Two autologous hematopoietic stem cell (HSC)–based gene therapies (GTs) are now commercially available for severe sickle cell disease and transfusion-dependent β-thalassemia. However, the safety and efficacy of a subsequent autologous HSC-based GT after graft failure with a previous allogeneic hematopoietic cell transplant (HCT) remains unclear. Some individuals who have experienced a failed first attempt at a potentially curative therapy might seek a second opportunity for cure via GT. In this article, we discuss various factors related to patient and HSC health that may influence feasibility, and shared decision-making regarding whether an individual who has previously received an allogeneic HCT and experienced graft failure could consider an autologous GT. Exposure to chronic inflammatory stress and conditioning chemotherapy may compromise HSC fitness, reduce hematopoietic reserve, accelerate HSC aging, and promote the accumulation of deleterious genetic mutations, all of which may adversely affect the safety and efficacy of the GT.
Introduction
Autologous genetically modified hematopoietic stem cell (HSC)–based therapies are now available for sickle cell disease (SCD) and transfusion-dependent β-thalassemia (TDT).1 These therapies involve collection of CD34+ HSCs and HSC progenitors (HSCPs) via apheresis from the patient after mobilization into the peripheral blood.2 The collected HSCPs are then genetically modified with a lentiviral vector or site-directed nucleases.2 Ultimately, the genetically modified HSCPs are infused back into the patient after bone marrow–ablating chemotherapy.2 Overall, this is a resource-intensive and high-risk, high-reward therapy that, if successful, can potentially cure certain individuals with severe SCD or TDT.
Although 2 HSC-based gene therapies are commercially available for individuals with severe SCD who experience frequent vaso-occlusive crises and for those with TDT, respectively, the detailed characteristics that define ideal candidacy for these therapies remain the subject of debate, especially with regard to the absolute contraindications.3-5 To date, clinical trials of gene therapy for SCD and TDT have strictly excluded patients who have received a previous HSC-based therapy such as an allogeneic hematopoietic cell transplant (HCT) or previous gene therapy.3 Therefore, the safety (and efficacy) of a subsequent autologous HSC-based gene therapy for patients who experience graft failure after an allogeneic HCT remains unclear. In this article, we discuss the various factors that may influence shared decision-making regarding whether an individual who has previously undergone an unsuccessful allogeneic HCT and experienced graft failure could proceed to autologous gene therapy.
Reasons for pursuing gene therapy after allogeneic HCT
Unmet need
Individuals with SCD who pursue an HCT often have severe disease features. They commonly have tried multiple disease-modifying therapies but with minimal success in controlling the disease symptoms. This is often why some individuals with SCD have elected to try a high-risk, high-reward therapy such as an allogeneic HCT in the first place. Individuals with TDT similarly pursue HCT to ameliorate the symptom burden associated with chronic blood transfusions and iron overload, as well as to preempt organ failure.6
Unfortunately, graft failure and disease recurrence can occur after an allogeneic HCT. Graft failure could be primary, defined by the lack of achievement of an absolute neutrophil count of >500/μL after HCT; or secondary, defined as hematopoietic functional decline after initial engraftment.7 Causes of graft failure include HCT-related factors (graft source and composition, including low graft stem cell dose), occurrence of infections in the early post-HCT period, exposure to myelosuppressive drugs, or immunologic graft rejection. Notably, the risk of graft failure after allogeneic HCT, although generally low, is not negligible for patients with TDT despite the use of multiagent myeloablative preparative approaches, and for those with SCD treated with reduced-intensity or subablative HCT conditioning regimens.8,9 In some instances, individuals experience mixed donor and recipient chimerism within different cellular lineages. When significant mixed chimerism or absence of donor chimerism (<5% myeloid and lymphoid lineages) occurs after HCT, patients experience poor graft function including dependence on transfusions, growth factor support, and eventual disease re-emergence. A second allogeneic HCT may be considered emergently if a patient experiences marrow aplasia, although more often a patient experiences hematopoietic reconstitution from their endogenous bone marrow.
