In this issue of Blood Advances, Sendker et al1 report on a retrospective cohort of pediatric patients with therapy-related acute myeloid leukemia (tAML), suggesting that treatment outcomes have improved dramatically over the last 26 years. Sendker et al examined the characteristics, treatment plans, and survival rates of 119 patients with tAML reported in the AML Berlin-Frankfurt-Munich (AML-BFM) registry between 1993 and 2019. The article outlines the clinical trials that were conducted during this time frame. In addition to therapy and outcome, the registry collected data on karyotype and, for a subset of patients, mutation status via next-generation sequencing for 54 AML-specific genes. Although the number of patients was relatively small, as is typical for rare populations, this report, to our knowledge, represents one of the largest collections of data on pediatric tAML published to date.
tAML in children is rare, comprising only ∼1% of all childhood malignancies.2-4 One of the most devastating late secondary complications of therapy, tAML, has historically reported an overall survival (OS) of ∼10%.5 Driven primarily by DNA damage after exposure to alkylating agents, anthracyclines, and topoisomerase inhibitors, the latency to tAML after exposure to these toxic agents is typically 1 to 8 years. In pediatric patients, tAML is most commonly observed after treatment for the most frequent pediatric malignancies, including leukemias, lymphomas, and central nervous system tumors. However, there is an increased risk of tAML in pediatric solid tumors that receive high doses of DNA-damaging agents, such as in children treated for sarcomas.4 These children are heavily pretreated, often with significant organ dysfunction, which can lead to challenges in administering the intensive induction regimens required to achieve remission.
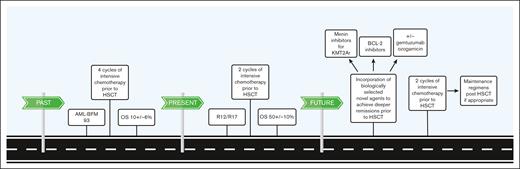
This large, multi-institution data set reported by Sendker et al describes several temporally stratified treatment approaches for individual patients in a longitudinal manner. The results reported in the article highlight improvements in OS over a 26-year period for children included in the AML-BFM registration trials. The OS of patients with tAML in the AML-BFM 93 registry (1993-1998) was 10% ± 6%, whereas children with tAML participating in the 2 registration trials AML-BFM 2012 and AML-BFM 2017 (2012-2019) had an OS of 50% ± 10%. This dramatic increase was attributed primarily to a change in treatment approach in which pediatric patients with tAML moved on to hematopoietic stem cell transplant (HSCT) after 2 induction chemotherapy cycles rather than 4. An additional critical take-home point from the data is the lack of differences in event-free survival (EFS) and OS between patients undergoing HSCT in complete remission (CR) and CR with incomplete count recovery (CRi). This suggests it may not be necessary to await full count recovery before proceeding to HSCT for pediatric patients with tAML.
Importantly, EFS and OS in children with tAML were nearly identical, suggesting that despite improvements in EFS, recurrence after the initial treatment is virtually unsalvageable. HSCT in patients with morphologic CR or CRi is therefore the best available therapy for pediatric patients with tAML. Lastly, the authors found that although de novo AML risk stratification criteria did not predict outcomes for patients with tAML in this study, there were some unique predictors of inferior outcomes. The 3 key indicators of poor prognosis in this cohort were latency before the diagnosis of tAML of ≤1 year, prior ionizing radiation (>35 Gy), and adverse cytogenetics.
One limitation of this study is the lack of minimal residual disease (MRD) data for a large percentage of the patients included. This prevents the authors from drawing meaningful conclusions regarding the prognostic impact of MRD at the end of the first or second induction before HSCT. However, data showing dramatic improvements in survival for children and young adults with tAML undergoing HSCT after the second induction with no evidence of leukemia by morphology advance the field significantly. Although it is currently standard for clinicians to achieve remission and get patients with tAML for HSCT after intensive chemotherapy, this article provides crucial data to help guide families, support clinical decision-making, and guide future research efforts to continue to improve outcomes for pediatric patients with tAML.
Patients with tAML are often excluded from cooperative group trials in the United States, which has hindered the collection of comprehensive pediatric data for this rare population. As enrollment in clinical trials is becoming more biologically informed, it no longer makes logical sense to exclude patients with tAML from clinical trials of agents that may further improve outcomes. Mutation and outcome data presented in Sendker et al support the practice of including these patients in biologically appropriate relapsed and refractory AML (R/R AML) trials, which may provide access to drugs with the potential to improve outcomes for these vulnerable patients.6
Important next steps are to prospectively acquire data on the response and outcome through the inclusion of children with tAML in clinical trials in the era of more targeted therapies and better supportive care (see figure). Venetoclax, menin inhibitors, and gemtuzumab oozagamicin (GO; Mylotarg) have the potential to improve outcomes for tAML.7-9 Venetoclax, a B-cell lymphoma 2 inhibitor, is commonly used in adults in combination with a hypomethylating agent for de novo AML, myelodysplastic syndrome, and tAML. Combinations of venetoclax with low-dose and intensive cytotoxic chemotherapy in adults with R/R AML or tAML are also being actively investigated. Further study of venetoclax, likely in combination with intensive chemotherapy, in children with tAML is warranted.7 Menin inhibitors are particularly promising for more than half of patients with tAML whose disease is driven by lysine methyltransferase 2A (KMT2A) rearrangements.8 Potential use of the CD33 targeted antibody drug conjugate GO is attractive given its efficacy in children with KMT2A rearranged AML.9 Study designs that incorporate GO for tAML should consider its proximity to consolidative HSCT given the potential for increased risk of sinusoidal obstruction syndrome, a potential life-threatening complication.10 All told, we are in an exciting era for therapeutic advances for pediatric tAML, and the data from Sendker et al help to bring improved outcomes to patients across the world.
Changes in treatment regimens have led to significant improvements in OS for children with tAML, and new opportunities on the horizon for additional forward movement. BCL-2, B-cell lymphoma 2; KMT2Ar, KMT2A rearrangement; R12/R17, registries 2012 and 2017.
Changes in treatment regimens have led to significant improvements in OS for children with tAML, and new opportunities on the horizon for additional forward movement. BCL-2, B-cell lymphoma 2; KMT2Ar, KMT2A rearrangement; R12/R17, registries 2012 and 2017.
Conflict-of-interest disclosure: A.M.S. reports research funding from Gilead Sciences Inc and AbbVie Inc. T.M.C. declares no competing financial interests.