Key Points
BCLAF1 is a novel transcriptional regulator that promotes HSC expansion during fetal development and after stem cell transplant.
BCLAF1 restrains the expression of stress response genes in hematopoietic progenitors via indirect or transient mechanisms.
Visual Abstract
Hematopoietic stem cells (HSCs) rapidly expand during fetal development and after stress. Here, we identify B-cell lymphoma-2-associated factor 1 (BCLAF1) as a regulator of HSC repopulation activity, with roles in the expansion of fetal HSCs and hematopoietic reconstitution after stem cell transplantation. Using mice with hematopoietic-specific and inducible deletion of Bclaf1, we find that BCLAF1 promotes fetal HSC development but is dispensable for the maintenance of adult HSCs at steady state. Loss of BCLAF1 in either fetal or adult HSCs significantly impairs their self-renewal and multilineage reconstitution activity after stem cell transplantation. Single-cell RNA sequencing of fetal hematopoietic progenitors reveals that loss of BCLAF1 reduces long-term HSCs and restrains the expression of stress response genes. BCLAF1 associates with chromatin throughout the genome of fetal and adult hematopoietic cells, likely through indirect mechanisms, to regulate transcriptional programs. These results establish a novel function for the transcriptional regulator BCLAF1 in limiting stress responses in HSCs, thereby preserving HSC development during embryogenesis and repopulation function after stem cell transplant.
Introduction
The generation of diverse hematopoietic cell populations relies on hematopoietic stem cell (HSC) properties of self-renewal and multipotent differentiation to committed cell fates. HSCs undergo self-renewing divisions to expand during fetal development and to regenerate their population after stress, such as stem cell transplantation or chemotherapy.1-6 Alternatively, HSCs can differentiate into multipotent progenitors (MPPs) that lack long-term self-renewal capacity and give rise to lineage-restricted myeloid, lymphoid, and erythroid progenitors.1,3,7 HSCs have distinct transcriptional networks that regulate self-renewal and multipotency, and these programs change from fetal to adult hematopoiesis.8-11
Fetal HSCs emerge from hemogenic endothelium and migrate to the liver to establish hematopoiesis. Fetal liver HSCs undergo limited self-renewal divisions to expand their numbers while retaining self-renewal capacity to reconstitute hematopoiesis after transplant.4,12-18 After birth, HSCs move to the bone marrow and progressively increase during the neonatal period before becoming largely quiescent during adulthood.4,16,19-24 Adult HSCs retain the capacity to repopulate hematopoiesis in transplantation. Several genes differentially regulate fetal and adult HSCs. Fetal HSCs uniquely express Sox17, Lin28b, and Ifg2bp1-3, whereas adult HSCs express Sox6, Cebpa, and Esr1.9,10,14,15,25-28 Other genes, such as Ash1l, Kmt2A/Mll1, and Kat8, are expressed in both fetal and adult HSCs but are only necessary for adult HSC self-renewal.29-31 Determining the developmental- and context-specific roles of a hematopoietic regulatory gene is critical to fully elucidating its function in HSCs.
Here, we define Bcl2-associated factor 1 (Bclaf1) as a novel regulator of fetal and adult HSC function. BCLAF1 is a transcriptional regulator that is ubiquitously expressed but has cell type–dependent functions in development and differentiation.32-37Bclaf1 expression was identified to be increased in acute myelogenous leukemia (AML).38-40 Suppression of Bclaf1 in AML blasts reduced proliferation, increased cell death, and induced differentiation toward a dendritic cell fate, suggesting that BCLAF1 supports neoplastic self-renewal.38 However, the specific function of BCLAF1 has not been defined. Given that normal HSCs and AML cells can share stemness programs, these prior studies raised the question of whether BCLAF1 regulates normal HSC self-renewal and differentiation.41-43
Using mice with germ line and conditional deletion of Bclaf1, we show that BCLAF1 promotes fetal HSC development but is dispensable for the maintenance of adult HSCs at steady state. Bclaf1-deficient fetal and adult HSCs both have severe defects in repopulating activity and self-renewal after HSC transplant. Single-cell RNA sequencing (RNA-seq) of fetal liver hematopoietic progenitors demonstrates that loss of BCLAF1 results in upregulation of stress response genes across stem and progenitor populations. These findings provide greater insight into the transcriptional regulation of HSC development and stress responses.
Methods
Mice
Mice were bred and housed according to institutional animal care and use committee standards at Washington University in St. Louis School of Medicine. Both sexes were used equivalently in all experiments. Mice were routinely genotyped before experiments using primers listed in supplemental Table 1.
Flow cytometric analyses and cell sorting
Flow cytometric analyses and sorting were performed on a BD LSRFortessa, Cytek Aurora, or BD Aria Fusion. Antibodies used for flow cytometry are listed in supplemental Table 2. Cell populations were defined based on surface marker expression, as defined in supplemental Table 3.
Transplantation
For all transplants, recipient CD45.1+ mice were given a split dose (≥ 3 hours apart) of lethal irradiation (11 Gy total). For primary transplants, mice were administered either sorted donor CD45.2+ HSCs mixed with 300 000 CD45.1+ adult bone marrow cells or 300 000 donor CD45.2+ bone marrow (or fetal liver) cells mixed with 300 000 CD45.1+ bone marrow cells. For secondary transplants, 3 million bone marrow cells from primary transplant recipients were transplanted into lethally irradiated secondary recipients. For all transplants, cells were administered via retroorbital injection, and recipients were treated with trimethoprim/sulfamethoxazole for 14 days after transplant.
Bulk RNA-seq
RNA was extracted from 1000 to 2000 HSCs and MPP3/4s. Complementary DNA amplification, library preparation, and sequencing were performed by the Washington University Genome Technology Access Center. Detailed methods are available in supplemental Materials.
Single-cell cellular indexing of transcriptomes and epitopes by sequencing
Fetal livers were pooled from 10 embryos per genotype at embryonic day 17.5. A total of 10 000 cells were stained with TotalSeq-B (BioLegend) antibodies (antibodies listed in supplemental Table 4) and loaded into a 10x Chromium Controller. Chromium Next GEM Single Cell 3′ version 3.1 (Dual Index) Kit with Feature Barcoding (PN-1000269; 10x Genomics), Dual Index NT Set A, and Dual Index TT Set A were used to further process samples according to the manufacturer’s instructions. Detailed methods are available in supplemental Materials.
CUT&RUN
A total of 50 000 E17.5 HSCs pooled from 24 to 26 embryos or 50 000 adult bone marrow Lineage-negative (Lin-) Sca1+c-Kit+ cells pooled from 2 to 3 mice were sorted into Iscove modified Dulbecco media with 10% fetal bovine serum. Cleavage under targets and release using nuclease (CUT&RUN) protocol was followed as in Janssens and Henikoff, using protocol option 1 with modifications.44-46 DNA libraries were prepared using the NEBNext Ultra II DNA Library Prep Kit for Illumina (catalog no. E7645; New England Biolabs), Agencourt AMPure XP magnetic beads (catalog no. A63880; Beckman Coulter), and NEBNext Multiplex Oligos for Illumina (Dual Index Primers Set 1; catalog no. E7600; New England Biolabs), with alterations to preserve small fragments.47 DNA was sequenced by Washington University Genome Technology Access Center. Detailed methods are available in supplemental Materials.