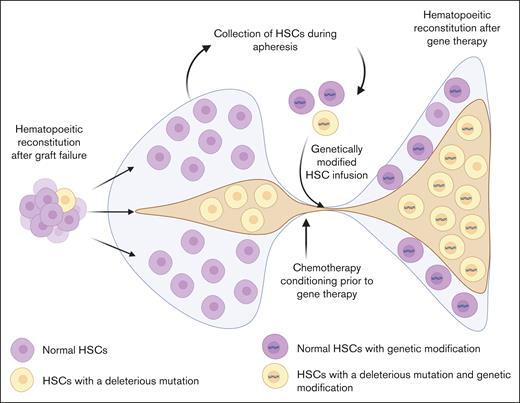
It is logical that those patients who have experienced a failed first attempt to transform their disease might seek a second opportunity in gene therapy. These individuals represent a cohort of patients who not only have high disease severity but also have a significant unmet need to be able to pursue HCT, given the relative paucity of HLA-matched donors, especially for individuals with SCD10 or TDT, and the significant potential toxicities associated with a second allogeneic HCT. Autologous gene therapy may appear an attractive alternative for those individuals who have experienced graft failure after a previous allogeneic HCT; however, pursuing autologous gene therapy may not be feasible because of several individual and HSC fitness-related issues (Figure 1).
Factors affecting subsequent autologous gene therapy after graft failure with a previous allogeneic HCT in individuals with SCD and thalassemia. Persistent systemic and bone marrow niche sterile inflammation, constant erythroid development pressure, iron overload, and associated toxicity from blood transfusions, as well as exposure to chemotherapy and radiation during an allogeneic HCT may cause damage to the HSCs, inducing accelerated senescence or clonal evolution. This may make the HSCs unsuitable for use in manufacturing of a genetically modified product. At the same time, exposure to alloantigens in the transfused blood units may induce anti-HLA antibodies, and damage to various organ systems from chemoradiotherapy exposure may affect the ability of an individual to receive a subsequent allogeneic HCT or autologous gene therapy.
Factors affecting subsequent autologous gene therapy after graft failure with a previous allogeneic HCT in individuals with SCD and thalassemia. Persistent systemic and bone marrow niche sterile inflammation, constant erythroid development pressure, iron overload, and associated toxicity from blood transfusions, as well as exposure to chemotherapy and radiation during an allogeneic HCT may cause damage to the HSCs, inducing accelerated senescence or clonal evolution. This may make the HSCs unsuitable for use in manufacturing of a genetically modified product. At the same time, exposure to alloantigens in the transfused blood units may induce anti-HLA antibodies, and damage to various organ systems from chemoradiotherapy exposure may affect the ability of an individual to receive a subsequent allogeneic HCT or autologous gene therapy.
General considerations for a subsequent HSC-based therapy
The decision to pursue a potentially curative therapy for hemoglobinopathies is challenging in itself.11 This decision becomes further complicated by the desire to pursue a life that is disease free after having experienced an unsuccessful previous allogeneic HCT. Hereafter, we provide a few considerations that support rational decision-making around feasibility of a second allogeneic HCT or autologous gene therapy.
