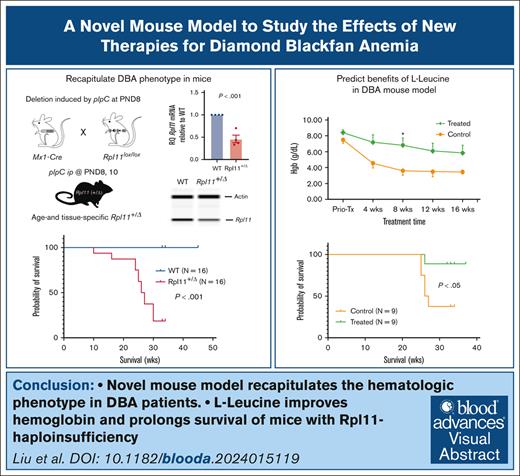
Visual Abstract
TO THE EDITOR:
Diamond-Blackfan anemia (DBA) is an inherited bone marrow (BM) failure characterized by macrocytic anemia, congenital malformations, and an increased predisposition to cancer.1,2 More than 90% of patients with DBA are diagnosed within the first year of life (median age, 12 weeks).3 The pathogenesis of DBA is linked to loss-of-function mutations in genes that encode ribosomal proteins (RPs), although mutations in 3 non-RP genes (GATA1, TSR2, and HEATR3) have been reported.1,4-6 Mutations in RPS19, RPL5, RPS26, RPL11, RPL35a, RPS10, RPS24, and RPS17 are responsible for ∼70% of the patients with DBA. Varying degrees of anemia are observed in 90% of patients with DBA at diagnosis and are the primary reason for treatment, which includes corticosteroids or red blood cell (RBC) transfusions. However, lifelong treatment results in morbidities, such as osteoporosis, diabetes, and iron overload.1,2,7 Currently, hematopoietic stem cell transplantation is the only curative therapy.3,8 The challenge in conducting preclinical studies for new therapies to treat DBA is the lack of mouse models that recapitulate its clinical features, including haploinsufficiency of ribosomal genes, severe anemia, and age of onset. Heterozygous mutations in RPL11 are found in 5% to 7% of patients with DBA.1,2 Previously published mouse models with Rpl11 haploinsufficiency have mild anemia and a normal life span,9,10 which have limitations for therapeutics development.
Anemia in patients with DBA presents with decreased RBC counts, hemoglobin (Hgb) concentrations, increased mean corpuscular values (MCV), and elevated erythrocyte adenosine deaminase activity.2,11 To develop a novel mouse model with Rpl11 haploinsufficiency and anemia, we generated mice carrying a single copy of mutant Rpl11 (Rpl11+/floxP) and Mx1-Cre+. The pups were born with normal body weight, regardless of genotype. Rpl11 deletion was induced as reported by intraperitoneal injection of 30 μL of 1 μg/μL polyinosinic-polycytidylic acid (pIpC) on postnatal days 8 and 10.12 Two weeks after pIpC injection, mice with Rpl11-haploinsufficiency (Rpl11+/▵) developed pale ears and macrocytic anemia. Blood genotyping confirmed that the allele with the Rpl11 deletion was detected only in mice carrying Rpl11+/floxP and Mx1-Cre+ (supplemental Methods; supplemental Figure 1A-C). Quantification of messenger RNA in blood nucleated cells from Rpl11+/▵ mice showed that Rpl11 expression was ∼50% of wild-type (WT) littermates (Rpl11+/floxPMx1-Cre–). Western blot analysis confirmed that Rpl11 protein levels were significantly decreased in BM cells from Rpl11+/▵ mice compared with their WT littermates (Figure 1A-C).
