi treatment initiation. (A) Comparison by best response (CR + PR) vs best response: progression (PD + SD) on duv/romi with standard censoring. (B) Comparison by best response (CR + PR) vs best response: progression (PD + SD) on duv/romi and allo-HSCT censored. P values calculated by log-rank test. PR, partial response; SD, stable disease.
EFS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show EFS estimates since duv/romi treatment initiation. (A) Overall cohort with standard censoring. (B) Overall cohort with allo-HSCT after duv/romi as censoring events. (C) Comparison by histological subtype (nTFH vs non-nTFH) with standard censoring. (D) Comparison by histological subtype (nTFH vs non-nTFH) and allo-HSCT censored. (E) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) with standard censoring. (F) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) and allo-HSCT censored. P values calculated by log-rank test. EFS, event-free survival.
EFS for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Kaplan-Meier curves show EFS estimates since duv/romi treatment initiation. (A) Overall cohort with standard censoring. (B) Overall cohort with allo-HSCT after duv/romi as censoring events. (C) Comparison by histological subtype (nTFH vs non-nTFH) with standard censoring. (D) Comparison by histological subtype (nTFH vs non-nTFH) and allo-HSCT censored. (E) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) with standard censoring. (F) Comparison by histological subtype (PTCL-NOS, nTFH subtype, and other) and allo-HSCT censored. P values calculated by log-rank test. EFS, event-free survival.
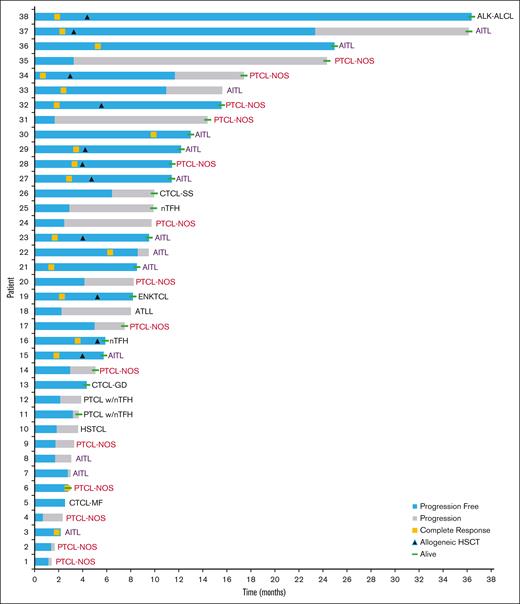
The overall median time to first response among responding patients was 1.9 months (interquartile range [IQR], 1.7-2.6), with a median DoR of 21 months (95% CI, 11 to NR), and was longer in nTFH (21 months; 95% CI, 8.6 to NR) compared with the non-nTFH subtype (11 months; 95% CI, 11 to NR). However, this difference was not statistically significant given the comparable follow-up time and time to next therapy. At the time of data cutoff, 3 of the 17 (18%) responders remained on active treatment (Table 5). Eleven (33%) patients (6 nTFH and 5 non-nTFH) immediately proceeded to allo-HSCT following combination treatment with duv/romi with curative intent. Among these 11 patients, the median age was lower (50 vs 66 years; P = .008), with no significant difference in PIT score compared with those who did not undergo allo-HSCT (P = .5). They had received a median of 1 previous line of therapy (IQR, 1-2), and their survival rate at the last follow-up was 100%, as opposed to 45% in the non–allo-HSCT group (P = .002), with a median follow-up time of 7.5 months (IQR, 4.2-12.2). Of the 11 patients, 10 (91%) patients proceeded to allo-HSCT and were in remission at day 100 after HSCT; 3 of the 10 (30%) patients remained in remission at day +290. Two patients demonstrated disease progression after day 200, and 1 patient was not yet evaluable. Two (n = 2/11 [18%]) patients experienced graft-versus-host disease (GVHD; acute stage 1 and 2 progressing to chronic) after allo-HSCT immediately following duv/romi, and GVHD was managed according to institutional standards. One patient with a CR to duv/romi died of bacterial sepsis. Of the 21 patients who developed PD on duv/romi, 6 died of lymphoma progression, 1 transitioned to hospice care, and 14 received subsequent salvage therapies. The most commonly used next-line treatment was CFT74455 (an IKZF1/3 degrader) in 3 patients, of whom, 1 (nTFH) achieved a CR followed by allo-HSCT, whereas 2 patients (PTCL-NOS) demonstrated PD. Among the 2 patients with PD, 1 (nTFH) received azacitidine and lenalidomide, achieved a CR, but eventually died of an infectious complication (Epstein–Barr virus reactivation leading to extranodal natural killer/T-cell lymphoma), while the other (Sézary syndrome) received alemtuzumab, achieved a CR, and proceeded to allo-HSCT. Three patients were unevaluable for their next line of therapy because they were transitioned to hospice soon after (nTFH on azacitidine alone, PTCL-NOS on gemcitabine, carboplatin, and dexamethasone, and PTCL-NOS on duv and ruxolitinib). Seven patients demonstrated PD on various different regimens spanning conventional chemotherapy, CFT74455, and mogamulizumab. A swimmer plot depicting responses of the cohort patients over time is shown in Figure 5.