Alternative donor availability
The first point to consider is whether other donors are available to facilitate a second allogeneic HCT. The risk of graft rejection is higher with a second allogeneic HCT from the same donor with whom the recipient has previously experienced complete immunologic graft rejection.12 Therefore, using a different donor is preferable to avoid the same outcome. With the advances in the field of transplantation, especially as they relate to using haploidentical donors,13 the donor pool has expanded to include partially matched family members who are readily available for almost any given patient. Using a haploidentical donor may offer the advantage of proceeding to a potentially curative therapy rather quickly, without the patient having to undergo autologous HSC collection for gene therapy and await product manufacture. At the same time, the presence of HLA-alloimmunization and donor-specific HLA antibodies (DSAs) in the haploidentical HCT setting may be prohibitive because of the associated high rates of graft rejection.14
Donor selection and immunologic challenges with an allogeneic graft source
Several factors affect future donor selection for individuals with SCD and TDT. Frequent blood transfusions expose recipients to foreign antigens, often leading to alloimmunization,15 which increases the risk of graft rejection with a first or subsequent allogeneic HCT.16 The presence of high mean fluorescence intensity anti-HLA antibodies poses a particular risk of graft rejection in recipients of HLA-mismatched donor transplants, in whom alloantibodies may be donor specific and target donor stem cells directly.17,18 Desensitization of the DSAs may be attempted before an allogeneic HCT but often requires additional immunochemotherapy and plasma exchange procedures.14 If the rejection risk cannot be mitigated through management of HLA-alloimmunization, either by identifying a subsequent donor for whom DSAs are not present or by successful desensitization with reduction of DSAs, using autologous HSCs for gene therapy may be preferred. In addition, the general presence of alloantibodies can cause complications such as delayed engraftment, poor graft function, immune-mediated hemolysis, increased blood and platelet transfusion needs, and other immunologic complications after an allogeneic HCT.19-21 Therefore, not only is identifying a suitable HSC donor for a second HCT challenging, but proceeding to a second HCT when a donor is identified is also a complex exercise. Given these risks, challenges, and the unmet need, an autologous gene therapy using the patient’s own HSCs appears to be a more feasible potentially curative therapy.
Organ function and patient fitness
Although several reduced-intensity conditioning regimens have been developed for allogeneic HCT for SCD or TDT, myeloablation before gene therapy still requires high-dose busulfan.3 Individuals who have received a previous HCT may have subclinical organ function deficits that put them at a cumulatively higher risk of treatment-related morbidity and mortality related to the use of high-dose chemotherapy before allogeneic HCT or the infusion of genetically modified cells. Hence, all such patients interested in pursuing a possible second HCT or autologous gene therapy after previous chemotherapy exposure must undergo a thorough organ function evaluation to assess for any dysfunction that may be exacerbated by subsequent therapy. If such dysfunction is detected, especially as it relates to hepatic or pulmonary compromise, HCT with an allogeneic donor and a busulfan-free, reduced-intensity or nonmyeloablative conditioning regimen might be preferable to autologous gene therapy.
Clonal hematopoiesis and donor chimerism
In addition to the usual organ function evaluations and infectious disease screening of all candidates for potentially curative therapies, individuals with a previous history of unsuccessful allogeneic HCT require additional evaluations to assess their risk of clonal hematopoiesis and secondary hematologic malignancies. These include an assessment of the bone marrow for cellularity and dysplasia, karyotyping and molecular diagnostics to screen for cytogenetic aberrations, and next-generation sequencing assays to look for clonal hematopoiesis or pathogenic mutations in the genes associated with myeloid malignancies. Although the detection of abnormalities in the aforementioned evaluations does not conclusively portend a worse prognosis for an individual if they pursue a submyeloablative allogeneic HCT or autologous gene therapy, such abnormalities may put these individuals at risk and warrant further evaluation.22 However, there are currently no established thresholds for detection depth or variant allele frequency for clonal hematopoiesis screening and further research is required to define meaningful parameters for clinical risk assessment.
Donor engraftment should be assessed via multilineage chimerism analyses in the bone marrow and/or peripheral blood in recipients of previous allogeneic HCT. The presence of persistent mixed recipient-donor chimerism, even in only a few cell lineages, indicates a degree of established immune tolerance between the donor and the recipient and requires attention when considering a second/subsequent HSC donor, preparative regimen, and graft-versus-host disease prophylaxis. Although immunologic complications such as graft-versus-host disease and graft rejection are typically not of concern with autologous HSC–based gene therapies, the selection of donor-derived HSCPs (in an individual with mixed donor host chimerism after prior allogeneic HCT) for gene modification may theoretically confer new risk upon expansion of genetically modified donor-derived cell lineages after HSC-based gene therapies. These laboratory findings may spark a discussion about the potential risks and benefits of pursuing various potentially curative therapies based on our current understanding of such abnormalities (which may change with time).