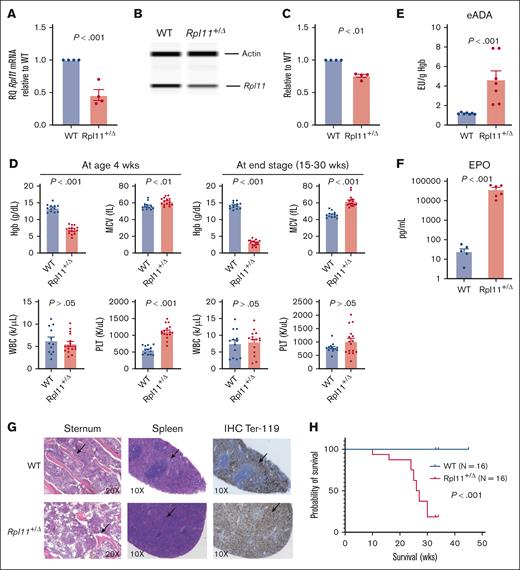
Rpl11-haploinsufficient mice recapitulate the hematologic phenotypes of patients with DBA. (A) Relative quantitative polymerase chain reaction showing Rpl11 messenger RNA levels in nucleated cells of the peripheral blood from Rpl11+/▵ mice and WT littermates. N = 4. (B-C) Representative western blot data show Rpl11 protein level in BM cells. N = 4. The area under the peak for Rpl11 and β-actin was calculated for the expression of Rpl11 protein relative to β-actin in BM-nucleated cells from Rpl11+/▵ mice and WT littermates. (D) Complete blood counts were performed using a HEMAVET 950FS analyzer. WT N = 13, Rpl11+/▵ N = 16. (E) Quantification of eADA in blood. The concentrations were normalized to blood Hgb levels. N = 7 per group. (F) Quantification of EPO concentrations in the plasma. N = 6 per group. (G) Representative morphology of the sternum and spleen. Hematoxylin and eosin–stained sections show that the sternum of Rpl11+/▵ mice were relatively hypocellular with substantially less trabecular bones compared with WT littermates, and their spleens lost the normal architecture without normal white pulp. Immunohistochemistry-stained sections show the abnormal distribution of Ter119+ cells in the spleens of diseased mice with Rpl11+/▵ (H) Kaplan-Meier analysis shows high penetrance (100%) of disease and a lethal impact of Rpl11- haploinsufficiency on survival of diseased mice with Rpl11+/▵ induced on postnatal day 8. The median survival age was 26.5 weeks in Rpl11+/▵ mice. N = 16 per group. Statistical differences between the groups were calculated using a 2-tailed Student t test. Data are presented as the mean ± standard error of the mean. More supportive data are provided in supplemental Figure 1. eADA, erythrocyte adenosine deaminase; MCV, mean corpuscular value; PLT, platelets; WBC, white blood cell counts; wks, weeks.
Rpl11-haploinsufficient mice recapitulate the hematologic phenotypes of patients with DBA. (A) Relative quantitative polymerase chain reaction showing Rpl11 messenger RNA levels in nucleated cells of the peripheral blood from Rpl11+/▵ mice and WT littermates. N = 4. (B-C) Representative western blot data show Rpl11 protein level in BM cells. N = 4. The area under the peak for Rpl11 and β-actin was calculated for the expression of Rpl11 protein relative to β-actin in BM-nucleated cells from Rpl11+/▵ mice and WT littermates. (D) Complete blood counts were performed using a HEMAVET 950FS analyzer. WT N = 13, Rpl11+/▵ N = 16. (E) Quantification of eADA in blood. The concentrations were normalized to blood Hgb levels. N = 7 per group. (F) Quantification of EPO concentrations in the plasma. N = 6 per group. (G) Representative morphology of the sternum and spleen. Hematoxylin and eosin–stained sections show that the sternum of Rpl11+/▵ mice were relatively hypocellular with substantially less trabecular bones compared with WT littermates, and their spleens lost the normal architecture without normal white pulp. Immunohistochemistry-stained sections show the abnormal distribution of Ter119+ cells in the spleens of diseased mice with Rpl11+/▵ (H) Kaplan-Meier analysis shows high penetrance (100%) of disease and a lethal impact of Rpl11- haploinsufficiency on survival of diseased mice with Rpl11+/▵ induced on postnatal day 8. The median survival age was 26.5 weeks in Rpl11+/▵ mice. N = 16 per group. Statistical differences between the groups were calculated using a 2-tailed Student t test. Data are presented as the mean ± standard error of the mean. More supportive data are provided in supplemental Figure 1. eADA, erythrocyte adenosine deaminase; MCV, mean corpuscular value; PLT, platelets; WBC, white blood cell counts; wks, weeks.