Results
Bclaf1 regulates fetal HSC expansion
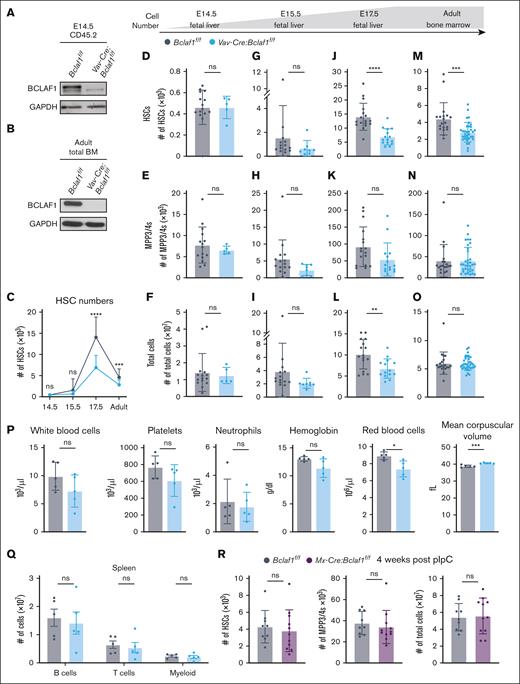
Germ line Bclaf1–/– mice die within a week after birth due to impaired lung development, but embryos are present in normal Mendelian ratios before birth.32 To assess whether Bclaf1 regulates HSC development, we quantified fetal HSCs (Lin-Sca1+c-kit+CD150+CD48–) and MPP3/4s (Lin-Sca1+c-kit+CD150–CD48+) in E17.5 livers (supplemental Figure 1).48-50Bclaf1–/– mice had significantly reduced HSC and MPP3/4 numbers despite normal fetal liver cellularity (supplemental Figure 2). To determine whether this reduction in HSCs is due to hematopoietic-specific functions of Bclaf1, we generated Vav-Cre:Bclaf1f/f mice, which have selective deletion of Bclaf1 in hematopoietic cells (Figure 1A-B).51-54Vav-Cre:Bclaf1f/f and control Bclaf1f/f mice had equivalent numbers and percentages of fetal liver HSCs at E14.5 and E15.5 (Figure 1C-I; supplemental Figure 3A-B). However, at E17.5, fetal HSC numbers and percentages were significantly reduced in Vav-Cre:Bclaf1f/f mice (Figure 1C,J-L; supplemental Figure 3A-C). Although both control and Bclaf1-deficient HSCs expanded from E14.5 to E17.5, Bclaf1-deficient HSCs increased to a lesser extent (Figure 1C; supplemental Figure 3B). Interestingly, fetal MPP2s, MPP3/4s, and erythroid progenitors were not different between E17.5 Vav-Cre:Bclaf1f/f and Bclaf1f/f mice (Figure 1E,H,K; supplemental Figure 3D-E). These data show that BCLAF1 does not regulate fetal HSC emergence but instead functions to support the increase in fetal HSC numbers during mid to late gestation. Furthermore, these results suggest that BCLAF1 has distinct activities in regulating fetal HSCs vs downstream MPPs.
Bclaf1 deficiency in hematopoietic cells reduces fetal HSC expansion but does not alter adult HSCs at steady state. (A-B) Western blot of BCLAF1 in sorted CD45.2+ hematopoietic cells from E14.5 fetal liver (A) or total adult bone marrow (BM) cells (B) of indicated genotypes. GAPDH shown as a loading control. (C) HSC numbers quantified by flow cytometry across developmental ages. (D-O) HSC, MPP3/4, and total cell numbers quantified by flow cytometry in E14.5 fetal liver (Bclaf1f/f, n = 15; Vav-Cre:Bclaf1f/f, n = 5) (D-F), E15.5 fetal liver (Bclaf1f/f, n = 15; Vav-Cre:Bclaf1f/f, n = 8) (G-I), E17.5 fetal liver (Bclaf1f/f, n = 17; Vav-Cre:Bclaf1f/f, n = 15) (J-L), and adult BM (Bclaf1f/f, n = 19; Vav-Cre:Bclaf1f/f, n = 34) (M-O). (P) Peripheral blood counts and red blood cell indices from adult Bclaf1f/f and Vav-Cre:Bclaf1f/f mice (n = 5 per genotype). (Q) Mature B220+ B, CD3+ T, and CD11b+ myeloid cell numbers in the spleen of adult Bclaf1f/f and Vav-Cre:Bclaf1f/f mice (n = 5 per genotype). (R) Adult (8-week-old) Bclaf1f/f and Mx-Cre:Bclaf1f/f mice were treated with pIpC, and BM hematopoietic cells were assessed 4 weeks after final dose of pIpC. HSC and MPP3/4 cell numbers were quantified by flow cytometry (Bclaf1f/f, n = 9; Mx-Cre:Bclaf1f/f, n = 11). Data in panels C-R are mean ± standard deviation (SD), and statistical significance was determined by unpaired 2-tailed Student t test. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant.
Bclaf1 deficiency in hematopoietic cells reduces fetal HSC expansion but does not alter adult HSCs at steady state. (A-B) Western blot of BCLAF1 in sorted CD45.2+ hematopoietic cells from E14.5 fetal liver (A) or total adult bone marrow (BM) cells (B) of indicated genotypes. GAPDH shown as a loading control. (C) HSC numbers quantified by flow cytometry across developmental ages. (D-O) HSC, MPP3/4, and total cell numbers quantified by flow cytometry in E14.5 fetal liver (Bclaf1f/f, n = 15; Vav-Cre:Bclaf1f/f, n = 5) (D-F), E15.5 fetal liver (Bclaf1f/f, n = 15; Vav-Cre:Bclaf1f/f, n = 8) (G-I), E17.5 fetal liver (Bclaf1f/f, n = 17; Vav-Cre:Bclaf1f/f, n = 15) (J-L), and adult BM (Bclaf1f/f, n = 19; Vav-Cre:Bclaf1f/f, n = 34) (M-O). (P) Peripheral blood counts and red blood cell indices from adult Bclaf1f/f and Vav-Cre:Bclaf1f/f mice (n = 5 per genotype). (Q) Mature B220+ B, CD3+ T, and CD11b+ myeloid cell numbers in the spleen of adult Bclaf1f/f and Vav-Cre:Bclaf1f/f mice (n = 5 per genotype). (R) Adult (8-week-old) Bclaf1f/f and Mx-Cre:Bclaf1f/f mice were treated with pIpC, and BM hematopoietic cells were assessed 4 weeks after final dose of pIpC. HSC and MPP3/4 cell numbers were quantified by flow cytometry (Bclaf1f/f, n = 9; Mx-Cre:Bclaf1f/f, n = 11). Data in panels C-R are mean ± standard deviation (SD), and statistical significance was determined by unpaired 2-tailed Student t test. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant.
Bclaf1 does not regulate adult HSCs at steady state
After birth, HSCs move to the bone marrow and increase their numbers during the neonatal period, then remain relatively quiescent and maintain steady-state numbers during adulthood.4,12-16,19-24 To determine whether Bclaf1 is required for maintaining adult HSCs, we assessed hematopoietic populations in 8- to 10-week-old mice. HSCs were reduced in adult Vav-Cre:Bclaf1f/f mice compared to controls (Figure 1C,M-O; supplemental Figure 3A-B). The relative reduction in Bclaf1-deficient HSCs is greater in E17.5 fetal livers than in adult bone marrow (Figure 1C,J,M), suggesting that Bclaf1-deficient HSCs do not decline further after fetal development but also do not recover to wild-type numbers. Despite the reduced HSCs, MPP2-4 populations were unchanged in adult Bclaf1-deficient mice (Figure 1N; supplemental Figure 3F). In addition, mature hematopoietic cell populations were not altered in Vav-Cre:Bclaf1f/f mice, as evidenced by similar white blood cell and platelet counts, as well as mature B, T, and myeloid cells in the spleen and lymph nodes, compared to controls (Figure 1P-Q; supplemental Figure 3G). Red blood cell numbers were slightly decreased in Bclaf1-deficient mice but within normal range, and red blood cell volume was not different (Figure 1P). Peritoneal B cells, which are derived from fetal liver HSCs, were similar between control and Vav-Cre:Bclaf1f/f mice (supplemental Figure 3H).55-57 These results show that loss of Bclaf1 does not alter mature hematopoietic cell numbers in young adults. These observations raise the question of whether the diminished HSC numbers in adult bone marrow simply reflect impaired HSC self-renewal during fetal development as opposed to an ongoing requirement for Bclaf1 in adulthood.
