Key Points
In non–low-risk AML, the addition of VEN to FLAI induction chemotherapy gives high rate of CR and limited toxicities.
In V-FLAI combination, VEN 600 mg was not markedly superior to VEN 400 mg in terms of remission rate or survival.
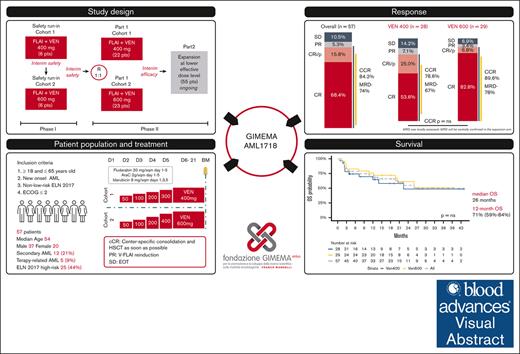
Visual Abstract
The standard induction treatment for acute myeloid leukemia (AML) has limited efficacy for patients with non–low-risk AML. We conducted a multicenter study phase 1b/2, Gruppo Italiano Malattie EMatologiche dell'Adulto AML1718, to investigate the safety and efficacy of venetoclax (VEN) combined with fludarabine, cytarabine, and idarubicin (V-FLAI) as an induction therapy for patients with non–low-risk AML aged <65 years and at intermediate or high European LeukemiaNet risk. After a safety run-in, patients were randomly allocated to VEN 400 mg or VEN 600 mg cohorts. The primary objectives were safety and composite complete remission (bone marrow blasts of <5% with any recovery). We report a predefined interim analysis after 57 patients. Median exposure to VEN during induction was 22 days. Effectiveness and safety were similar between VEN 400 mg and VEN 600 mg cohorts. The 60-day mortality rate was 5.8%. Prolonged aplasia was observed in patients receiving high doses of cytarabine during consolidation. Composite CR was achieved in 84% of patients. With a median follow-up of 20.6 months, 1-year overall survival was 71%, 1-year disease-free survival was 66.2%, and 1-year cumulative incidence of relapse was 24%. V-FLAI is an effective induction therapy for young and fit patients. Fifty-five more patients will be enrolled in part 2; they will receive VEN 400 mg + FLAI as predefined and will be evaluated centrally for measurable residual disease. This trial was registered at www.clinicaltrials.gov as #NCT03455504.
Introduction
The standard protocol for initial chemotherapy treatment of acute myeloid leukemia (AML) involves the administration of a combination of cytarabine (Ara-C) and an anthracycline. This approach is commonly referred to as frontline induction chemotherapy, or “3+7,” and is widely accepted in clinical practice.1 Although complete remission (CR) is achievable in 60% to 75% of patients with AML who are aged <60 years, the incidence of relapse remains high at ∼50%.2 Furthermore, only a small fraction of patients, roughly 30% to 40%, are able to achieve a survival period of >5 years.2,3 Results worsen in patients with intermediate or high-risk biology; with standard induction, candidate patient with non–low-risk AML achieve remission in ∼50% of cases.4-6 Overall, the frontline therapeutical approach to AML remains unsatisfactory, and needs to be ameliorated.
A multiagent induction regimen consisting of fludarabine, high-dose Ara-C, and idarubicin with granulocyte colony-stimulating factor priming (FLAG-IDA) or without (FLAI [fludarabine, Ara-C, and idarubicin]) is a highly effective therapeutic strategy. In comparison to alternative induction regimens, the use of FLAG-IDA or FLAI as a frontline treatment result in composite CR (cCR) rates of up to 80%, along with a decreased cumulative incidence of relapse when compared with 3+7.3,7,8 Even reaching these end points, FLAI/FLAG-IDA failed to demonstrate a significant overall survival (OS) benefit3 for the augmented myelosuppression and for the quality of the CR, that reached measurable residual disease (MRD) negativity rates of only 35%.9
In the unfit or older population, the B-cell lymphoma 2 inhibitor–BH3 mimetic drug venetoclax (VEN) demonstrated a relevant synergism in frontline regimens containing hypomethylating agents,10,11 low and intermediate dose chemotherapy,12-15 thus establishing the rationale for including VEN in frontline therapy for younger patients.16 Three pivotal single-center experiences reported high effectiveness in terms of CR rate and survival with VEN added to 3+7,17 FLAG-IDA,18,19 or CLIA (cladribine, idarubicin, and Ara-C)20 in a fit population. Remarkably, in these experiences, MRD negativity rate after induction was 68%, 93%, and 72%, respectively, suggesting a superior antileukemic activity of the VEN combination.17-20 Concurrently, it was shown that addition of VEN improved prognosis of patients with myelodysplasia-related gene mutation.21
We hypothesized that the addition of VEN to high-intensity induction chemotherapy could diminish apoptotic threshold of blasts, generating a deeper remission that translate into a prolonged survival. To prove this hypothesis, we designed a multicenter safety run-in and phase 2 study, which combined VEN with FLAI (V-FLAI). In the safety run-in, we tested the feasibility of VEN 400 or 600 mg in combination with FLAI. In part 1, patients were randomly allocated to a cohort with VEN 400 mg + FLAI or VEN 600 mg + FLAI. Herein, we present the preliminary results of the first planned interim efficacy analysis, at the end of part 1.