Feasibility and safety of gene therapy after graft failure
Low residual hematopoietic reserve
It is unclear whether individuals who have experienced graft failure after a previous allogeneic HCT have sufficient hematopoietic reserve to enable enough HSCs to be collected for manufacturing a genetically modified product. Collecting adequate numbers of healthy HSCs is generally the rate-limiting step in manufacturing a genetically modified cellular product.23 Not only are a large number of CD34+ cells required at the outset because of the significant losses expected during the manufacturing process,24 but collecting high-quality, long-term repopulating HSCs is essential for long-term engraftment and is particularly challenging in individuals with SCD or TDT.25,26 With current collection practices, individuals with hemoglobinopathies (who have not received previous chemotherapy or HCT) often require multiple mobilization and apheresis collection cycles to achieve an adequate CD34+ cell quantity for manufacturing of a minimum goal cell dose.4,27-29 Proximity to previous chemotherapy exposure and/or previous HCT may contribute further to the state of the HSC pool and hence HSC mobilization and subsequent gene therapy manufacturing.
Granulocyte colony–stimulating factor, the cytokine most commonly used to mobilize HSCs from the bone marrow to the peripheral circulation, is contraindicated in individuals with SCD because of the risk of inducing a life-threatening vaso-occlusive crisis.30 Therefore, plerixafor, a CXCR4 antagonist with no such associated risk, is the only HSC mobilizing agent currently available for individuals with SCD.31 However, plerixafor is not a potent mobilization agent when used alone, and the yield after apheresis is often insufficient, given the relatively high collection requirement for gene therapy product manufacture.25 The mobilization potential is better for individuals with TDT because both granulocyte colony–stimulating factor and plerixafor can be used. Nevertheless, a combination of chronic hemolysis, defective or ineffective erythropoiesis, chronic oxidative stress, and iron overload in SCD and TDT not only affects the bone marrow milieu and resident HSCs but contributes to technical difficulties with the apheresis process due to separation interference from excess leukocytosis, marked reticulocytosis, and hemolytic debris.32,33 Because previous chemotherapy and radiation exposure are added risk factors for subsequent poor mobilization,34-36 individuals who have previously received an HCT may not mobilize well owing to these previous exposures. A future consideration may be the application of newer and potentially more potent mobilization agents such as the CXCR4 receptor inhibitor motixafortide, which remains in clinical trial evaluation for SCD and for HSC-based gene therapy.37,38 However, the relatively high CD34+ cell collection requirements, paired with the lack of efficient mobilization agents, technical barriers with apheresis, and decreased reserve as a consequence of chemotherapy/radiation exposure associated with a previous allogeneic HCT may currently make such an individual a poor candidate for gene therapy because of the potential failure of the HSC collection step.
Accelerated aging of HSCs after chemotherapy and radiation exposure during HCT
Individuals with SCD experience chronic hematologic stress due to persistent sterile inflammation secondary to chronic microvascular ischemia, both systemically and in the bone marrow niche.39-43 These insults, along with the high erythropoietic demand because of the rapid turnover of the sickled red blood cells, contribute to accelerated aging of the HSCs.44,45 Indeed, in 1 study, the incidence of clonal hematopoiesis, which may be a marker of HSC senescence and chronic damage, was noted to be higher in individuals with SCD than in the general population.46 Large population-based studies have indicated that, in addition to having a probably higher than usual incidence of clonal hematopoiesis, individuals with SCD have a risk of hematologic malignancies that is 2- to 11-fold higher than in the general population, suggesting that the accumulating HSC damage may predispose some individuals to myeloid neoplasms.47,48
In contrast to SCD, TDT is characterized by ineffective erythropoiesis with erythroid hyperplasia and significant iron overload. In conjunction with transfusion-related iron, chronic hypoxia and ineffective erythropoiesis further drive iron loading and toxic oxidative stress in HSCs.49 Epidemiologic data regarding TDT and cancers are scarce, although TDT has been associated with increased risk of solid cancers, namely hepatocellular carcinomas, because of iron-induced oxidative toxicity and viral hepatitis. Some population-based studies have found a higher incidence of hematologic malignancies in patients with TDT than in the general population; this is attributable, at least in part, to marrow oxidative stress,50-52 which has been hypothesized to contribute to accelerated erythroid differentiation, eryptosis, and senescence.