To further test whether Bclaf1 is required to maintain adult HSCs, we generated Mx-Cre:Bclaf1f/f mice, then treated 8-week-old mice with polyinosinic:polycytidylic acid (pIpC) to delete Bclaf1 in established HSCs. Bclaf1f/f mice were also treated with pIpC as controls. Subsequent analyses were performed 4 weeks after pIpC to ensure recovery from pIpC before assessing effects of Bclaf1 deletion.58,59 After pIpC, BCLAF1 was depleted in bone marrow cells, and 86% of HSCs in Mx-Cre:Bclaf1f/f mice had homozygous Bclaf1 deletion (supplemental Figure 3I-J). HSC and MPP3/4 numbers remained equivalent in Mx-Cre:Bclaf1f/f and Bclaf1f/f mice after pIpC treatment (Figure 1R). In combination with findings in fetal mice, these data demonstrate that BCLAF1 has a critical role in fetal HSC development but is dispensable for adult HSC maintenance at steady state.
Bclaf1 supports HSC self-renewal after transplantation
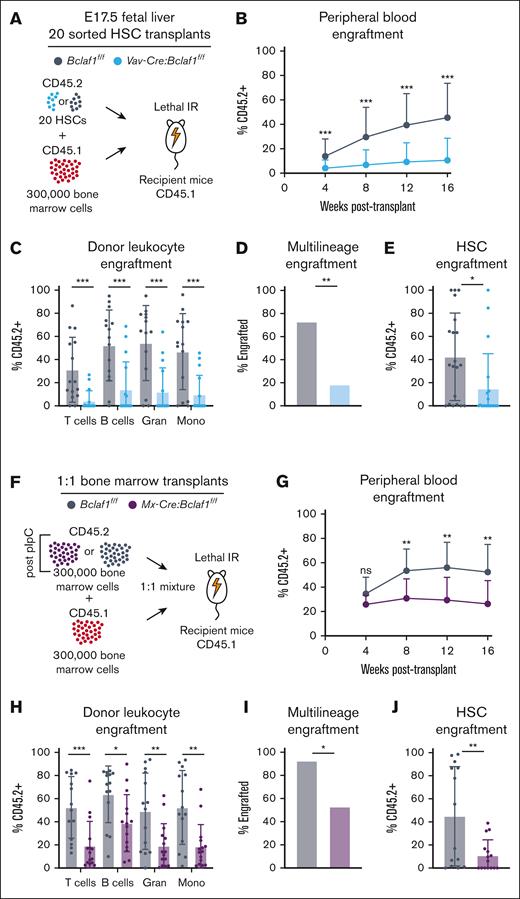
To test whether BCLAF1 supports fetal HSC self-renewal capacity, we performed competitive transplants and tracked donor cell engraftment in peripheral blood and bone marrow. To account for differences in HSC frequencies, we transplanted 20 HSCs from fetal livers of E17.5 control Bclaf1f/f or Vav-Cre:Bclaf1f/f mice (CD45.2+), along with wild-type competitor adult bone marrow cells (CD45.1+), into lethally irradiated CD45.1+ recipient mice (Figure 2A). Recipients of Vav-Cre:Bclaf1f/f fetal HSCs had significantly lower donor peripheral blood leukocytes at all times after transplant than recipients of Bclaf1f/f HSCs (Figure 2B). Chimerism of multiple lineages (T cells, B cells, granulocytes, and monocytes) was reduced, and 20% of mice that received Vav-Cre:Bclaf1f/f donor HSCs had multilineage donor engraftment compared to 75% of mice that received control donor HSCs (Figure 2C-D). Furthermore, donor Vav-Cre:Bclaf1f/f HSCs were significantly reduced in the bone marrow of recipient mice at 18 weeks after transplant (Figure 2E). These results are consistent with abnormal HSC expansion with loss of BCLAF1 rather than a defect in a specific differentiation pathway. Parallel experiments using sorted fetal HSCs or total fetal liver cells from germ line E17.5 Bclaf1–/– or Bclaf1+/+ mice also demonstrated reduced hematopoietic reconstitution by Bclaf1-deficient HSCs (supplemental Figure 4A-H). Thus, Bclaf1 helps maintain fetal HSC repopulating activity.
Bclaf1 is required for fetal and adult HSC repopulation activity. (A-E) A total of 20 sorted HSCs from donor CD45.2+Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal livers were transplanted with 300 000 CD45.1+ wild-type recipient BM cells into lethally irradiated CD45.1+ recipient mice (Bclaf1f/f, n = 15; Vav-Cre:Bclaf1f/f, n = 16). (A) Schematic of 20 HSC competitive transplant. (B) Peripheral blood donor engraftment at indicated times after transplant. (C) Donor-derived chimerism in peripheral blood leukocyte populations at 16 weeks after transplant. (D) Percentage of mice with multilineage engraftment (defined as ≥5% CD45.2+ donor in all cell lineages) at 16 weeks after transplant. (E) Frequency of donor-derived HSCs at 18 weeks after transplant. (F-J) Donor adult CD45.2+Bclaf1f/f or Mx-Cre:Bclaf1f/f mice were treated with pIpC, and then 4 weeks later, 300 000 BM cells from these mice were transplanted with 300 000 CD45.1+ wild-type recipient BM cells into lethally irradiated CD45.1+ recipient mice (Bclaf1f/f, n = 14; Mx-Cre:Bclaf1f/f, n = 15). (F) Schematic of 1:1 competitive transplant. (G) Peripheral blood donor engraftment at indicated times after transplant. (H) Donor-derived chimerism in peripheral blood leukocytes at 16 weeks after transplant. (I) Percentage of mice with multilineage engraftment (defined as ≥5% CD45.2+ donor in all cell lineages) at 16 weeks after transplant. (J) Frequency of donor-derived HSCs at 18 weeks after transplant. All transplants were conducted as 3 independent experiments of 5 to 10 mice per genotype, and data were combined for analyses. Data in panels B-C,E,G-H,J are mean ± SD, and statistical significance was determined by unpaired 2-tailed Student t test. Statistical significance in panels D,I was determined by Fisher exact test. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001. Gran, granulocytes; IR, irradiation; Mono, monocytes; ns, not significant.