Two weeks after pIpC injection, Rpl11+/▵ mice, regardless of gender, developed moderate macrocytic anemia with a mean Hgb concentration of 6.9 g/dL and mean corpuscular value of 61 fL, whereas white blood cell counts were comparable to those of WT littermates (Figure 1D; supplemental Figure 1D). Hgb progressively decreased in Rpl11+/▵ mice, resulting in severe anemia by the age of 15 to 35 weeks (hereafter termed as the end stage). The platelet counts initially increased in Rpl11+/▵ mice but returned to normal at the end stages. Similar to patients with DBA,13,14 peripheral blood reticulocyte counts were significantly decreased in Rpl11+/▵ mice (mean 0.3 vs 0.4 M/mL; P < .001) (supplemental Figure 1E). Blood erythrocyte adenosine deaminase concentrations were also significantly elevated in Rpl11+/▵ mice compared with their WT littermates (mean 5 vs 1 EU/g Hgb; P < .001), and plasma erythropoietin (EPO) concentrations were significantly increased (mean 38 350 vs 25 pg/mL; P < .01) (Figure 1E-F). The body weights of Rpl11+/▵ mice were significantly lower than those of their WT littermates at the time of evaluation (supplemental Figure 1F). Necropsy showed that Rpl11+/▵ mice had significant splenomegaly, whereas the liver size was normal (supplemental Figure 1G-H). Histology of the BM revealed relative hypocellularity with less trabecular bones in the sternum of Rpl11+/▵ mice than in WT littermates, and the spleens from Rpl11+/▵ mice lost the normal architecture with substantially increased Ter119+ erythrocytes (Figure 1G). Without intervention, mice with Rpl11+/▵ started to die at 15 weeks of age, with a median survival of 26.5 weeks (equivalent to adult age in humans, Figure 1H). Therefore, compared with previously reported mouse models with Rpl11 mutations and other mutations,9,10,15 this model recapitulates the haploinsufficiency of RPs and the hematologic phenotype of patients with DBA (supplemental Table 1).
To investigate whether anemia in Rpl11+/▵ mice results from aberrant erythropoiesis in the BM, we conducted colony formation assays. BM cells from Rpl11+/▵ mice derived significantly fewer numbers of colonies for burst-forming unit-erythroid (BFU-E) in the presence of 1 or 10 units/mL of EPO (Figure 2A). We further evaluated the immunophenotype of BM cells using flow cytometry. Lineage-negative cells were significantly increased in the BM of Rpl11+/▵ mice, whereas hematopoietic stem cells and multipotent progenitors were significantly reduced compared with their WT littermates (Figure 2B; supplemental Figure 2). We also analyzed the BM cell subpopulations from erythroid progenitors (EPs) to reticulocytes using the I to V system published by Doty et al.16 Populations I to V represent sequential maturation during erythropoiesis. Cells in the I population correspond to EPs that functionally define the BFU-E and colony-forming unit erythroid colonies.16-19 Populations I and II were significantly increased, whereas populations IV and V were decreased in the BM of the Rpl11+/▵ mice. Our data demonstrated that cells in the early stages of erythroid differentiation (EPs and erythroblasts in populations of I-II) were significantly increased in the BM of Rpl11+/▵ mice. In contrast, the more mature erythroid precursors in populations IV and V were significantly decreased (Figure 2C-D), suggesting that the early EPs are preserved, and the maturation defect occurs in the late precursor Ter119+ cells during differentiation. This is consistent with the data showing that erythroid maturation is preserved in EPs and early precursors in BM cells from patients with DBA-carrying mutations in RPL genes.20 The loss of hematopoietic stem cells and multipotent progenitors in BM of Rpl11+/▵ mice is likely due to cell exhaustion, driven by compensatory efforts to increase RBC production. Overall, our data suggest that defective differentiation of erythropoiesis in the BM contributes to lethal macrocytic anemia in Rpl11-haploinsufficient mice.