Notably, previous chemotherapy and radiation exposure accelerate HSC senescence and decrease long-term reconstitution capacity in animal models.53,54 Therefore, it is plausible that after a previous HCT, the remaining host HSCs, which are already ageing expeditiously, especially in the setting of SCD or TDT, may be accelerated even further in ageing, thus making them intolerant and unsuitable for use in manufacturing a gene therapy product.
Accumulation of deleterious mutations and clonal expansion during regenerative phases
The subtle damage to HSCs in SCD and TDT may become more apparent during regenerative phases when a few HSCs expand to reconstitute the entire hematopoietic system, such as might occur after a graft failure.55 Indeed, analysis of cryopreserved pretransplant bone marrow samples from patients with SCD who underwent an allogeneic HCT and subsequently developed a myeloid malignancy after experiencing graft failure found TP53 mutations at very low variant allele frequencies.56 These mutant clones expanded rapidly and preferentially during the regenerative hematopoiesis after graft failure when the few remaining HSCs that escaped chemoablation expanded to reconstitute the hematopoietic system.55 Another study of gene therapy recipients for SCD found mutations in DNMT3A and EZH2 to be present in genetically modified cells after infusion at a much higher frequency than at baseline, suggesting there was positive selection for these mutant clones after the infusion of the genetically modified cells and their subsequent expansion to reconstitute the hematopoietic system.57 In yet another report, somatic mutations in RUNX1 and PTPN11, and monosomy 7 were noted in 2 patients who developed leukemia after gene addition in the HGB-206 clinical trial of gene therapy for SCD.58,59 It is unclear whether these mutations were present at baseline. The incidence of hematologic malignancies is increased in patients with TDT, with some overlapping contributory risk being hypothesized, as with SCD.60 The role of clonal hematopoiesis before and after gene therapy for TDT, although not yet well described, should not be discounted.
Overall, these findings prompt caution regarding the risk of preexisting clones with deleterious mutations, which may be present in the host HSCs before allogeneic HCT or be acquired by host HSCs after chemoirradiation. Such clones may expand in the autologous recovery phase after graft failure and ultimately affect the quality of the HSCs collected via apheresis for manufacturing a genetically modified product (Figure 2). If present in the apheresis product and in the subsequent gene therapy product, these HSCs with acquired and/or accumulated mutations in critical genes may preferentially expand clonally during the regenerative phase after infusion of the gene therapy product, predisposing recipients to malignant transformation. Finally, accumulated deleterious mutations in HSCs may affect their ability to tolerate genetic modification by making them susceptible to deleterious preferential gene insertion at oncogenic sites or to off-target editing.61,62
Clonal expansion and evolution after graft failure and autologous gene therapy. HSCs can acquire deleterious mutations during the normal course of life of an individual or as a result of exposure to chemotherapy and radiation during conditioning for an allogeneic HCT. HSCs that have acquired deleterious mutations may preferentially expand during the phase of regenerative hematopoiesis after graft failure with an allogeneic HCT. These clonally expanded HSCs, along with normal stem cells, may be collected during apheresis, be genetically modified, and then be reinfused into the patient to reconstitute the entire hematopoietic system after gene therapy. During this second cycle of regenerative hematopoiesis, HSCs with a mutation that confers a relative growth advantage may continue to expand clonally and predispose the individual to developing a hematologic malignancy.