Bclaf1 is required for fetal and adult HSC repopulation activity. (A-E) A total of 20 sorted HSCs from donor CD45.2+Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal livers were transplanted with 300 000 CD45.1+ wild-type recipient BM cells into lethally irradiated CD45.1+ recipient mice (Bclaf1f/f, n = 15; Vav-Cre:Bclaf1f/f, n = 16). (A) Schematic of 20 HSC competitive transplant. (B) Peripheral blood donor engraftment at indicated times after transplant. (C) Donor-derived chimerism in peripheral blood leukocyte populations at 16 weeks after transplant. (D) Percentage of mice with multilineage engraftment (defined as ≥5% CD45.2+ donor in all cell lineages) at 16 weeks after transplant. (E) Frequency of donor-derived HSCs at 18 weeks after transplant. (F-J) Donor adult CD45.2+Bclaf1f/f or Mx-Cre:Bclaf1f/f mice were treated with pIpC, and then 4 weeks later, 300 000 BM cells from these mice were transplanted with 300 000 CD45.1+ wild-type recipient BM cells into lethally irradiated CD45.1+ recipient mice (Bclaf1f/f, n = 14; Mx-Cre:Bclaf1f/f, n = 15). (F) Schematic of 1:1 competitive transplant. (G) Peripheral blood donor engraftment at indicated times after transplant. (H) Donor-derived chimerism in peripheral blood leukocytes at 16 weeks after transplant. (I) Percentage of mice with multilineage engraftment (defined as ≥5% CD45.2+ donor in all cell lineages) at 16 weeks after transplant. (J) Frequency of donor-derived HSCs at 18 weeks after transplant. All transplants were conducted as 3 independent experiments of 5 to 10 mice per genotype, and data were combined for analyses. Data in panels B-C,E,G-H,J are mean ± SD, and statistical significance was determined by unpaired 2-tailed Student t test. Statistical significance in panels D,I was determined by Fisher exact test. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001. Gran, granulocytes; IR, irradiation; Mono, monocytes; ns, not significant.
To determine whether Bclaf1 is also necessary to maintain the repopulating activity of adult HSCs, we performed competitive transplants using 20 HSCs from 8-week-old Vav-Cre:Bclaf1f/f and Bclaf1f/f mice. Similar to experiments with fetal HSCs, mice that received Bclaf1-deficient HSCs had significantly lower donor-derived leukocytes at all times and reduced multilineage engraftment at 16 weeks after transplant (supplemental Figure 4I-L). Furthermore, although induced deletion of BCLAF1 in adult Mx1-Cre:Bclaf1f/f mice did not alter HSC numbers (Figure 1R), these Bclaf1-deficient HSCs were defective in reconstituting hematopoiesis after transplant (Figure 2F-J). Collectively, these studies show that loss of Bclaf1 impairs both fetal and adult HSC repopulating capacity after transplant.
To assess activity of BCLAF1 in regulating long-term HSC (HSCLT) self-renewal, we performed secondary transplants using bone marrow from recipients of primary transplants. Given the reduced repopulation activity of Bclaf1-deficient HSCs in primary transplants, we identified recipient mice of Bclaf1+/+ or Bclaf1–/– fetal HSCs that had approximately equivalent donor chimerism (∼40%) in peripheral blood leukocytes at 16 weeks after primary transplants. Whole bone marrow from these primary recipient mice was transplanted into lethally irradiated secondary recipients (supplemental Figure 5A). Average Bclaf1+/+ donor chimerism ranged from 40% to 70% over time, whereas average Bclaf1–/– chimerism remained <20% (supplemental Figure 5B). At 20 weeks after transplant, the percentage of Bclaf1–/– donor chimerism was low in all leukocyte populations, and <20% of mice had multilineage engraftment, whereas control Bclaf1+/+ donor cells repopulated in 100% of mice (supplemental Figure 5C-D). Similar results were observed in secondary transplants using bone marrow from primary transplants with pIpC-treated Mx-Cre:Bclaf1f/f and Bclaf1f/f adult bone marrow (supplemental Figure 5E-G). Together, the primary and secondary transplants demonstrate that BCLAF1 has critical functions in HSC repopulation and self-renewal.
Loss of Bclaf1 does not impair HSC response to chemotherapy
HSC repopulation activity also helps regenerate bone marrow and peripheral blood after chemotherapy.60,61 To determine whether BCLAF1 regulates HSC function in this context, we treated adult Mx-Cre:Bclaf1f/f and Bclaf1f/f mice with pIpC and then with 4 sequential doses of 5-fluorouracil (5-FU) administrated at 3-week intervals (supplemental Figure 6A). Treatment with 5-FU depletes hematopoietic cells, and in response, HSCs regenerate progenitor cells and mature hematopoietic populations.60-62 Peripheral blood and bone marrow cells were equivalently suppressed in control (Bclaf1f/f) and Bclaf1-deficient (Mx-Cre:Bclaf1f/f) mice after the first dose of 5-FU (supplemental Figure 6B-D). Three weeks after the fourth dose of 5-FU, there were no differences in peripheral leukocyte or bone marrow HSCs between control Bclaf1f/f and Bclaf1-deficient Mx-Cre:Bclaf1f/f mice (supplemental Figure 6E-F). All mice in both groups survived. Thus, loss Bclaf1 does not alter HSC function in hematopoietic recovery after 5-FU.
To assess the kinetics of HSC recovery after chemotherapy, we treated Mx-Cre:Bclaf1f/f and Bclaf1f/f mice with pIpC and a higher dose of 5-FU (250 mg/kg; supplemental Figure 6G). Because 5-FU is known to alter surface marker expression during early recovery phases, we included an antibody to endothelial protein C receptor (EPCR/CD201) to quantify HSCs (supplemental Figure 1C).49 At 9 days after 5-FU, HSCs were lower in Mx-Cre:Bclaf1f/f mice than Bclaf1f/f controls (supplemental Figure 6G). By 14 days after 5-FU, HSCs were equivalent in both groups (supplemental Figure 6G).49,50 Although there may be slight differences in the kinetics of HSC recovery, Bclaf1-deficient HSCs can repopulate the bone marrow and peripheral blood after chemotherapy, which contrasts with the obligate role of Bclaf1 in sustaining HSC function after transplantation.
Loss of Bclaf1 does not alter HSC response to inflammatory stimulus
Inflammatory signals, such as type I interferon, induce HSC entry into the cell cycle.63 After exposure to pIpC, adult Bclaf1f/f and Vav-Cre:Bclaf1f/f HSCs had equivalent increase in the percentage of cells in S phase and a concomitant decrease in quiescent cells in G1 (supplemental Figure 6H). These results show that loss of BCLAF1 does not impair HSC proliferative response to an inflammatory stimulus, further supporting that BCLAF1 has distinct functions in HSCs after transplantation.
Bclaf1 does not regulate HSC homing
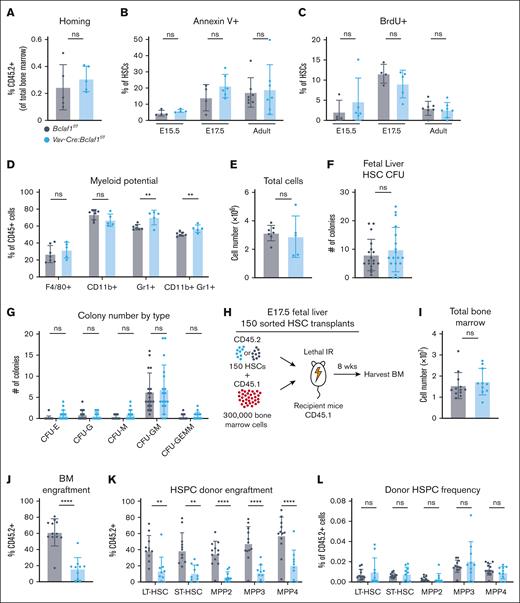
The differences in repopulating activity of Bclaf1-deficient HSCs after transplant vs chemotherapy could be due to altered HSC homing to the bone marrow. To test this, Lin-Sca1+c-Kit+ hematopoietic stem and progenitor cells (HSPCs) from adult Vav-Cre:Bclaf1f/f or Bclaf1f/f donors were transplanted into lethally irradiated recipients, and donor cells were quantified in the bone marrow 16 hours later. Percentages of donor Vav-Cre:Bclaf1f/f and Bclaf1f/f cells in recipient bone marrow were equivalent (Figure 3A), indicating that Bclaf1-deficient HSPCs do not have aberrant homing to the bone marrow after transplant.