Patient outcomes status after combination therapy with duv/romi in multicenter R/R cohort
| Clinical outcomes . | All (N = 38) . |
|---|---|
| Outcomes in responders following duv/romi, n (%) | 17/38 (45) |
| On active duv/romi therapy | 3/17 (18) |
| PR on duv/romi, PD despite switch to pralatrexate | 1/17 (6) |
| Discontinued duv/romi due to patient preference | 1/17 (6) |
| Bridged to allo-HSCT | 11/17 (65) |
| CR at day 100 | 10/11 (91) |
| CR at day +290 | 3/10 (30) |
| PD after day 200 | 2/10 (20)∗ |
| Not yet evaluable (day <100) | 1/11 (9) |
| Death due to duv/romi AE | 1/17 (6) |
| Outcomes in PD, n (%) | 21/38 (55) |
| Salvage therapy after duv/romi | 14/21 (67) |
| Death due to lymphoma | 6/21 (29) |
| Hospice care, no known death at data cutoff | 1/21 (5) |
| Therapy received directly after duv/romi progression and response, n (%) | 14/21 (66) |
| CFT74455 (IKZF1/3 degrader) | 3/21 (14) |
| CR + bridge to allo-HSCT | 1 (5) |
| PD | 2 (10) |
| Azacitidine, not evaluable, transition to hospice | 1/21 (5) |
| Azacitidine + lenalidomide, CR† | 1/21 (5) |
| Alemtuzumab, CR + bridge to allo-HSCT | 1/21 (5) |
| Brentuximab vedotin + gemcitabine, PD | 1/21 (5) |
| Dexamethasone + methotrexate,‡ PD | 1/21 (5) |
| GVD, PD | 1/21 (5) |
| GCD, not evaluable, transition to hospice | 1/21 (5) |
| GemDOx, PD | 1/21 (5) |
| Gemcitabine, PD | 1/21 (5) |
| Mogamulizumab, PD | 1/21 (5) |
| Duv + ruxolitinib, not evaluable, transition to hospice | 1/21 (5) |
| Clinical outcomes . | All (N = 38) . |
|---|---|
| Outcomes in responders following duv/romi, n (%) | 17/38 (45) |
| On active duv/romi therapy | 3/17 (18) |
| PR on duv/romi, PD despite switch to pralatrexate | 1/17 (6) |
| Discontinued duv/romi due to patient preference | 1/17 (6) |
| Bridged to allo-HSCT | 11/17 (65) |
| CR at day 100 | 10/11 (91) |
| CR at day +290 | 3/10 (30) |
| PD after day 200 | 2/10 (20)∗ |
| Not yet evaluable (day <100) | 1/11 (9) |
| Death due to duv/romi AE | 1/17 (6) |
| Outcomes in PD, n (%) | 21/38 (55) |
| Salvage therapy after duv/romi | 14/21 (67) |
| Death due to lymphoma | 6/21 (29) |
| Hospice care, no known death at data cutoff | 1/21 (5) |
| Therapy received directly after duv/romi progression and response, n (%) | 14/21 (66) |
| CFT74455 (IKZF1/3 degrader) | 3/21 (14) |
| CR + bridge to allo-HSCT | 1 (5) |
| PD | 2 (10) |
| Azacitidine, not evaluable, transition to hospice | 1/21 (5) |
| Azacitidine + lenalidomide, CR† | 1/21 (5) |
| Alemtuzumab, CR + bridge to allo-HSCT | 1/21 (5) |
| Brentuximab vedotin + gemcitabine, PD | 1/21 (5) |
| Dexamethasone + methotrexate,‡ PD | 1/21 (5) |
| GVD, PD | 1/21 (5) |
| GCD, not evaluable, transition to hospice | 1/21 (5) |
| GemDOx, PD | 1/21 (5) |
| Gemcitabine, PD | 1/21 (5) |
| Mogamulizumab, PD | 1/21 (5) |
| Duv + ruxolitinib, not evaluable, transition to hospice | 1/21 (5) |
GCD, gemcitabine, carboplatin, and dexamethasone; GVD, gemcitabine, vinorelbine, and doxorubicin with pralatrexate in cycle 1 only; GemDOx, gemcitabine, oxaliplatin, and dexamethasone.
One patient had relapsed disease after bridging to allo-HSCT, for which they were retreated with combination duv/romi. They achieved a response to retreatment, bridged to a second allo-HSCT, and remain in remission.
Patient developed Epstein–Barr virus viremia–related extranodal natural killer/T-cell lymphoma from severe immunocompromise during cycle 2 and was thus not bridged to allo-HSCT.
Patient developed central nervous system involvement and hence methotrexate was added.
Swimmer plot of patient outcomes over time for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Each horizontal bar represents an individual patient’s treatment timeline, with the x-axis denoting time (months since duv/romi treatment initiation) and the y-axis listing individual patients. Colored segments within the bars indicate different responses (blue denotes progression-free and gray denotes progression). Symbols indicate key clinical events (yellow square denotes complete response, dark blue triangle denotes allogeneic HSCT, and green line denotes alive patient status at the time of data cutoff). AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK–, anaplastic lymphoma kinase–negative; ATLL, adult T-cell leukemia/lymphoma; ENKTCL, extranodal natural killer/T-cell lymphoma; GD, gamma-delta; HSTCL, hepatosplenic T-cell lymphoma; MF, mycosis fungoides; SS, Sézary syndrome; w/nTFH, with nodal T follicular helper phenotype.
Swimmer plot of patient outcomes over time for real-world patients with R/R PTCL and CTCL receiving combination duv/romi. Each horizontal bar represents an individual patient’s treatment timeline, with the x-axis denoting time (months since duv/romi treatment initiation) and the y-axis listing individual patients. Colored segments within the bars indicate different responses (blue denotes progression-free and gray denotes progression). Symbols indicate key clinical events (yellow square denotes complete response, dark blue triangle denotes allogeneic HSCT, and green line denotes alive patient status at the time of data cutoff). AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ALK–, anaplastic lymphoma kinase–negative; ATLL, adult T-cell leukemia/lymphoma; ENKTCL, extranodal natural killer/T-cell lymphoma; GD, gamma-delta; HSTCL, hepatosplenic T-cell lymphoma; MF, mycosis fungoides; SS, Sézary syndrome; w/nTFH, with nodal T follicular helper phenotype.
AEs
Treatment was overall well tolerated, with no cases of gr 3 to 4 elevated bilirubin, or elevated alkaline phosphatase in 36 evaluable patients (Table 6). Notable AEs were mostly hematologic, affecting 72% (26/36) of patients. Neutropenia occurred in 42% (15/36) of patients, with gr 3 and 4 occurring in 14% and 28%, respectively. The median time to onset of neutropenia was 0.5 months (IQR, 0.2-1.4). Febrile neutropenia was less common, occurring in 11% (4/36) of patients, with a median onset of 2.2 months (IQR, 2.1-2.7). Gr 3 anemia was observed in 17% (6/36) of patients, with a median time to onset of 1.9 months (IQR, 0.2-4.2). Thrombocytopenia occurred in 28% (10/36) of patients, with gr 3 and 4 comprising 6% and 22%, respectively, with a median onset of 1.6 months (IQR, 0.4-1.9). Lymphopenia was observed in 42% (15/36) of patients, with gr 3 events (28%) being more common than gr 4 events (14%). The median time to lymphopenia onset was 2.2 months (IQR, 0.8-3.0). Leukocytosis occurred in 11% (4/36) of patients, mostly gr 3 (8%) and 4 (3%), with a median onset of 0.2 months (IQR, 0-1.2).