Patients and methods
Study design
On behalf of GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) study group, this multicenter, safety run-in and randomized phase 2 trial was approved by the ethical committee of Istituto di Ricovero e Cura a Carattere Scientifico, Istituto Romagnolo per lo Studio dei Tumori “Dino Amadori” S.r.l., and by all the enrolling institutions (ClinicalTrials.gov identifier: NCT03455504). In the safety run-in phase, patients were recruited from 3 institutions, in the randomized phase 2 part 1 from 15 institutions across Italy. We allowed in the study patients aged from 18 and 65 years. Within inclusion criteria, the study required a diagnosis of non-M3 AML, according to World Health Organization 2016 criteria,22 graded non–low-risk according to European LeukemiaNet (ELN) 2017 criteria (via exclusion of NPM1 mutation and core binding factor rearrangements or confirmation of a high-risk–defining cytogenetic or molecular abnormalities).23 Patients should have never received any treatment for AML apart of hydroxyurea or 6-mercaptopurine administered for cytoreduction. We included patients with an Eastern Cooperative Oncology Group performance status score of ≤2, adequate hepatic and renal function, and absence of pancreatic enzyme elevation. All nonhematological adverse events must have resolved to grade ≤2 before starting therapy. All patients were required to use effective methods of contraception. Patients affected by secondary or therapy-related AML were eligible for this study; however, the duration of antecedent myeloid disorder was limited to 6 months to avoid excessive toxicities. According to exclusion criteria, the study did not enroll patients with evidence of active central nervous system leukemia; history of another neoplastic disease unless in persistent long-term remission; and any severe and/or uncontrolled medical condition that could be jeopardized by treatment including, but not limited to, severe uncontrolled cardiological, pulmonary, renal diseases, active infections not controlled by antimicrobial therapy, and HIV positivity. Patients who had received prior bcl2 inhibitors and patients who refused blood transfusion were also not included.
The study used a modified 2-stage Simon design comprising 6 sequential patient safety run-in cohorts (SRI-C1 and SRI-C2) with single-patient dose-limiting toxicity (DLT) periods; followed by a part 1 phase with 2 extension cohorts (P1-C1 and P1-C2); and a confirmatory cohort (part 2). Predefined interim analyses were conducted after SRI-C1 and SRI-C2, and part 1. In SRI-C1, the safety of daily VEN at 400 mg administered in combination with FLAI was assessed, whereas in SRI-C2, the VEN dose was escalated to 600 mg per day. After a favorable safety review, patients were randomized into P1-C1 or P1-C2, which differed in the VEN doses administered with FLAI (400 or 600 mg, respectively; Figure 1). If both P1-C1 and P1-C2 met predefined efficacy criteria, the study protocol mandated an extension at the lower dosages with demonstrated effectiveness (ie, VEN 400 mg) into a confirmatory cohort, termed part 2 (P2), currently waiting for mature follow-up. The randomization was open label with a 1:1 allocation ratio, stratified by known ELN risk, using the randomization scheme in blocks.
CONSORT diagram of the AML1718 trial safety run-in and part 1. CRi, complete response without platelet and neutrophils recovery; PR, partial response; pts, patients; Rel, relapse; SCT, stem cell transplant; SD, stable disease.
CONSORT diagram of the AML1718 trial safety run-in and part 1. CRi, complete response without platelet and neutrophils recovery; PR, partial response; pts, patients; Rel, relapse; SCT, stem cell transplant; SD, stable disease.