Loss of Bclaf1 does not alter HSC homing, survival, proliferation, or differentiation. (A) To assess BM homing, 30 000 HSPCs from adult CD45.2+Bclaf1f/f or Vav-Cre:Bclaf1f/f BM were sorted and transplanted into lethally irradiated CD45.1+ recipients. At 16 hours after transplant, percentage of live, Lin-CD45.2+ cells in the BM was quantified by flow cytometry (Bclaf1f/f, n = 5; Vav-Cre:Bclaf1f/f, n = 5). (B) Cell death assessed by percentage of annexin V–positive HSCs in E15.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 4), E17.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 6), and adult BM (Bclaf1f/f, n = 7; Vav-Cre:Bclaf1f/f, n = 7). (C) Proliferation assessed by in vivo BrdU incorporation in HSCs of E15.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 5), E17.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 5), and adult BM (Bclaf1f/f, n = 7; Vav-Cre:Bclaf1f/f, n = 7). (D-E) Myeloid potential was assessed by culturing sorted HSCs from adult Bclaf1f/f or Vav-Cre:Bclaf1f/f BM in myeloid differentiation media for 6.5 days; cells were then stained for indicated myeloid cell markers (D) and total numbers were enumerated (Bclaf1f/f, n = 7; Vav-Cre:Bclaf1f/f, n = 5) (E). (F-G) Bclaf1f/f or Vav-Cre:Bclaf1f/f E17.5 fetal HSCs were sorted and cultured in complete MethoCult media. Total CFUs (F) and number of CFU with each classification (G) were quantitated at day 10 of culture (Bclaf1f/f, n = 18; Vav-Cre:Bclaf1f/f, n = 18). (H-L) A total of 150 sorted HSCs from donor CD45.2+Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal livers were transplanted with 300 000 CD45.1+ wild-type recipient BM cells into lethally irradiated CD45.1+ recipient mice. All analyses were conducted at 8 weeks after transplant (Bclaf1f/f, n = 12; Vav-Cre:Bclaf1f/f, n = 10). (H) Schematic of 150 HSC competitive transplant. (I) Total BM counts. (J) Frequency of CD45.2+ donor-derived HSCs. (K) Percentage of CD45.2+ donor-derived cells within each HSPC population. BM cells were gated on each HSPC population then on CD45.2. (L) HSPC populations as percentage of donor CD45.2+ BM cells. BM cells were gated on CD45.2+, then on each HSPC group within that donor population. Data in all panels are mean ± SD and statistical significance was determined by unpaired 2-tailed Student t test. ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001. CFU, colony-forming unit; CFU-E, colony-forming unit–erythroid; CFU-G, colony-forming unit–granulocyte; CFU-GEMM, colony-forming unit–granulocyte-erythroid-macrophage-megakaryocyte; CFU-GM, colony-forming unit–granulocyte-macrophage; CFU-M, colony-forming unit–macrophage; IR, irradiation; ns, not significant; ST, short-term.
Loss of Bclaf1 does not alter HSC homing, survival, proliferation, or differentiation. (A) To assess BM homing, 30 000 HSPCs from adult CD45.2+Bclaf1f/f or Vav-Cre:Bclaf1f/f BM were sorted and transplanted into lethally irradiated CD45.1+ recipients. At 16 hours after transplant, percentage of live, Lin-CD45.2+ cells in the BM was quantified by flow cytometry (Bclaf1f/f, n = 5; Vav-Cre:Bclaf1f/f, n = 5). (B) Cell death assessed by percentage of annexin V–positive HSCs in E15.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 4), E17.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 6), and adult BM (Bclaf1f/f, n = 7; Vav-Cre:Bclaf1f/f, n = 7). (C) Proliferation assessed by in vivo BrdU incorporation in HSCs of E15.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 5), E17.5 fetal liver (Bclaf1f/f, n = 4; Vav-Cre:Bclaf1f/f, n = 5), and adult BM (Bclaf1f/f, n = 7; Vav-Cre:Bclaf1f/f, n = 7). (D-E) Myeloid potential was assessed by culturing sorted HSCs from adult Bclaf1f/f or Vav-Cre:Bclaf1f/f BM in myeloid differentiation media for 6.5 days; cells were then stained for indicated myeloid cell markers (D) and total numbers were enumerated (Bclaf1f/f, n = 7; Vav-Cre:Bclaf1f/f, n = 5) (E). (F-G) Bclaf1f/f or Vav-Cre:Bclaf1f/f E17.5 fetal HSCs were sorted and cultured in complete MethoCult media. Total CFUs (F) and number of CFU with each classification (G) were quantitated at day 10 of culture (Bclaf1f/f, n = 18; Vav-Cre:Bclaf1f/f, n = 18). (H-L) A total of 150 sorted HSCs from donor CD45.2+Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal livers were transplanted with 300 000 CD45.1+ wild-type recipient BM cells into lethally irradiated CD45.1+ recipient mice. All analyses were conducted at 8 weeks after transplant (Bclaf1f/f, n = 12; Vav-Cre:Bclaf1f/f, n = 10). (H) Schematic of 150 HSC competitive transplant. (I) Total BM counts. (J) Frequency of CD45.2+ donor-derived HSCs. (K) Percentage of CD45.2+ donor-derived cells within each HSPC population. BM cells were gated on each HSPC population then on CD45.2. (L) HSPC populations as percentage of donor CD45.2+ BM cells. BM cells were gated on CD45.2+, then on each HSPC group within that donor population. Data in all panels are mean ± SD and statistical significance was determined by unpaired 2-tailed Student t test. ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001. CFU, colony-forming unit; CFU-E, colony-forming unit–erythroid; CFU-G, colony-forming unit–granulocyte; CFU-GEMM, colony-forming unit–granulocyte-erythroid-macrophage-megakaryocyte; CFU-GM, colony-forming unit–granulocyte-macrophage; CFU-M, colony-forming unit–macrophage; IR, irradiation; ns, not significant; ST, short-term.
Loss of Bclaf1 does not alter HSC cell death or proliferation
Loss of BCLAF1 could alter HSC cell death or proliferation. To assess cell survival, we measured annexin V expression on fetal and adult HSCs. The percentages of annexin V–positive HSCs were equivalent in Bclaf1f/f and Vav-Cre:Bclaf1f/f HSCs at all ages (Figure 3B). To measure fetal HSC proliferation, we performed in vivo BrdU (5-bromo-2′-deoxyuridine) labeling in fetal and adult mice. At all developmental stages, BrdU incorporation in HSCs was similar in Bclaf1-deficient and control HSCs (Figure 3C). These findings demonstrate that Bclaf1 does not have significant functions in regulating HSC survival or proliferation.
Loss of Bclaf1 does not alter HSC differentiation
The decrease in fetal HSC numbers and self-renewal function of Bclaf1-deficient HSCs could be a consequence of altered myeloid differentiation, namely increased differentiation at the expense of self-renewal. To test myeloid potential, we cultured Bclaf1f/f or Vav-Cre:Bclaf1f/f HSCs ex vivo in conditions to promote myeloid differentiation. Compared to Bclaf1f/f, Vav-Cre:Bclaf1f/f HSCs had a small increase in the percentage of Gr1+ myeloid cell output (Figure 3D). This increase in the percentage of Gr1+ cells was not due to altered proliferation, because total cell numbers were similar in both groups (Figure 3E). To further investigate differentiation, colony-forming assays were performed with Bclaf1f/f or Vav-Cre:Bclaf1f/f E17.5 fetal HSCs. Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal HSCs generated similar numbers and types of colonies (Figure 3F-G). These results support that Bclaf1-deficient HSCs have similar myeloid capacity as control HSCs.