Gr 3 to 4 AEs associated with combination therapy with duv/romi in multicenter R/R cohort
| AE . | n (%) (n = 36) . | Time to event∗ (mo), median (IQR) . | Dose delay† due to AE, n (%) . |
|---|---|---|---|
| Hematologic | 26 (72) | 5/36 (14) | |
| Neutropenia | 15 (42) | 0.5 (0.2-1.4) | 2/36 (6) |
| Gr 3 | 5 (14) | 1RD (3) | |
| Gr 4 | 10 (28) | 1RD, 1D (6) | |
| Febrile neutropenia | 4 (11) | 2.2 (2.1-2.7) | 0 |
| Gr 3 | 3 (8) | 2RD‡ (6) | |
| Gr 4 | 1 (3) | ||
| Anemia, gr 3 | 6 (17) | 1.9 (0.2-4.2) | 0 |
| Thrombocytopenia | 10 (28) | 1.6 (0.4-1.9) | 4/36 (11) |
| Gr 3 | 2 (6) | 1RD (3) | |
| Gr 4 | 8 (22) | 1RD, 2R (8) | |
| Lymphopenia | 15 (42) | 2.2 (0.8-3.0) | 0 |
| Gr 3 | 10 (28) | ||
| Gr 4 | 5 (14) | ||
| Leukocytosis | 4 (11) | 0.2 (0-1.2) | 0 |
| Gr 3 | 3 (8) | — | |
| Gr 4 | 1 (3) | ||
| Gastrointestinal | 9 (25) | 5/36 (14) | |
| Transaminitis (ALT elevation) | 6 (17) | 2.0 (1.7-2.6) | 5/36 (14) |
| Gr 3 | 5 (14) | 2RD,§ 2D (6) | |
| Gr 4 | 1 (3) | 1RD,D (3) | |
| Transaminitis (AST elevation) | 6 (17) | 1.1 (1.0-1.7) | 3/36 (8) |
| Gr 3 | 1RD,D, 2D (8) | ||
| Enterocolitis | 2 (6) | 2.8 (2.5-3.2) | 1/36 (3) |
| Gr 3 | 1D‖ (3) | ||
| Noninfectious diarrhea | 3.39 | 1/36 (3) | |
| Gr 4 | 1 (3) | 1D‖ (3) | |
| Alkaline phosphatase elevation | 0 | — | 0 |
| Infectious | 9/29 (38) | 3/36 (8) | |
| Cytomegalovirus viremia¶ | 5/29 (17) | 1.2 (1.1-1.4) | 0 |
| TB reactivation | 1 (3) | — | 1R (3) |
| COVID-19 infection | 2 (6) | — | 2RD (6) |
| Pseudomonas bacteremia | 1 (3) | — | 0 |
| Klebsiella bacteremia | 1 (3) | — | 0 |
| Other gr 3 and 4 AEs | 10 (28) | 3/36 (8) | |
| Rash | 5 (14) | 9.5 (1.2-9.9) | 1R (3) |
| Fatigue (gr 3) | 6 (17) | 2.1 (1.5-2.4) | 1D, 1RD (6) |
| Anorexia | 0 | — | 0 |
| AE . | n (%) (n = 36) . | Time to event∗ (mo), median (IQR) . | Dose delay† due to AE, n (%) . |
|---|---|---|---|
| Hematologic | 26 (72) | 5/36 (14) | |
| Neutropenia | 15 (42) | 0.5 (0.2-1.4) | 2/36 (6) |
| Gr 3 | 5 (14) | 1RD (3) | |
| Gr 4 | 10 (28) | 1RD, 1D (6) | |
| Febrile neutropenia | 4 (11) | 2.2 (2.1-2.7) | 0 |
| Gr 3 | 3 (8) | 2RD‡ (6) | |
| Gr 4 | 1 (3) | ||
| Anemia, gr 3 | 6 (17) | 1.9 (0.2-4.2) | 0 |
| Thrombocytopenia | 10 (28) | 1.6 (0.4-1.9) | 4/36 (11) |
| Gr 3 | 2 (6) | 1RD (3) | |
| Gr 4 | 8 (22) | 1RD, 2R (8) | |
| Lymphopenia | 15 (42) | 2.2 (0.8-3.0) | 0 |
| Gr 3 | 10 (28) | ||
| Gr 4 | 5 (14) | ||
| Leukocytosis | 4 (11) | 0.2 (0-1.2) | 0 |
| Gr 3 | 3 (8) | — | |
| Gr 4 | 1 (3) | ||
| Gastrointestinal | 9 (25) | 5/36 (14) | |
| Transaminitis (ALT elevation) | 6 (17) | 2.0 (1.7-2.6) | 5/36 (14) |
| Gr 3 | 5 (14) | 2RD,§ 2D (6) | |
| Gr 4 | 1 (3) | 1RD,D (3) | |
| Transaminitis (AST elevation) | 6 (17) | 1.1 (1.0-1.7) | 3/36 (8) |
| Gr 3 | 1RD,D, 2D (8) | ||
| Enterocolitis | 2 (6) | 2.8 (2.5-3.2) | 1/36 (3) |
| Gr 3 | 1D‖ (3) | ||
| Noninfectious diarrhea | 3.39 | 1/36 (3) | |
| Gr 4 | 1 (3) | 1D‖ (3) | |
| Alkaline phosphatase elevation | 0 | — | 0 |
| Infectious | 9/29 (38) | 3/36 (8) | |
| Cytomegalovirus viremia¶ | 5/29 (17) | 1.2 (1.1-1.4) | 0 |
| TB reactivation | 1 (3) | — | 1R (3) |
| COVID-19 infection | 2 (6) | — | 2RD (6) |
| Pseudomonas bacteremia | 1 (3) | — | 0 |
| Klebsiella bacteremia | 1 (3) | — | 0 |
| Other gr 3 and 4 AEs | 10 (28) | 3/36 (8) | |
| Rash | 5 (14) | 9.5 (1.2-9.9) | 1R (3) |
| Fatigue (gr 3) | 6 (17) | 2.1 (1.5-2.4) | 1D, 1RD (6) |
| Anorexia | 0 | — | 0 |
Superscript “RD” represents romi and duv delay, whereas “D” and “R” indicate duv and romi delay, respectively.
Time to event was measured from treatment start date to date of AE and only reported in patients who had an AE.
Dose delays were defined as a held dose due to an AE resulting in a deviation from the treatment plan for either duv, romi, or both in any treatment cycle. If a patient experienced dose delays in multiple treatment cycles due to a single AE, they were counted as 1 delay. Dose delays often but not always coincided with dose modifications.