Treatment and procedures
In every patient, we administered fludarabine 30 mg/m2 from day 1 to day 5 over a 1-hour infusion; Ara-C 2000 mg/m2 from day 1 to day 5 over a 4-hour infusion; and idarubicin 8 mg/m2 on days 1, 3, and 5 over a 30-minute infusion. Dose reductions for induction chemotherapy were predefined for patients with significant comorbidities or organ distress due to leukemia at physician discretion (fludarabine 30 mg/m2 from day 1 to day 3, Ara-C 2000 mg/m2 from day 1 to day 3, and idarubicin 8 mg/m2 on day 1; supplemental Figure 1). VEN was administered from day 2 in a 4-day ramp-up to the target dose of 400 or 600 mg, according to the cohort and has been planned to be continued (supplemental Figure 2); an early VEN suspension at day 21 has been suggested for those patients with an already documented leukemia-free bone marrow to facilitate blood count recovery. After V-FLAI induction, continuous administration of VEN was restarted at count recovery (if previously suspended). Azoles were specifically allowed during the treatment, with appropriate VEN dose adjustment. Posaconazole was suggested as prophylaxis during induction24 and, in such cases, VEN dose was managed with a predefined, eightfold dose reduction scheme (maximum allowed VEN dose was 50 mg for VEN 400 mg, and 70 mg for VEN 600 mg cohorts). Antibacterial prophylaxis with levofloxacin and granulocyte colony-stimulating factor use after demonstrated empty marrow remission were suggested but administered at center discretion.
Patients with a documented partial response after induction (ie, blast reduction of ≥50% with count recovery) were allowed to receive a second induction course at the same dosages, whereas patients with stable disease, progression, or unacceptable toxicities were mandated to withdraw from the study. We suggested performing allogeneic stem cell transplant (HSCT) as soon as possible, administering center-specific consolidation if required. Consolidation courses were administered according to center-specific guidelines for patients who were not able to receive transplant after induction or for whom transplant was not indicated. VEN was suspended 5 days before any further chemotherapy course or transplant; thus, VEN was not administered during consolidation courses or transplant conditioning. VEN maintenance was administered to patients not undergoing HSCT, whereas after-HSCT maintenance was not allowed. A centralized biobank was issued for study samples, at which we performed post hoc next-generation sequencing panels (supplemental Methods).
Outcomes
Remission status was defined with bone marrow evaluation after course 1 as follows: CR if bone marrow blasts of <5% and complete recovery of platelets and neutrophils; CR without platelet recovery (CRp) if patients experienced a CR maintaining a platelet count of <100 × 109/L and neutrophils >5 × 109/L; CR with insufficient recovery of peripheral counts was defined for patients in CRp with neutrophils of <0.5 × 109/L. Response was upgraded whenever peripheral recovery followed a negative bone marrow evaluation within 2 weeks. MRD was defined by each enrolling institution with internal procedures by flow cytometry (threshold, 0.1%); a centralized analysis is ongoing on the confirmatory part 2. During the safety run-in, DLT was defined as any grade 4 or 5 noninfective and nonhematological severe adverse event. Hematological adverse events included decrease in any blood count. The DLT observation period was 28 days after induction. Late count recovery was not considered a DLT.
The primary outcome of the study was the bone marrow cCR (cCR = CR + CR with insufficient recovery of peripheral counts + CRp) rate after induction course. Key secondary end points were OS, defined as the time between the first study drug administration and death from any cause; disease free survival (DFS), estimated for patients in CR and defined as the time between the date of response and any event including disease progression or death; cumulative incidence of relapse; and MRD negativity rate after each course. Safety data were collected across the entire study as number and incidence of adverse events according to National Cancer Institute common terminology criteria for adverse events, version 5.0.25
Statistical analysis
In this study, we considered an expected cCR rate of 50% with 3+7-based standard-of-care chemotherapy in the target population2,26 and decided to consider the primary end point of this study met if a cCR rate of >65% would have been obtained. The sample calculation was performed to yield a type-1 error of 0.05 and power of 0.8 in defining the expected augment in cCR. At the end of part 1, the study predefined an analysis of the primary outcome for patients treated with VEN 400 mg or VEN 600 mg. Here, we present the results of this planned interim analysis. In the analysis, we included all the patients that received at least 1 drug in cycle 1, day 1. Incidence of remission was defined as a rate. Survival analyses were reported descriptively with Kaplan-Meier representation. All analyses were performed using the SAS system software (version 9.4) and R (R Foundation for Statistical Computing, Vienna, Austria). All tests were 2-sided, accepting P ≤ .05 as indicative of a statistically significant difference. Study data were collected and managed using the Research Electronic Data Capture (REDCap) tools hosted at the GIMEMA Foundation REDCap.27,28
The study was approved by all the institutional review boards of all institutions involved in the clinical trial, according to Italian regulation.