To assess whether loss of BCLAF1 altered HSC differentiation in vivo, we quantitated donor cell engraftment in HSPCs at 8 weeks after competitive transplant. HSCs from E17.5 Bclaf1f/f and Vav-Cre:Bclaf1f/f fetal livers were transplanted with wild-type competitor adult bone marrow cells into lethally irradiated recipient mice (Figure 3H). Compared to recipients of Bclaf1f/f fetal HSCs, recipients of Vav-Cre:Bclaf1f/f fetal HSCs had significantly lower donor cells in all populations assessed (Figure 3I-K). HSCs and MPPs were present at equivalent frequencies within the donor population in recipients of both wild-type and Bclaf1-deficient HSCs (Figure 3L). Thus, Bclaf1-deficient HSCs differentiate to lineage-restricted progenitors similarly to wild-type HSCs. Cumulatively, these studies show that the differences in fetal HSC numbers and HSC self-renewal observed with Bclaf1 deficiency are not due to changes in HSC differentiation.
Bclaf1-deficient HSCs do not have altered gene expression by bulk RNA-seq
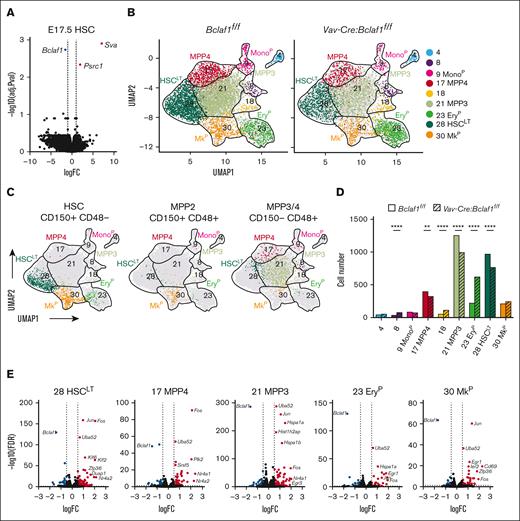
BCLAF1 functions as a transcriptional repressor in early B cells and other cell populations.37,64 We performed RNA-seq on bulk-sorted HSCs and MPP3/4s from E17.5 Vav-Cre:Bclaf1f/f and Bclaf1f/f fetal livers to determine whether loss of BCLAF1 alters gene expression in these populations. As expected, lineage-specific genes (ie, Mecom, Cd48, and Slamf1) were differentially expressed between HSCs and MPP3/4s from both Bclaf1-deficient and control mice (supplemental Figure 7A). However, only 2 genes, Sva and Psrc1, which do not have known roles in HSCs or hematopoiesis, were differentially expressed in Bclaf1-deficient HSCs or MPP3/4s compared to controls (Figure 4A; supplemental Figure 7B). These studies raised the question of whether BCLAF1 has activities in a subpopulation of HSCs that is not resolved by assessment of bulk populations.
Bclaf1 maintains fetal HSCLT
We next performed single-cell cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq)65 on sorted Lin-Sca1+c-Kit+ hematopoietic cells from control Bclaf1f/f and Vav-Cre:Bclaf1f/f E17.5 fetal livers to determine why loss of Bclaf1 leads to HSC depletion. Iterative Clustering and Guide-gene Selection version 2 was used to cluster cells in an unsupervised manner based on differential gene expression into 9 different clusters (Figure 4B). Using expression of population-specific genes and surface marker phenotypes inferred from barcoded features, we were able to assign cell identities to each cluster (Figure 4B-C; supplemental Figure 7C-D).66,67 HSCLT predominantly occupied cluster 28, with MPP3s in cluster 21, MPP4s in cluster 17, erythroid precursors (EryP) in cluster 23, and megakaryocyte progenitors (MkP) in cluster 30 (Figure 4B).
Bclaf1 loss results in reduced HSCLT and increased stress response gene expression signature. (A) Volcano plot shows differentially expressed genes in Vav-Cre:Bclaf1f/f (n = 3) vs Bclaf1f/f (n = 4) E17.5 fetal HSCs determined by RNA-seq of bulk-sorted populations. Red and blue dots indicate significantly (P < .05; log2FC ≥1) increased and decreased genes, respectively. (B-G) Single-cell CITE-seq on E17.5 Lin-Sca1+c-Kit+ HSPCs from Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal livers (cells from 10 embryos pooled for each genotype). (B) UMAP shows Iterative Clustering and Guide-gene Selection version 2 (ICGS2) results of progenitor populations (Bclaf1f/f, 5551 cells; Vav-Cre:Bclaf1f/f, 3246 cells). (C) Overlay of surface-marked HSC, MPP2, and MPP3/4 populations defined from barcoded CD150 and CD48 antibodies. (D) Bar graph shows numbers of cells in each ICGS2 cluster by genotype. Genotypes were normalized to cell number in Vav-Cre:Bclaf1f/f group. Statistical significance was determined by χ2 analysis. (E) Volcano plots show differentially expressed genes in Vav-Cre:Bclaf1f/f vs Bclaf1f/f cells of indicated hematopoietic populations/clusters determined by pseudobulk gene expression analysis. Red and blue dots indicate significantly (P < .05; log2FC ≥1) increased and decreased genes, respectively. (F) Stress score applied to each cell and overlayed on single-cell RNA-seq UMAP. Higher score (green) indicates increased expression of stress response genes. (G) Quantitation of stress score in Bclaf1f/f and Vav-Cre:Bclaf1f/f cells in each cluster. ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001. FC, fold change; FDR, false discovery rate; MkP, megakaryocyte progenitors; Mono, monocyte precursor; UMAP, uniform manifold approximation and projection.
Bclaf1 loss results in reduced HSCLT and increased stress response gene expression signature. (A) Volcano plot shows differentially expressed genes in Vav-Cre:Bclaf1f/f (n = 3) vs Bclaf1f/f (n = 4) E17.5 fetal HSCs determined by RNA-seq of bulk-sorted populations. Red and blue dots indicate significantly (P < .05; log2FC ≥1) increased and decreased genes, respectively. (B-G) Single-cell CITE-seq on E17.5 Lin-Sca1+c-Kit+ HSPCs from Bclaf1f/f or Vav-Cre:Bclaf1f/f fetal livers (cells from 10 embryos pooled for each genotype). (B) UMAP shows Iterative Clustering and Guide-gene Selection version 2 (ICGS2) results of progenitor populations (Bclaf1f/f, 5551 cells; Vav-Cre:Bclaf1f/f, 3246 cells). (C) Overlay of surface-marked HSC, MPP2, and MPP3/4 populations defined from barcoded CD150 and CD48 antibodies. (D) Bar graph shows numbers of cells in each ICGS2 cluster by genotype. Genotypes were normalized to cell number in Vav-Cre:Bclaf1f/f group. Statistical significance was determined by χ2 analysis. (E) Volcano plots show differentially expressed genes in Vav-Cre:Bclaf1f/f vs Bclaf1f/f cells of indicated hematopoietic populations/clusters determined by pseudobulk gene expression analysis. Red and blue dots indicate significantly (P < .05; log2FC ≥1) increased and decreased genes, respectively. (F) Stress score applied to each cell and overlayed on single-cell RNA-seq UMAP. Higher score (green) indicates increased expression of stress response genes. (G) Quantitation of stress score in Bclaf1f/f and Vav-Cre:Bclaf1f/f cells in each cluster. ∗∗P ≤ .01; ∗∗∗∗P ≤ .0001. FC, fold change; FDR, false discovery rate; MkP, megakaryocyte progenitors; Mono, monocyte precursor; UMAP, uniform manifold approximation and projection.
