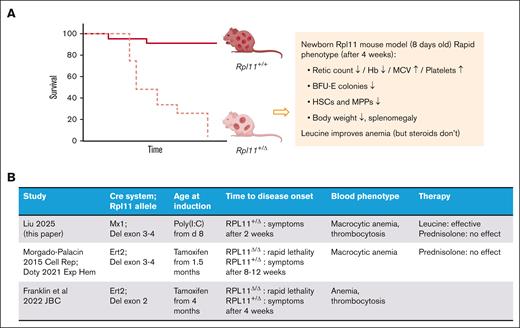
In this issue of Blood Advances, Liu et al present a new mouse model of Diamond-Blackfan anemia (DBA) syndrome (see figure panel A).1 They use a poly I:C-inducible Mx1-Cre system to generate heterozygous Rpl11 knockout (KO) mice, employing a previously established mouse model where loxP sites flank exons 3 and 4 of the Rpl11 gene.2 DBA syndrome is an inherited bone marrow failure disorder caused in the majority of cases by heterozygous loss-of-function mutations in ribosomal protein (RP) genes.3 Approximately 60% of patients with DBA have mutations in 1 of the 4 RP genes, RPS19, RPL5, RPL11, and RPS26. DBA classically presents with isolated erythroid failure in infancy, whereas some older patients can develop bone marrow hypocellularity, multilineage cytopenias, and immunodeficiency.
Rpl11 mouse models to recapitulate DBA syndrome. (A) overall survival and phenotype of Mx-Cre inducible Rpl11 haploinsufficient mouse model. (B) Summary of studies employing Rpl11 mouse models.
Rpl11 mouse models to recapitulate DBA syndrome. (A) overall survival and phenotype of Mx-Cre inducible Rpl11 haploinsufficient mouse model. (B) Summary of studies employing Rpl11 mouse models.
The enigma of how RP mutations selectively impair erythropoiesis has captivated hematologists for decades. Experimental models that accurately recapitulate DBA in vivo have been challenging to develop, limiting our understanding of disease mechanisms and evaluation of novel therapeutics. Constitutional heterozygous disruption of Rps19 failed to produce a phenotype in 1 of the first animal models of DBA.4 Subsequently, an inducible short hairpin RNA–based knockdown of Rps19 was able to induce macrocytic anemia and leukocytopenia.5 Two other groups developed conditional KO of Rpl11 using tamoxifen-inducible (Ert2) Cre recombinase2,6 (see figure panel B). Serrano et al2 showed that treatment with tamoxifen treatment at 6 weeks of life in mice with Rpl11+/lox resulted in mild anemia, impaired erythroid maturation, normal survival, restrained p53 activity, and increased lymphomagenesis following irradiation. These mice maintained ∼60% of wildtype Rpl11 messenger RNA levels. More recently, Franklin et al6 used a similar Ert2 Cre system to induce Rpl11 haploinsufficiency in 4-month-old mice resulting in severe anemia, increased platelet count, normal white cell count, splenomegaly, increased p53 activity, and modest reduction in survival. The phenotypic discrepancies between the different models, and between human DBA and existing animal models have stimulated continued interest in developing more accurate models.7
Liu et al developed a new conditional KO model of DBA using Mx1-Cre, which restricts the Rpl11 gene KO to mainly hematopoietic tissue, producing a model with severe anemia that is associated with inflammation (where administration of polyI:C induces an interferon response, activating the Mx1 promoter). Similar to the model created by Serrano et al,2 the authors use loxP sites flanking exons 3 and 4 of Rpl11 gene. Unlike the previous approaches where KO was induced in adult mice, the authors induced Mx1-Cre at postnatal day 8 to more accurately recapitulate human DBA, which typically presents in infancy. This timing of induction resulted in severe macrocytic anemia, reticulocytopenia, impaired erythroid maturation, elevated erythropoietin, increased erythrocyte adenosine deaminase, and reduced survival. The authors highlight the utility of this model by testing L-leucine supplementation, which improves anemia and survival in this Rpl11 mouse model. Interestingly, corticosteroids, a widely used and effective medication in patients with DBA syndrome, did not ameliorate the anemia phenotype, which aligns with previous report in another Rpl11-haploinsufficient model.8 Immunophenotypic analysis demonstrated reduced hematopoietic stem cells (HSC) and multipotent progenitor cells in these mice. Although further studies are warranted, these findings are consistent with impaired HSC maintenance seen in some older patients with DBA syndrome and in our CRISPR/Cas9–based model of DBA.9,10
This latest addition to the repertoire of animal models of DBA has the potential to address several unanswered questions. Does Rpl11 haploinsufficiency lead to reduced Gata1 translation? What is the role of inflammation in DBA? Is there an increased p53 activation in Rpl11-haploinsufficient mice? Recent studies using patient bone marrow cells suggest differences in phenotype between RPS19 DBA and those due to RPL5 and RPL11 mutations.11 Careful dissection of mechanisms underlying hematopoietic defects in new animal models could inform these phenotypic differences. Multiple previous studies suggest that RPL5 and RPL11, along with 5S ribosomal RNA, form a complex called 5S ribonucleoprotein, which can sense RP imbalance and lead to p53 activation by sequestering MDM2, a p53 ubiquitin ligase.12,13 This suggests DBA due to RPL5 and RPL11 mutations likely involves mechanisms that are different than those due to mutations in other RP genes. This Rpl11-haploinsufficient mouse model offers an excellent opportunity to address this question. Furthermore, as demonstrated by the authors using L-leucine, this model can be used for preclinical evaluation of pharmacotherapies and newer therapeutic strategies including gene therapy and antibody-based conditioning for HSC transplant.
Conflict-of-interest disclosure: The authors declare no competing financial interests.