Clonal expansion and evolution after graft failure and autologous gene therapy. HSCs can acquire deleterious mutations during the normal course of life of an individual or as a result of exposure to chemotherapy and radiation during conditioning for an allogeneic HCT. HSCs that have acquired deleterious mutations may preferentially expand during the phase of regenerative hematopoiesis after graft failure with an allogeneic HCT. These clonally expanded HSCs, along with normal stem cells, may be collected during apheresis, be genetically modified, and then be reinfused into the patient to reconstitute the entire hematopoietic system after gene therapy. During this second cycle of regenerative hematopoiesis, HSCs with a mutation that confers a relative growth advantage may continue to expand clonally and predispose the individual to developing a hematologic malignancy.
Shared decision-making
Because demonstration of feasibility or safety of HSC-based gene therapy after failed allogeneic HCT has not been achieved, discussions surrounding such decision-making may benefit from involvement of subject matter expert colleagues in the field. Although no specific contraindications with regard to autologous gene therapies for SCD and TDT after previous allogeneic HCT are included in the US Food and Drug Administration prescriber information; commercial entities, insurance carriers and payors, and clinical practice groups may choose to limit the use of autologous genetically modified HSC-based products after receipt of a previous cell-based therapy until more information is known given the ultrahigh cost, uncertain feasibility, and unknown but potentially significant risks associated with such an endeavor that we have detailed earlier.
In many cases, the evaluations listed earlier may not provide a clear reason to pursue one potentially curative therapy over another, and the decision may ultimately come down to personal preference, and shared decision-making between an informed patient and their trusted medical provider after considering all the factors listed above. Ultimately, the decision whether to pursue an autologous gene therapy after previous unsuccessful allogeneic HCT may need to be made on a case-by-case basis. Irrespective of the patient’s eventual choice, they should be followed up closely for an extended period (several years) after receiving either of these therapies to ensure that there are no untoward outcomes.
Acknowledgments
The authors thank Keith A. Laycock, a senior scientific editor employed by St. Jude Children’s Research Hospital, for scientific editing of the manuscript. The figures were created with BioRender.com. Sharma A. (2025) https://BioRender.com/sdoomj3.
A.S. acknowledges support from the American Lebanese Syrian Associated Charities for his work at St. Jude Children’s Research Hospital, as well as grant support (1U01HL163983) from the National Institutes of Health/National Heart, Lung, and Blood Institute.
The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Authorship
Contribution: A.S. and T.J. participated in the development of the concept of this article and discussed the content outline, and are responsible for all aspects of the article, from conception to completion; A.S. wrote the first draft of the manuscript; and T.J. revised the manuscript with several rounds of modifications.
Conflict-of-interest disclosure: A.S. received consultant fees from Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, Editas Medicine, BioLineRx, and Pfizer; is a medical monitor for an RCI BMT CSIDE clinical trial for which he receives financial compensation; received research funding from CRISPR Therapeutics; received honoraria from Vindico Medical Education and Blackwood CME; is the St. Jude Children’s Research Hospital site principal investigator for clinical trials of genome editing for sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (ClinicalTrials.gov identifier: NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880), and the industry sponsors provide funding for the clinical trial, which includes salary support paid to the institution of A.S.; and has no direct financial interest in these therapies and the conflicts are managed through the Compliance Office at St. Jude Children’s Research Hospital, in accordance with their conflict-of-interest policy. T.J. has participated as an advisory board member and has received consulting fees from bluebird bio, Vertex Pharmaceuticals, and BioLineRx; is a medical monitor for the BMT CTN 2001 GRASP and BMT CTN CRISPR_SCD001 studies, for which she receives compensation; and is the Stanford site principal investigator for clinical trials of genome editing sponsored by Beam Therapeutics (ClinicalTrials.gov identifier: NCT05456880), and has no direct financial interest in this therapy.
Correspondence: Akshay Sharma, Department of Bone Marrow Transplantation and Cellular Therapy, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS1130, Memphis, TN 38105; email: akshay.sharma@stjude.org.