Vav-Cre:Bclaf1f/f fetal hematopoietic progenitors had similar cluster heterogeneity compared to Bclaf1f/f controls, indicating that differentiation trajectories remain intact in Bclaf1 deficiency (Figure 4B). However, comparison of distribution across the clusters revealed a distinct reduction of Bclaf1-deficient cells in HSCLT (cluster 28) and MPP3 clusters (cluster 21), with increased representation in the EryP cluster (cluster 23; Figure 4D). The increase in Bclaf1-deficient cells in transcriptionally defined erythroid precursors (EryP) contrasts with the finding of normal surface-marked erythroid precursors (supplemental Figure 3D) and suggests that loss of BCLAF1 may result in a distinct gene expression despite normal surface markers in these populations. The reduction in HSCLT in Vav-Cre:Bclaf1f/f is consistent with the decreased fetal HSCs defined by surface markers (Figure 1J). These findings complement the in vivo studies and support that BCLAF1 functions to maintain HSCLT fidelity.
BCLAF1 restrains expression of stress response genes in hematopoietic progenitors
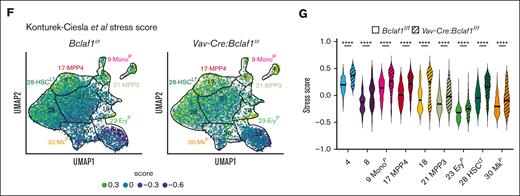
We next used pseudobulk gene expression analysis to identify transcriptional changes between wild-type and Bclaf1-deficient HSPCs identified by CITE-seq, with the aim of identifying programs that could account for altered HSC function. Within the HSCLT population (cluster 28), Vav-Cre:Bclaf1f/f cells had significantly increased expression of stress response genes, including Jun, Fos, Nr4a2, Uba52, Klf2, and Klf6 (Figure 4E). Interestingly, stress response genes were also differentially increased in Vav-Cre:Bclaf1f/f cells in MPP3 (cluster 21), MPP4 (cluster 17), EryP (cluster 23), and MkP (cluster 30) clusters (Figure 4E). To better define the dysregulation of these genes across Bclaf1-deficient hematopoietic precursors, we applied a stress score, based on previously defined stress response gene signature, to each cell.68Bclaf1-deficient cells had higher stress scores than wild-type cells across all populations and clusters (Figure 4F-G). In particular, Bclaf1-deficient HSCLT had strikingly increased stress scores relative to wild-type HSCLT (Figure 4G). Stress response genes are transiently expressed in response to hematopoietic insults and function to reduce HSC self-renewal and promote myeloid differentiation to preserve hematopoiesis.68-74 Aberrant or persistent activation of stress pathways results in depletion of HSCLT and impaired hematopoiesis, consistent with our findings of reduced repopulation activity of Bclaf1-deficient HSCs.71,72,75 These findings suggest that BCLAF1 functions to restrain stress response genes to maintain HSC fidelity and function.
BCLAF1 associates with chromatin in HSCs at sites enriched for E26 transformation-specific:interferon regulatroy factor consensus sequence
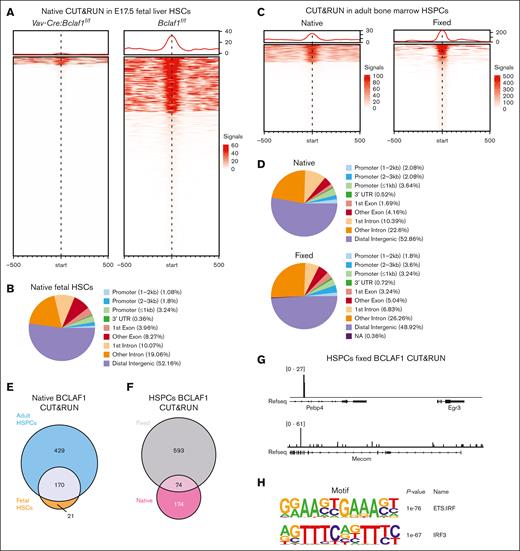
To determine whether BCLAF1 binds chromatin in HSCs, we used CUT&RUN to profile BCLAF1 binding in wild-type (Bclaf1f/f) E17.5 fetal HSCs (Figure 5A). BCLAF1 CUT&RUN was also performed in Bclaf1-deficient fetal (Vav-Cre:Bclaf1f/f) HSCs to serve as a negative control. BCLAF1 binds throughout the genome in fetal HSCs, predominantly at intronic and distal intergenic regions (Figure 5B). BCLAF1 peaks were not present in proximity to the differentially expressed stress response genes identified by CITE-seq, suggesting that BCLAF1 may not directly regulate their transcription. Alternatively, BCLAF1 may transiently bind DNA or complex with other transcription factors, as in early B cells.64 These alternative binding profiles are generally weaker chromatin associations and may not be captured by standard CUT&RUN.
BCLAF1 associates with chromatin in hematopoietic progenitor cells. (A) Heat map of signal intensity for BCLAF1 peaks identified by native CUT&RUN in Vav-Cre:Bclaf1f/f and Bclaf1f/f E17.5 fetal HSCs. Vav-Cre:Bclaf1f/f HSCs included as negative control. (B) Annotation of genomic location for BCLAF1 peaks identified in Bclaf1f/f HSCs in panel A. (C) Heat map of signal intensity for BCLAF1 peaks identified by native and fixed CUT&RUN in adult BM HSPCs (Lin-Sca+Kit+) from adult Bclaf1f/f mice. (D) Annotation of genomic location for BCLAF1 peaks identified in native and fixed CUT&RUN in panel C. (E) Venn diagram comparing BCLAF1 peaks identified by native CUT&RUN in fetal liver HSCs in panel A vs native adult BM HPSCs in panel C. (F) Venn diagram comparing BCLAF1 peaks identified by native vs fixed CUT&RUN in the BM HSPCs in panel C. (G) Representative tracks at indicated regions for BCLAF1 peaks identified by fixed CUT&RUN in adult BM HSPCs in panel C. (H) Top 2 results from Hypergeometric Optimization of Motif EnRichment (HOMER)76 known motif analysis of BCLAF1 peaks identified in Bclaf1f/f HSCs in panel A. NA, not available; Refseq, reference sequence; UTR, untranslated region.
BCLAF1 associates with chromatin in hematopoietic progenitor cells. (A) Heat map of signal intensity for BCLAF1 peaks identified by native CUT&RUN in Vav-Cre:Bclaf1f/f and Bclaf1f/f E17.5 fetal HSCs. Vav-Cre:Bclaf1f/f HSCs included as negative control. (B) Annotation of genomic location for BCLAF1 peaks identified in Bclaf1f/f HSCs in panel A. (C) Heat map of signal intensity for BCLAF1 peaks identified by native and fixed CUT&RUN in adult BM HSPCs (Lin-Sca+Kit+) from adult Bclaf1f/f mice. (D) Annotation of genomic location for BCLAF1 peaks identified in native and fixed CUT&RUN in panel C. (E) Venn diagram comparing BCLAF1 peaks identified by native CUT&RUN in fetal liver HSCs in panel A vs native adult BM HPSCs in panel C. (F) Venn diagram comparing BCLAF1 peaks identified by native vs fixed CUT&RUN in the BM HSPCs in panel C. (G) Representative tracks at indicated regions for BCLAF1 peaks identified by fixed CUT&RUN in adult BM HSPCs in panel C. (H) Top 2 results from Hypergeometric Optimization of Motif EnRichment (HOMER)76 known motif analysis of BCLAF1 peaks identified in Bclaf1f/f HSCs in panel A. NA, not available; Refseq, reference sequence; UTR, untranslated region.