Abnormal erythropoiesis in Rpl11+/▵ mice and L-leucine treatment. (A) Burst-forming unit-erythroid (BFU-E) colony counts. BM cells (2 ×105) in 1 mL of MethoCult M3234 medium supplemented with 1 or 10 units/mL recombinant mouse EPO. Number of BFU-E colonies (containing >10 cells) was counted on days 8 to 10 using STEMvision (STEMCELL Technologies). N = 5 per group. (B) Frequency analysis of hematopoietic stem cells/progenitor cells by flow cytometry. Fresh single BM cells were collected from the femurs and tibias. LIN−, lineage negative (CD4–CD8–B220-Gr1-CD11b–Ter119–); LSK, LIN– Scal-1+ c-Kit+; hematopoietic stem cells, LIN-Scal1+cKit+CD48–CD150+, multipotent progenitors, LIN-Scal1+cKit+CD48–CD150–. N = 5 per group. (C) Representative flow cytometry analysis of erythroid differentiation trajectory in BM cells from Rpl11+/▵ mice and WT littermates. LIN− population (CD4–CD8–B220-Gr1-CD11b–) was analyzed and gated as previously reported16 to identify the LNPCs and erythroblast populations I to V, which include BFU-E deriving cells and proerythroblasts (I), basophilic erythroblasts (II), polychromatic erythroblasts (III), orthochromatic erythroblasts (IV), and reticulocytes and mature RBCs (V). The data revealed a differentiation block of BFU-E-derived cells/proerythroblasts (I-II) before polychromatic erythroblasts (III). (D) Frequency analysis of flow cytometry data for the populations of erythroid differentiation trajectory in the BM from Rpl11+/▵ mice and WT littermates. N = 7 per group. (E) Mice treated with 1.5% l-leucine in drinking water at the age of 4 to 6 weeks for 16 weeks. Blood Hgb concentration was monitored every 4 weeks. (F) Kaplan-Meier analysis shows that L-leucine treatment significantly prolonged the survival of the treated group (P < .05). Statistical differences between groups were calculated using a 2-tailed Student t test. Data are presented as mean ± standard error of the mean, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. More supportive data are provided in supplemental Figures 2 and 3. FSC, forward scatter; LNPC, LIN− precursor cells; wks, weeks.
Abnormal erythropoiesis in Rpl11+/▵ mice and L-leucine treatment. (A) Burst-forming unit-erythroid (BFU-E) colony counts. BM cells (2 ×105) in 1 mL of MethoCult M3234 medium supplemented with 1 or 10 units/mL recombinant mouse EPO. Number of BFU-E colonies (containing >10 cells) was counted on days 8 to 10 using STEMvision (STEMCELL Technologies). N = 5 per group. (B) Frequency analysis of hematopoietic stem cells/progenitor cells by flow cytometry. Fresh single BM cells were collected from the femurs and tibias. LIN−, lineage negative (CD4–CD8–B220-Gr1-CD11b–Ter119–); LSK, LIN– Scal-1+ c-Kit+; hematopoietic stem cells, LIN-Scal1+cKit+CD48–CD150+, multipotent progenitors, LIN-Scal1+cKit+CD48–CD150–. N = 5 per group. (C) Representative flow cytometry analysis of erythroid differentiation trajectory in BM cells from Rpl11+/▵ mice and WT littermates. LIN− population (CD4–CD8–B220-Gr1-CD11b–) was analyzed and gated as previously reported16 to identify the LNPCs and erythroblast populations I to V, which include BFU-E deriving cells and proerythroblasts (I), basophilic erythroblasts (II), polychromatic erythroblasts (III), orthochromatic erythroblasts (IV), and reticulocytes and mature RBCs (V). The data revealed a differentiation block of BFU-E-derived cells/proerythroblasts (I-II) before polychromatic erythroblasts (III). (D) Frequency analysis of flow cytometry data for the populations of erythroid differentiation trajectory in the BM from Rpl11+/▵ mice and WT littermates. N = 7 per group. (E) Mice treated with 1.5% l-leucine in drinking water at the age of 4 to 6 weeks for 16 weeks. Blood Hgb concentration was monitored every 4 weeks. (F) Kaplan-Meier analysis shows that L-leucine treatment significantly prolonged the survival of the treated group (P < .05). Statistical differences between groups were calculated using a 2-tailed Student t test. Data are presented as mean ± standard error of the mean, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. More supportive data are provided in supplemental Figures 2 and 3. FSC, forward scatter; LNPC, LIN− precursor cells; wks, weeks.