Febrile neutropenia gr 3 and neutropenia gr 4 with secondary Pseudomonas (n = 1) and Klebsiella bacteremia (n = 1) led to discontinuation of duv/romi therapy in 2 patients.
Transaminitis with ALT elevation led to discontinuation of duv/romi therapy (n = 1).
Duv-associated gr 3 enterocolitis and gr 4 noninfectious diarrhea led to duv/romi treatment discontinuation (n = 1).
Nine patients had no cytomegalovirus polymerase chain reaction values available during duv/romi regimen, and therefore, 29 patients were evaluable.
Gastrointestinal AEs occurred in 25% (9/36) of patients, with ALT elevation observed in 17% (6/36). Most cases were gr 3 (n = 5), with a single gr 4 case. The median time to onset was 2 months (IQR, 1.7-2.6). Similarly, AST elevation was observed in 17% (6/36) of patients, was entirely of gr 3 severity, and had a median time to onset of 1.1 months (IQR, 1.0-1.7). Drug-mediated noninfectious gr 3 enterocolitis was observed in 2 patients and noninfectious diarrhea in 3% (1/36), with an onset of 2.8 months (IQR, 2.5-3.2) and 3.4 months. Among infectious AEs, observed in 38% (9/38) of patients, cytomegalovirus viremia without organ involvement was the most common and noted in 17% (5/29) of evaluable patients, with a median onset of 1.2 months (IQR, 1.1-1.4). Tuberculosis (TB) reactivation was reported in 1 patient from a TB-endemic region (3%). COVID-19 infection occurred in 2 patients (7%), and Pseudomonas bacteremia and Klebsiella bacteremia occurred in 1 patient (3%) each. Other notable gr 3 and 4 AEs observed in 28% (10/36) of patients included rash in 14% (5/36), with a notably delayed median onset of 9.5 months (IQR, 1.2-9.9) and gr 3 fatigue in 17% (6/36) of patients, with a median onset of 2.1 months (IQR, 1.5-2.4). No cases of anorexia were reported.
Overall, hematologic toxicities were the most prevalent AEs, with neutropenia and leukocytosis occurring early in the treatment course. Gastrointestinal and infectious complications were less frequent but remained clinically relevant. Rash, although less common, was a late-onset toxicity.
Dose delays/reductions/discontinuations
Sixteen patients experienced dose interruptions due to gr 3 to 4 AEs, most commonly ALT and AST transaminitis (14% [5/36] and 8% [3/36], respectively) and thrombocytopenia (11% [4/36]). Gr 3 transaminitis led to varying periods of duv-only interruption for 3 patients, ranging from 3 to 28 days. One patient with gr 3 AST and gr 4 ALT transaminitis experienced 1 week of interruption for both duv/romi followed by a 3-week duv interruption, and 1 patient discontinued duv and then romi because of gr 3 colitis. No dose reductions due to transaminitis occurred.
Thrombocytopenia led to dose interruptions of both duv and romi in 2 patients (1 gr 3 and 1 gr 4), 1 of whom also had neutropenia. Thrombocytopenia also led to romi-only delay in 1 patient (gr 4) who also had neutropenia, and to discontinuation of the romi day 8 dose for the remainder of therapy in 1 patient (gr 4). Thrombocytopenia was associated with romi-only dose reduction from 10 to 8 mg/m2 in cycles 2 and 4 in 2 patients (1 gr 3 and 1 gr 4), 1 of whom (gr 4) also had a romi dose interruption. Thrombocytopenia did not lead to duv-only interruption in the cohort. Thrombocytopenia (gr 4) and neutropenia (gr 4) during duv lead-in in 1 patient led to duv delay for 1 week and dose reductions from 75 to 25 mg twice daily. Remaining duv-only dose reductions to 50 mg were observed in cycle 1 and were primarily due to pancytopenia (gr 4) and neutropenia (gr 3) in 1 patient each. Remaining romi-only dose reductions to 8 mg/m2 were due to gr 3 fatigue in cycle 4 for 1 patient.
Dose interruptions occurred because of infectious complications. This included COVID-19 in 2 patients (6% [2/32]), in which duv/romi was held for 1 cycle (n = =1) and 1 dose each per cycle for 2 cycles in another patient (n = 1). TB reactivation occurred in 1 patient (3% [1/32]), for whom 1 dose of romi was withheld. Duv/romi treatment was discontinued in 4 patients, including one with gr 3 autoimmune enterocolitis and associated gr 4 noninfectious diarrhea in cycle 4, and another with gr 3 ALT transaminitis in cycle 3 (duv) then colitis in cycle 4 (romi). The remaining 2 discontinuations were due to gr 3 febrile neutropenia and gr 4 neutropenia, 1 in cycle 1 (following 5 cycles of duv lead-in) with Pseudomonas bacteremia, and 1 in cycle 2 with Klebsiella bacteremia. One patient died of septic shock secondary to duv-associated pancytopenia, following a dose reduction in cycle 1 from 75- to 50-mg duv.
Dose schedule
Twenty-eight (74%) patients started duv at 75 mg twice daily, and the majority (19/28 [68%]) maintained this dose for at least half of their total treatment cycles (lead-in or combination). Eight (8/28 [29%]) patients received 75 mg twice daily in cycles 1 and 2 and 25 mg twice daily in cycle 3 per the PRIMO-EP dosing schedule. At cycle 3, day 1, 35% (9/26) of patients on treatment continued to receive 75-mg duv twice daily. Among these patients, 1 started duv at cycle 1, day 14; 1 received 25-mg duv during cycle 1; and 1 resumed duv treatment after a 6-week dose interruption in cycles 1 and 2 due to motor vehicle trauma. For the remaining 6 patients receiving 75-mg duv twice daily at cycle 3, day 1, no specific reason for the sustained 75-mg dose was cited in provider notes. By cycle 4, day 1, 18% (3/17) of patients still on the doublet received duv 75 mg twice daily, whereas the remaining 76% (13/17) received 25 mg (n = 12) or 50 mg (n = 1). Of the 38 patients, 7 (18%) started at 25 mg twice daily duv in cycle 1, but 3 of 7 increased to 75 mg by cycle 2. Three patients (3/38) started at 50 mg twice daily and remained on this dose (n = 2) or lower (n = 1) until treatment end.
In cycle 1, 15 (15/31 [48%]) of the patients receiving romi started at 10 mg/m2, with the rest receiving greater doses up to 14 mg/m2. By cycle 2, most patients (18/32 [56%]) on romi received 10 mg/m2. Romi modifications due to gr 3 to 4 AEs were more commonly dose delays than dose reductions.