Results
Patient population
Between February 2019 and November 2021, 57 patients were enrolled in the study; the first 12 patients were sequentially assigned to SRI-C1 (n = 6) and SRI-C2 (n = 6), respectively, thereafter we performed a positive safety review, and the remaining 45 patients were randomly assigned to VEN 400 mg + FLAI (P1-C1, n = 22) or VEN 600 mg + FLAI (P1-C2, n = 23; Figure 1). All enrolled patients received the predefined treatment. Cumulatively, 28 patients received VEN 400 mg + FLAI, and 29 patients received VEN 600 mg + FLAI.
Overall, 37 patients (65%) were male. The median age at diagnosis was 54 years (range, 18-65). According to baseline risk definition, 32 patients (56%) and 25 patients (44%) were at intermediate and high ELN 2017 risk, respectively; 7 patients (12%) had secondary AML, and 5 (9%) a therapy-related disease. Six patients (12%) harbored FLT3 mutation; no patient was NPM1 positive. No statistically significant difference between VEN 400 mg + FLAI and 600 mg + FLAI cohorts has been documented with respect to the aforementioned factors (Table 1).
Patient characteristics
| Characteristic . | Overall, N = 57 . | VEN dosage . | |
|---|---|---|---|
| VEN 400 mg, n = 28 . | VEN 600 mg, n = 29 . | ||
| Age, median (range), y | 54 (18-66) | 55 (30-66) | 53 (18-66) |
| Male, n (%) | 37 (65) | 17 (61) | 20 (69) |
| ELN 2017 intermediate risk, n (%)∗ | 32 (56) | 15 (54) | 17 (59) |
| ELN 2017 high risk, n (%)∗ | 25 (44) | 13 (46) | 12 (41) |
| White blood cells, median (range), ×109/L | 3 (0-274) | 3 (0-120) | 3 (1-274) |
| FLT3 mutation, n (%) | |||
| ITD | 4 (7.7) | 1 (3.9) | 3 (11.5) |
| TKD | 1 (1.9) | 0 (0) | 1 (3.8) |
| ITD and TKD | 2 (3.9) | 0 (0) | 2 (7.8) |
| WT | 45 (86.5) | 26 (96.1) | 20 (76.9) |
| Unknown† | 5 | 2 | 3 |
| NPM1, n (%) | |||
| WT | 46 (100) | 24 (100) | 22 (100) |
| Unknown† | 11 | 4 | 7 |
| Complex/high-risk cytogenetic, n (%) | 25 (44) | 13 (46) | 12 (41) |
| Secondary AML, n (%) | 12 (21.1) | 8 (28.6) | 4 (13.8) |
| Therapy-related AML, n (%) | 5 (9) | 3 (10.7) | 2 (6.9) |
| Characteristic . | Overall, N = 57 . | VEN dosage . | |
|---|---|---|---|
| VEN 400 mg, n = 28 . | VEN 600 mg, n = 29 . | ||
| Age, median (range), y | 54 (18-66) | 55 (30-66) | 53 (18-66) |
| Male, n (%) | 37 (65) | 17 (61) | 20 (69) |
| ELN 2017 intermediate risk, n (%)∗ | 32 (56) | 15 (54) | 17 (59) |
| ELN 2017 high risk, n (%)∗ | 25 (44) | 13 (46) | 12 (41) |
| White blood cells, median (range), ×109/L | 3 (0-274) | 3 (0-120) | 3 (1-274) |
| FLT3 mutation, n (%) | |||
| ITD | 4 (7.7) | 1 (3.9) | 3 (11.5) |
| TKD | 1 (1.9) | 0 (0) | 1 (3.8) |
| ITD and TKD | 2 (3.9) | 0 (0) | 2 (7.8) |
| WT | 45 (86.5) | 26 (96.1) | 20 (76.9) |
| Unknown† | 5 | 2 | 3 |
| NPM1, n (%) | |||
| WT | 46 (100) | 24 (100) | 22 (100) |
| Unknown† | 11 | 4 | 7 |
| Complex/high-risk cytogenetic, n (%) | 25 (44) | 13 (46) | 12 (41) |
| Secondary AML, n (%) | 12 (21.1) | 8 (28.6) | 4 (13.8) |
| Therapy-related AML, n (%) | 5 (9) | 3 (10.7) | 2 (6.9) |
ITD, internal tandem duplication; TKD, tyrosine kinase domain; WT, wild type.