L-leucine has been reported to improve anemia and growth in some patients with DBA, as well as in mouse models with short hairpin RNA-mediated Rps19 knockdown.21-23 To test whether Rpl11+/▵ mice can serve as a model to predict treatment response, we treated Rpl11+/▵ mice with drinking water containing 1.5% L-leucine for 16 weeks, starting at age 4 weeks. Rpl11+/▵ mice treated with L-leucine maintained higher Hgb levels during treatment than the control group (water alone) (Figure 2E). Importantly, L-leucine significantly prolonged the survival of treated Rpl11+/▵ mice (P < .05; Figure 2F). Our data suggest that L-leucine may be beneficial for improving the survival of patients with DBA. We also treated Rpl11+/▵ mice with 25 mg/mL of prednisolone in drinking water,16 or 3 to 10 mg/kg body weight administered by daily gavage in 5% milk for 10 days and repeated every 21 days for 3 cycles. As previously reported, prednisolone had no impact on Hgb concentration in treated mice16 (supplemental Figure 3). This is most likely due to the impact of glucocorticoids on red cell production through stimulation of the earliest progenitors (BFU-E deriving cells).24
In conclusion, this mouse model exhibits hematologic characteristics of patients with DBA with Rpl11 haploinsufficiency, including disease severity and early age of onset. Importantly, we demonstrate that this model is useful for studying DBA pathogenesis and for testing novel therapies.
Acknowledgments: The authors thank Nan Wang, Kailen Mark, Carolyn Wong, and Hye Na Kim for their assistance in the experiments for data collection and analysis, and Rhonda Perriman for her critical editing of the final version of the manuscript. The authors are also indebted to Bertil Glader, Raymond T. Doty, Mark C. Wilkes, and Janis L. Abkowitz for their helpful suggestions throughout the project.
K.M.S. is supported by National Institutes of Health grants (R01 DK107286, R25 DK130827, R01 DK136961, U2C DK133488), American Society of Hematology, Department of Defense Bone Marrow Failure Program (BM180024), Diamond-Blackfan anemia Foundation, Stanford SPARK program in Translational Research, Stanford Maternal Child Health Research Institute, and California Institute of Regenerative Medicine (CIRM 12475).
Contribution: Y.L.L. conceived the project, designed/performed/supervised experiments, analyzed data, prepared figures and tables, and wrote the manuscript; N.N. performed genotyping experiments and prepared supplemental Figure 1; K.M.S. helped to conceive the project, designed/supervised experiments, analyzed data, and wrote the manuscript; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kathleen M. Sakamoto, Division of Hematology/Oncology, Department of Pediatrics, Stanford University School of Medicine, CCSR-1215C, 269 Campus Dr, Stanford, CA 94305-5162; email: kmsakamo@stanford.edu.
References
Author notes
Data are available on request from the corresponding author, Kathleen M. Sakamoto (kmsakamo@stanford.edu).
The full-text version of this article contains a data supplement.