Discussion
In this less-selected, real-world patient population, the data confirm earlier observations regarding the safety, tolerability, and high ORRs and CRRs with the duv/romi combination for the treatment of R/R TCLs, which remains a challenging group of malignancies to date. Specifically, the responses observed in patients with the nTFH subtype are noteworthy and consistent with previous observations from early-phase clinical trial data using the combination and from SA studies. Although the robust responses in patients with the nTFH subtype are not surprising (albeit higher than those observed in the phase 1b/2a trial data), the demonstration of a relatively high ORR of 43% in the non-nTFH subtype, including 29% CRR, positions duv/romi favorably as a novel strategy for patients with a broad range of nodal PTCLs, including the common PTCL-NOS subtype.4 The difference in ORR between the nTFH and non-nTFH groups persisted even with comparable baseline prognostic indices such as IPI, PIT, and PIRT, acknowledging the limitations of a small sample size. Our data demonstrate the efficacy and safety of this regimen in patients aged up to 89 years, who received up to 8 prior lines of therapy and exhibited intermediate to high-risk disease features. This further highlights the potential of duv/romi as another viable strategy for patients aged >60 years, a subset with reportedly poor outcomes due to advancing age and comorbidities. Of note are the responses observed among patients previously exposed to epigenetic modifiers including romi and azacitidine administered as SAs and as a combination. All 3 patients with previous refractoriness to azacitidine and romi achieved a durable CR on duv/romi. One patient bridged to allo-HSCT and remains in remission at 11 months after HSCT with no GVHD. Two others remain in CR on active therapy: one is planned to undergo allo-HSCT, and the other declined future HSCT. For 2 patients who experienced relapsed lymphoma after their first allo-HSCT, duv/romi led to a durable remission and enabled a second allo-HSCT, with both patients alive at day +100.
Our study, which used broader inclusion criteria than the phase 1b/2a trial (including patients with cytopenias, prior treatment toxicities, and no mandated washout period) demonstrates comparable ORR and CRR, thereby enhancing the generalizability of these findings to real-world, high-risk patient populations.4 It is worth noting that in this multicenter cohort, many patients experienced brief interruptions and dose reductions in duv from cycle 3 onward. Despite this, and in contrast to the higher doses permitted in the phase 1b/2a trial, we did not observe any reduction in overall efficacy, including ORR or CRR. This suggests an alternative plausible approach whereby a decrease in dose could be permitted if a CR is achieved after 2 cycles of therapy to mitigate toxicity. It is plausible that this strategy could allow a potentially longer duration of treatment for patients who are transplant-ineligible. AE profiles noted with duv/romi suggest that among gr 3 to 4 toxicities, hematologic toxicities are the most common, with gr 3 to 4 lymphopenia and neutropenia each observed in 42% of patients, similar to the phase 1b/2a trial.4 Bacteremia secondary to gr 3 febrile neutropenia and gr 4 neutropenia led to discontinuation in 2 patients. Although we observed a higher incidence of gr 3 to 4 transaminitis than previously reported, it led to discontinuation of therapy in only 1 patient, suggesting that appropriate management with dose interruptions, reductions, use of short-term steroids, and growth factor support can enable continued use. With appropriate prophylactic use of antibiotics and preemptive cytomegalovirus monitoring when feasible, no cases of disseminated viral infections with organ involvement were observed. No instance of fungal infection was observed despite the lack of prophylactic antifungal antibiotics for most patients. However, 1 case of TB reactivation in a patient from a TB-endemic region warrants consideration of chemoprophylaxis with isoniazid-like antibiotics to prevent active TB disease in high-risk populations. Thus, our study provides robust data on observed gr 3 to 4 AEs, timeline of occurrence, and the associated dose changes, delays, discontinuations, management, and outcomes after treatment with the combination. This provides valuable information and facilitates clinical decisions and optimization of therapy in this high-risk population. It is worth noting that no patients who experienced a PR after cycle 2 subsequently achieved a CR, despite continuation of therapy. In fact, all patients with PR displayed progression of their lymphoma within 6 weeks, leading to alternative strategies. Thus, according to our small multicenter cohort with a median time to response of 1.9 months, a CR after cycle 2 might serve as a surrogate marker for a meaningful response to this combination.
We reviewed the literature on other contemporary combination therapies for R/R PTCL and CTCL, including phase 1 to 3 trials and real-world studies, as reported in Table 7. The OS ranged from 10.2 to 32.9 months, PFS from 2.2 to 23.2 months, and DoR from 8.2 to 25.3 months.8-18 ORRs ranged from 35.7% to 78% and CRRs from 13% to 55%.8-18 In 4 of the 11 studies that reported subgroup data, nTFH subtype response rates were consistently superior to those in the overall cohort, except with the duv/ruxolitinib combination, for which the CRR was lower but ORR higher in nTFH.8,13-15 In 8 of the 11 studies that mentioned allo-HSCT, histology was rarely reported, and the rate of allo-HSCT ranged from 0% to 55%.8-11,13,14,17,18 The most commonly reported gr 3 to 4 AEs were thrombocytopenia (range of event rates, 11%-53%) and neutropenia (8%-45%).8-18 Although no direct comparison can be made across these doublets, we believe that the duv/romi combination is a tolerable and effective option for patients with R/R PTCL. However, randomized studies will be critical in identifying whether 1 regimen is superior to others for patients with R/R PTCL. Further, systematic next-generation sequencing–based studies will play a critical role in defining subpopulations likely to benefit from 1 combination over another based on inherent molecular vulnerabilities and heterogeneity across the 3 nTFH subtypes. Thus, it is also plausible that enrollment of patients in a biomarker-driven master trial will aid in the nomination of ideal doublets tailored to nTFH subsets across the available options. Prospective longitudinal samples (including cell-free DNA collected from diagnosis through treatment, relapse, and after allo-HSCT) have been collected for our cohort. Diagnostic and relapsed tumor tissues are undergoing whole exome and cell-free DNA–based sequencing and analyses for noninvasive tracking of personalized somatic variants underlying response and resistance. These findings will be reported soon as part of a larger multicenter effort to define molecular residual disease in nodal PTCL, and will provide genomic information to support precision therapy in PTCL. Nevertheless, the robust reporting of responses leading to curative-intent allo-HSCT (without increased incidence of GVHD or toxicity after HSCT), relative to the underreporting and lack of details in other doublet studies, positions duv/romi as another novel option for transplant-eligible patients with R/R PTCL.