To assess for indirect or transient DNA binding, we compared BCLAF1 chromatin association using a modified CUT&RUN with fixation to standard native CUT&RUN (Figure 5C).44,45,77 HSPCs from wild-type adult Bclaf1f/f bone marrow were used due to decreased DNA yield with fixed CUT&RUN, which precluded use of fetal HSCs. BCLAF1 binding was enriched in intronic and intergenic regions in both fixed and native hematopoietic cells (Figure 5D). BCLAF1 chromatin association was similar in fetal HSCs and adult HSPCs (Figure 5E). Comparison of annotated genes in proximity of BCLAF1 binding peaks in native vs fixed CUT&RUN in adult HSPCs revealed distinct genes associated with each condition, highlighting the complementarity of the 2 CUT&RUN approaches (Figure 5F). Notably, fixed CUT&RUN identified BCLAF1 peaks near stress response genes (ie, Egr3) and developmental genes (ie, Mecom), which were differentially expressed in CITE-seq analyses (Figure 5G). Cumulatively, these results suggest that BCLAF1 associates with chromatin in HSPCs through direct and indirect mechanisms. The different binding profiles in native vs fixed CUT&RUN may reflect distinct BCLAF1 binding sites in HSCs and MPPs, which are more abundant in HSPCs.
BCLAF1 peaks in both native and fixed CUT&RUN in HSCs were enriched for the E26 transformation-specific:interferon regulatroy factor composite DNA element (Figure 5H), suggesting that BCLAF1 may not directly bind DNA but rather is recruited to chromatin by other transcription factors.78 Collectively, these results suggest that BCLAF1 localizes to chromatin to modulate transcriptional programs in hematopoietic progenitors.
Discussion
We show that BCLAF1 has critical functions in fetal HSC development and in preserving HSC self-renewal after transplant. In late-stage fetal livers, BCLAF1-deficient mice have reduced HSC numbers. During embryogenesis, fetal HSCs develop from an immature, pre-HSC population and undergo a limited expansion to increase their numbers.4-6 BCLAF1 may function in either of these processes to support fetal HSC development. Bone marrow HSC numbers increase in the immediate neonatal period and then maintain steady-state levels through adulthood.4,79,80 Interestingly, loss of BCLAF1 early in development results in reduced HSC numbers in adult mice; however, deletion of BCLAF1 in established adult hematopoietic cells does not alter the HSC population. Thus, BCLAF1 has roles in increasing HSC numbers during development but is not critical for maintaining adult HSCs at steady state.
After transplant, BCLAF1-deficient fetal and adult HSCs fail to expand to the same levels as control cells. After transplant, limited number of HSCs must expand to repopulate the hematopoietic niche and establish mature blood cell populations. In contrast, HSC responses after chemotherapy and inflammatory stimulation are not dependent on BCLAF1. During development and after transplant, HSCs undergo self-renewal to varying degrees to increase their population.81-87 Although self-renewal is still active after chemotherapy or inflammation, it is less essential for repopulation of the bone marrow and reconstitution of hematopoiesis. It is conceivable that loss of BCLAF1 could lead to HSC divisions that favor differentiation rather than self-renewal. However, we find that Bclaf1-deficient fetal HSCs do not have accelerated differentiation during development or after transplant. Thus, our findings suggest that BCLAF1 has distinct activities in promoting fetal HSC development and in preserving repopulating function of both fetal and adult HSCs.
We find that BCLAF1 represses expression of stress response genes in fetal HSCs. Stress response programs are induced to promote HSC differentiation and regenerate mature populations.68-74,88 However, persistent activation of stress responses blocks HSC self-renewal, which impairs establishment of long-term hematopoiesis.71,72,75,88 Notably, although loss of BCLAF1 impaired fetal HSC expansion, it did not result in altered differentiation. Thus, BCLAF1-mediated restraint of stress response genes could function to preserve HSC self-renewal, rather than restrict differentiation, to permit HSCLT expansion and repopulation. It is interesting to consider how these findings may apply to impaired repopulating activity of Bclaf1-deficient HSCs after transplant. Increased expression of stress response genes, including Jun and Fos, limits HSC self-renewal and reconstitution of hematopoiesis after transplant, similar to the phenotype of Bclaf1-deficient HSCs.71,72,75,88 How dysregulation of the individual or combined stress response genes in Bclaf1-deficient HSCs contributes to aberrant HSC function is being investigated in ongoing studies.
Stress response genes were increased in many Bclaf1-deficient hematopoietic precursor subpopulations. BCLAF1 may regulate expression of this stress program in these downstream progenitors. Alternatively, increased stress signature may be imprinted on cells differentiated from Bclaf1-deficient HSCs. Further research is needed to distinguish between these possibilities and to define the function(s) of BCLAF1 in other hematopoietic progenitor populations.
BCLAF1 binding was only identified in proximity to a few stress response genes, suggesting that BCLAF1 does not directly modulate transcription of this full program but rather acts through indirect mechanisms. Namely, BCLAF1 may alter the expression of other transcription factors, such as AP-1, that in turn coordinate the broader stress response program.70-74 Interestingly, we find that BCLAF1 binds similar genomic regions in fetal HSCs and adult HSPCs. Other factors may modulate BCLAF1 function in these populations, accounting for the differential phenotypes. BCLAF1 binding sites were enriched for the sequence bound by ETS family transcription factors, such as ETV6 and PU.1, which are known regulators of HSC stress responses.89-92 These findings suggest that BCLAF1 may complex with ETS transcription factors to regulate gene expression in HSCs, similar to its activity in early B cells.64 The binding partners of BCLAF1 and its recruitment to gene regulatory elements are active areas of study.
In summary, we have shown that BCLAF1 has critical functions in HSCs during fetal development and after stem cell transplant but is dispensable for steady-state hematopoiesis. We propose that BCLAF1 transcriptionally limits stress response genes to preserve HSC fidelity and self-renewal.
Acknowledgments
The authors thank the Genome Technology Access Center at the McDonnell Genome Institute at Washington University School of Medicine for help with genomic analysis. The center is partially supported by National Cancer Institute (NCI) Cancer Center Support grant P30 CA91842 to the Siteman Cancer Center from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
This work was supported by grants from the NIH (National Institute of Allergy and Infectious Diseases [NIAID] R56AI153234 [J.J.B.], NIAID R01AI173077 [J.J.B.], NIAID T32AI007163 [S.J.C. and H.S.], NHLBI F31HL165908-01A1 [S.J.C.], NHLBI R01HL152180 [J.A.M.], and NHLBI R01HL55337 [K.C.]). J.J.B. was supported by the American Society of Hematology, St. Louis Children’s Hospital Foundation, the Children's Discovery Institute, Hyundai Hope on Wheels, and Kelsie’s Hope Foundation. This publication was supported by the Washington University NIH/NCI Specialized Programs in Research Excellence in Leukemia grant 1P50CA171063 (J.J.B.). J.A.M. is a Scholar of the Leukemia & Lymphoma Society.
This publication is solely the responsibility of the authors and does not necessarily represent the official view of NIAID, NCI, NCRR, or NIH.
Authorship
Contribution: J.J.B. conceived the project; S.J.C. and J.J.B. designed experiments and wrote the manuscript; S.J.C., L.S.W., W.Y., J.W., H.S., and Y.L. performed experiments and data analyses; K.C. and J.A.M. provided expertise and assisted with experimental design and data analyses; J.J.B. supervised the project, interpreted experiments, and secured funding; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey J. Bednarski, Department of Pediatrics, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8208, St. Louis, MO 63110; email: bednarski_j@wustl.edu.
References
Author notes
The full-text version of this article contains a data supplement.