Efficacy and safety of 2-drug combination therapies in R/R PTCL and CTCL
| Drug combination . | Drug targets . | Trial phase . | Enrolled patients with TCL (n) . | Previous treatment lines, median (range) . | Patients evaluated (n) . | ORR (%) . | CRR (%) . | OS, median (mo) . | PFS, median (mo) . | DoR, median (mo) . | Most prevalent gr 3-4 AEs . | AEs, n (%) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Romi + azacitidine∗ | HDAC + DNMT1 | Real-world | Overall: 27† | 1 (0-5) | Overall: 26 | 76.9 | 53 | NR | 7.07 | NR | Thrombocytopenia‡ | 14 (51) | 8 |
| AITL: 19 | AITL + TFH: 23 | 69.5 | 60.8 | Nausea‡ | 11 (40) | ||||||||
| TFH: 3 | Neutropenia‡ | 10 (37) | |||||||||||
| PTCL-NOS: 1 | Fatigue‡ | 8 (29) | |||||||||||
| ATLL: 2 | Anemia‡ | 6 (22) | |||||||||||
| TFH-PTCL + DLBCL: 1 | |||||||||||||
| ALK– ALCL + FL: 1 | |||||||||||||
| Romi + azacitidine† | HDAC + DNMT1 | 2 | Overall: 14 | 2 (1-6) | Overall: 13 | 54 | 38 | 20.6 | 8.0 | 13.5 | Thrombocytopenia | 12 (48) | 9 |
| AITL: 9 | Neutropenia | 10 (40) | |||||||||||
| PTCL-NOS: 2 | Lymphopenia | 8 (32) | |||||||||||
| ALCL: 1: | Anemia | 4 (16) | |||||||||||
| EATL: 1 | Febrile neutropenia | 3 (12) | |||||||||||
| ENKTCL: 1 | |||||||||||||
| Romi + azacitidine§ | HDAC + DNMT1 | 1 | Overall: 11 | 6 (1-15) | Overall: 11 | 73 | 55 | — | NR | — | Lymphopenia | 11 (42) | 10 |
| AITL: 3 | Neutropenia | 11 (42) | |||||||||||
| PTCL-NOS: 2 | Thrombocytopenia | 7 (27) | |||||||||||
| ATLL: 2 | Leukopenia | 6 (23) | |||||||||||
| ALCL: 1 | Anemia | 5 (19) | |||||||||||
| CTCL: 1 | |||||||||||||
| EATL: 1 | |||||||||||||
| ENKTCL: 1 | |||||||||||||
| Romi + lenalidomide|| | HDAC + E3 ubiquitin | 2 | Overall: 27 | 3 (1-12) | Overall: 24 | 50 | 13 | 18.3 | 4.8 | 15.7 | Thrombocytopenia | 26 (53) | 11 |
| AITL: 3 | PTCL: 15 | 53 | 13 | Lymphopenia | 25 (51) | ||||||||
| PTCL-NOS: 5 | CTCL: 9 | 44 | 11 | Neutropenia | 24 (49) | ||||||||
| ATLL: 7 | Leukopenia | 22 (45) | |||||||||||
| ENKTCL: 1 | Anemia | 13 (27) | |||||||||||
| T-PLL: 1 | |||||||||||||
| CTCL, MF: 7 | |||||||||||||
| CTCL-SS: 3 | |||||||||||||
| Romi + pembrolizumab | HDAC + PD1 | 2 | Overall: 38 | 2 (1-6) | Overall: 38 | 47.3 | 37.3 | 21.3 | — | — | Infection, NOS‡,¶ | 11 (28) | 12 |
| Thrombocytopenia | 10 (26) | ||||||||||||
| Romi + pralatrexate# | HDAC + DHFR | 2 | Overall: 18 | 2 (0-7) | Overall: 14 | 35.7 | 14.3 | 20.2 | 3.56 | 8.2 | Infection∗∗ | 4 (24) | 13 |
| PTCL-NOS: 8 | PTCL-NOS: 6 | 33 | Sepsis | 1 (6) | |||||||||
| PTCL with TFH phenotype/AITL: 4 | PTCL with TFH phenotype/AITL: 3 | 50 | Anemia | 1 (6) | |||||||||
| ATLL: 2 | ATLL: 2 | Heart failure | 1 (6) | ||||||||||
| ENKTCL: 1 | ENKTCL: 1 | Sinus tachycardia | 1 (6) | ||||||||||
| CTCL: 1 | CTCL: 1 | ||||||||||||
| Subcutaneous panniculitis PTCL: 2 | Subcutaneous panniculitis PTCL: 1 | ||||||||||||
| Duv + azacitidine | PI3K-δ + DNMT1 | 1 | Overall: 14 | 2 (1-21) | Overall: 14 | 46 | 31 | 10.2 | 2.2 | — | Neutropenia‡ | 4 (28) | 14 |
| TFH: 4 | TFH: 4 | 100 | 100 | Anemia‡ | 3 (21) | ||||||||
| CTCL: 2 | Transaminitis | 2 (14) | |||||||||||
| Thrombocytopenia‡ | 2 (14) | ||||||||||||
| Leukopenia‡ | 1 (7) | ||||||||||||
| Duv + ruxolitinib†† | PI3K-δ + JAK | 1 | Overall: 49 | ‡ | Overall: 49 | 41 | 24 | — | — | NR | Neutropenia | (38) | 15 |
| PTCL-NOS: 13 | PTCL-NOS: 13 | 23 | 15 | Anemia | (16) | ||||||||
| TFH: 14 | TFH: 14 | 79 | 64 | Thrombocytopenia | (12) | ||||||||
| T-PLL: 3 | T-PLL: 3 | 60 | 0 | Transaminitis | (4) | ||||||||
| T-LGL: 3 | T-LGL: 3 | 67 | 33 | Hypertension | (4) | ||||||||
| MF: 7 | MF: 7 | 14 | 0 | ||||||||||
| ATLL: 1 | |||||||||||||
| ALCL, ALK–: 3 | |||||||||||||
| ALCL, ALK+: 1 | |||||||||||||
| MEITL: 1 | |||||||||||||
| Durvalumab + lenalidomide | PD1 + E3 ubiquitin | 2 | Overall: 13 | 3‡‡ | Overall: 12 | 75 | — | — | NR | — | Neutropenia | 1 (8) | 16 |
| CTCL: 13 | CTCL: 12 | 75 | |||||||||||
| Chidamide + sintilimab | HDAC + PD1 | 1b/2 | Overall: 38 | 1 (1-2) | Overall: 37 | 59.5 | 48.6 | 32.9 | 23.2 | 25.3 | Neutropenia | 11 (29) | 17 |
| ENKTCL, phase 1b: 9 | ENKTCL, phase 1b: 9 | 66.7 | 55.6 | Leukopenia | 3 (8) | ||||||||
| ENKTCL, phase 2: 28 | ENKTCL, phase 2: 28 | 57.1 | 46.4 | Lymphopenia | 3 (8) | ||||||||
| Thrombocytopenia | 4 (11) | ||||||||||||
| Azacitidine + nivolumab | PD1 + DNMT1 | NA | Overall: 9 | 1 (1-2) | Overall: 9 | 78 | 33 | — | — | — | Neutropenia | 1 (11) | 18 |
| AITL: 9 | AITL: 9 | 78 | 33 | Anemia | 1 (11) | ||||||||
| Colitis | 1 (11) |
| Drug combination . | Drug targets . | Trial phase . | Enrolled patients with TCL (n) . | Previous treatment lines, median (range) . | Patients evaluated (n) . | ORR (%) . | CRR (%) . | OS, median (mo) . | PFS, median (mo) . | DoR, median (mo) . | Most prevalent gr 3-4 AEs . | AEs, n (%) . | Reference . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Romi + azacitidine∗ | HDAC + DNMT1 | Real-world | Overall: 27† | 1 (0-5) | Overall: 26 | 76.9 | 53 | NR | 7.07 | NR | Thrombocytopenia‡ | 14 (51) | 8 |
| AITL: 19 | AITL + TFH: 23 | 69.5 | 60.8 | Nausea‡ | 11 (40) | ||||||||
| TFH: 3 | Neutropenia‡ | 10 (37) | |||||||||||
| PTCL-NOS: 1 | Fatigue‡ | 8 (29) | |||||||||||
| ATLL: 2 | Anemia‡ | 6 (22) | |||||||||||
| TFH-PTCL + DLBCL: 1 | |||||||||||||
| ALK– ALCL + FL: 1 | |||||||||||||
| Romi + azacitidine† | HDAC + DNMT1 | 2 | Overall: 14 | 2 (1-6) | Overall: 13 | 54 | 38 | 20.6 | 8.0 | 13.5 | Thrombocytopenia | 12 (48) | 9 |
| AITL: 9 | Neutropenia | 10 (40) | |||||||||||
| PTCL-NOS: 2 | Lymphopenia | 8 (32) | |||||||||||
| ALCL: 1: | Anemia | 4 (16) | |||||||||||
| EATL: 1 | Febrile neutropenia | 3 (12) | |||||||||||
| ENKTCL: 1 | |||||||||||||
| Romi + azacitidine§ | HDAC + DNMT1 | 1 | Overall: 11 | 6 (1-15) | Overall: 11 | 73 | 55 | — | NR | — | Lymphopenia | 11 (42) | 10 |
| AITL: 3 | Neutropenia | 11 (42) | |||||||||||
| PTCL-NOS: 2 | Thrombocytopenia | 7 (27) | |||||||||||
| ATLL: 2 | Leukopenia | 6 (23) | |||||||||||
| ALCL: 1 | Anemia | 5 (19) | |||||||||||
| CTCL: 1 | |||||||||||||
| EATL: 1 | |||||||||||||
| ENKTCL: 1 | |||||||||||||
| Romi + lenalidomide|| | HDAC + E3 ubiquitin | 2 | Overall: 27 | 3 (1-12) | Overall: 24 | 50 | 13 | 18.3 | 4.8 | 15.7 | Thrombocytopenia | 26 (53) | 11 |
| AITL: 3 | PTCL: 15 | 53 | 13 | Lymphopenia | 25 (51) | ||||||||
| PTCL-NOS: 5 | CTCL: 9 | 44 | 11 | Neutropenia | 24 (49) | ||||||||
| ATLL: 7 | Leukopenia | 22 (45) | |||||||||||
| ENKTCL: 1 | Anemia | 13 (27) | |||||||||||
| T-PLL: 1 | |||||||||||||
| CTCL, MF: 7 | |||||||||||||
| CTCL-SS: 3 | |||||||||||||
| Romi + pembrolizumab | HDAC + PD1 | 2 | Overall: 38 | 2 (1-6) | Overall: 38 | 47.3 | 37.3 | 21.3 | — | — | Infection, NOS‡,¶ | 11 (28) | 12 |
| Thrombocytopenia | 10 (26) | ||||||||||||
| Romi + pralatrexate# | HDAC + DHFR | 2 | Overall: 18 | 2 (0-7) | Overall: 14 | 35.7 | 14.3 | 20.2 | 3.56 | 8.2 | Infection∗∗ | 4 (24) | 13 |
| PTCL-NOS: 8 | PTCL-NOS: 6 | 33 | Sepsis | 1 (6) | |||||||||
| PTCL with TFH phenotype/AITL: 4 | PTCL with TFH phenotype/AITL: 3 | 50 | Anemia | 1 (6) | |||||||||
| ATLL: 2 | ATLL: 2 | Heart failure | 1 (6) | ||||||||||
| ENKTCL: 1 | ENKTCL: 1 | Sinus tachycardia | 1 (6) | ||||||||||
| CTCL: 1 | CTCL: 1 | ||||||||||||
| Subcutaneous panniculitis PTCL: 2 | Subcutaneous panniculitis PTCL: 1 | ||||||||||||
| Duv + azacitidine | PI3K-δ + DNMT1 | 1 | Overall: 14 | 2 (1-21) | Overall: 14 | 46 | 31 | 10.2 | 2.2 | — | Neutropenia‡ | 4 (28) | 14 |
| TFH: 4 | TFH: 4 | 100 | 100 | Anemia‡ | 3 (21) | ||||||||
| CTCL: 2 | Transaminitis | 2 (14) | |||||||||||
| Thrombocytopenia‡ | 2 (14) | ||||||||||||
| Leukopenia‡ | 1 (7) | ||||||||||||
| Duv + ruxolitinib†† | PI3K-δ + JAK | 1 | Overall: 49 | ‡ | Overall: 49 | 41 | 24 | — | — | NR | Neutropenia | (38) | 15 |
| PTCL-NOS: 13 | PTCL-NOS: 13 | 23 | 15 | Anemia | (16) | ||||||||
| TFH: 14 | TFH: 14 | 79 | 64 | Thrombocytopenia | (12) | ||||||||
| T-PLL: 3 | T-PLL: 3 | 60 | 0 | Transaminitis | (4) | ||||||||
| T-LGL: 3 | T-LGL: 3 | 67 | 33 | Hypertension | (4) | ||||||||
| MF: 7 | MF: 7 | 14 | 0 | ||||||||||
| ATLL: 1 | |||||||||||||
| ALCL, ALK–: 3 | |||||||||||||
| ALCL, ALK+: 1 | |||||||||||||
| MEITL: 1 | |||||||||||||
| Durvalumab + lenalidomide | PD1 + E3 ubiquitin | 2 | Overall: 13 | 3‡‡ | Overall: 12 | 75 | — | — | NR | — | Neutropenia | 1 (8) | 16 |
| CTCL: 13 | CTCL: 12 | 75 | |||||||||||
| Chidamide + sintilimab | HDAC + PD1 | 1b/2 | Overall: 38 | 1 (1-2) | Overall: 37 | 59.5 | 48.6 | 32.9 | 23.2 | 25.3 | Neutropenia | 11 (29) | 17 |
| ENKTCL, phase 1b: 9 | ENKTCL, phase 1b: 9 | 66.7 | 55.6 | Leukopenia | 3 (8) | ||||||||
| ENKTCL, phase 2: 28 | ENKTCL, phase 2: 28 | 57.1 | 46.4 | Lymphopenia | 3 (8) | ||||||||
| Thrombocytopenia | 4 (11) | ||||||||||||
| Azacitidine + nivolumab | PD1 + DNMT1 | NA | Overall: 9 | 1 (1-2) | Overall: 9 | 78 | 33 | — | — | — | Neutropenia | 1 (11) | 18 |
| AITL: 9 | AITL: 9 | 78 | 33 | Anemia | 1 (11) | ||||||||
| Colitis | 1 (11) |
ALK+, anaplastic lymphoma kinase–positive; DHFR, dihydrofolate reductase; DLBCL, diffuse large B-cell lymphoma; DNMT1, DNA methyltransferase 1; EATL, enteropathy-associated T-cell lymphoma; FL, follicular lymphoma; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; NA, not applicable; PD1, programmed cell death protein 1; SS, Sézary syndrome; T-LGL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia.
Three (3/27) patients of unspecified histology were treatment-naive and included in all treatment response and AE measures. R/R-specific outcomes were not reported by the authors.
AE rates include treatment-naive patients.
Gr of AE not reported.
Median lines of therapy and AE rates include treatment-naive patients and those with Hodgkin and B-cell lymphomas.
Median lines of therapy and DoR include patients with R/R Hodgkin and B-cell lymphomas.
Infection type not reported.
Three (3/18) patients of unspecified histology were treatment-naive and included in all treatment response and AE measures. R/R-specific outcomes were not reported by the authors.
Gr 3 to 4 infection included staphylococcal infection, lymph node infection, and upper respiratory infection, and appendicitis.
Treatment response and AE measures include treatment-naive patients of unknown count.
Median lines of therapy included systemic therapies only (range not reported).
This study has several limitations. These include systematic errors based on the partially retrospective nature of the analyses, notably varied documentation over the span of 8 years, and lack of centralized pathology/treatment response review, among others. This is a small, prospective and retrospective real-world study, and is therefore insufficient on its own to support broader conclusions. However, it contributes to the evidence supporting the overall activity of this regimen in a patient population with very limited treatment options. We note that definitive conclusions cannot be drawn in the absence of a randomized trial, and further research is needed, such as a randomized phase 3 trial of duv/romi vs investigators’ choice in the upfront and/or relapsed/refractory setting. To ascertain the role of this combination in CTCL, further studies or observations from use in real-world settings are warranted. Until these studies provide more data (including insight into the mechanisms by which duv/romi overcomes resistance in patients previously unresponsive to epigenetic modifiers), we consider this combination a very viable option for transplant-eligible and even ineligible patients with R/R PTCL who cannot be enrolled into clinical trials. We believe that these data also support further investigation of this combination or its individual agents as a treatment option for transplant-ineligible patients, preferably guided by minimal residual disease–based assays.
Acknowledgments
This work was supported by the Center for Lymphoma Research Funds. S.J. is supported by National Cancer Institute K08 Career Development award K08CA230498. E.J. is supported by the Reid Family Fund for Lymphoma Research.
Authorship
Contribution: J.G.F., M.J.K., A.W.L., C.M., K.C., M.N.S., A.M., M.M., A.B.R., A.R.S., S.M.M., R.A.N., M.I., K.M.K., S.C., J.B., S.M., Y.-B.C., E.J., and S.J. designed the research; J.G.F., M.J.K., M.N.S., A.W.L., C.M., K.C., A.M., and S.J. analyzed data; J.G.F., M.J.K., and S.J. wrote the manuscript; and all authors contributed to and performed the research and reviewed the manuscript.
Conflict-of-interest disclosure: M.N.S. reports research funding from Secura Bio and Daiichi Sankyo. E.J. reports research funding from Celgene, Merck, Pharmacyclics, and Hoffman-La Roche; honoraria from Merck, Daiichi Sankyo, Bristol Myers Squibb, and Bayer; and patents and royalties from UpToDate. S.J. reports consultancy for Mersana Therapeutics, Myeloid Therapeutics, SIRPant Immunotherapeutics, and Abcuro, Inc; research funding from SIRPant Immunotherapeutics, Abcuro, Inc, Daiichi Sankyo, and Acrotech Biopharma Inc; and membership on an entity’s board of directors or advisory committees for Mersana Therapeutics, Myeloid Therapeutics, SIRPant Immunotherapeutics, Abcuro, Inc, Secura Bio, Daiichi Sankyo, and CRISPR Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Salvia Jain, Jon and Jo Ann Hagler Center for Lymphoma, Massachusetts General Hospital Cancer Center, 55 Fruit St, Boston, MA 02114-2696; email: salvia.Jain@mgh.harvard.edu; and Eric Jacobsen, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: eric_jacobsen@dfci.harvard.edu.
References
Author notes
J.G.F. and M.J.K. contributed equally to this study.
Presented at the 66th annual meeting poster session of the American Society of Hematology, San Diego, CA, 9 December 2024; and the poster session of the 15th T-Cell Lymphoma Forum Annual Conference, La Jolla, CA, 6 June 2024.
Original data are available on request from the corresponding author, Salvia Jain (salvia.jain@mgh.harvard.edu). Deidentified patient data can be shared. External data requests will be answered within 1 month.
The full-text version of this article contains a data supplement.