Karyotypes are reported in supplemental Table 2.
Extended next-generation sequencing characterization was performed as a post-hoc analysis on centralized and biobanked samples (54/57 analyzable; 3/57 insufficient material). We confirmed that no NPM1 hot spot mutation was present in NPM1, and no new FLT3 mutation was detected. IDH1 was mutated in 4 of 54 (7.4%), IDH2 in 9 of 54 (16.6%), TP53 in 8 of 54 (14.8%) patients. Accounting for genetic abnormalities defined post hoc, ELN 2022 risk stratification is fully applicable in 55 patients, and 42 of 55 patients (76.3%) are classified as high risk.
Treatment and response
At induction, 54 patients received V-FLAI without reductions. In 2 of 57 patients (3.5%) we administered FLAI3 chemotherapy (a reduced schedule; supplemental Figure 3) and in 1 of 57 (1.8%) we reduced VEN dose because of clinical distress and subsequent medical decision; VEN dose was adjusted per azole coadministration in 52 of 57 patients (91.2%).
cCR was observed in 48 of 57 patients (84%, 95% confidence interval [CI], 72-92). As for the primary end point, the cCR rate was 22 of 28 (79%) in VEN 400 mg + FLAI arm and 26 of 29 (90%) in VEN 600 mg + FLAI arm; responses at induction are summarized in Table 2. MRD negativity status after 1 course of induction was documented in 28 of 38 tested patients (74%; 95% CI, 56-86); specifically, 67% in the VEN 400 mg + FLAI arm, and 78% in the VEN 600 mg + FLAI arm. cCR was similar between intermediate and high ELN risk patients (29/32, 90.6% and 19/25, 76.0%, respectively).
Response after induction
| Response . | Overall N = 57 . | Arm . | P value . | |
|---|---|---|---|---|
| VEN 400 mg n = 28 . | VEN 600 mg n = 29 . | |||
| CR | 39 (68.4%)∗ | 15 (53.6%) | 24 (82.8%)∗ | 0.11 |
| CRp | 7 (12.3%) | 6 (21.4%) | 1 (3.4%) | |
| CRi | 2 (3.5%) | 1 (3.6%) | 1 (3.4%) | |
| PR | 3 (5.3%) | 2 (7.1%) | 1 (3.4%) | |
| SD | 4 (7.0%) | 2 (7.1%) | 2 (6.9%) | |
| Failure | 2 (3.5%) | 2 (7.1) | 0 (0%) | |
| cCR | 48 (84.2%)∗ | 22 (78.6%) | 26 (89.6%)∗ | 0.30 |
| Response . | Overall N = 57 . | Arm . | P value . | |
|---|---|---|---|---|
| VEN 400 mg n = 28 . | VEN 600 mg n = 29 . | |||
| CR | 39 (68.4%)∗ | 15 (53.6%) | 24 (82.8%)∗ | 0.11 |
| CRp | 7 (12.3%) | 6 (21.4%) | 1 (3.4%) | |
| CRi | 2 (3.5%) | 1 (3.6%) | 1 (3.4%) | |
| PR | 3 (5.3%) | 2 (7.1%) | 1 (3.4%) | |
| SD | 4 (7.0%) | 2 (7.1%) | 2 (6.9%) | |
| Failure | 2 (3.5%) | 2 (7.1) | 0 (0%) | |
| cCR | 48 (84.2%)∗ | 22 (78.6%) | 26 (89.6%)∗ | 0.30 |
CRi, complete response without platelet and neutrophils recovery; PR, partial response; SD, stable disease.
One patient obtained PR after first induction and CR after second V-FLAI.
After induction, 1 patient died, 5 patients went off study because of disease refractoriness (n = 4) or toxicity (n = 1), and 1 patient went off treatment for medical decision. We administered a second induction course in 5 patients not in cCR (3 in the VEN 400 mg + FLAI arm, and 2 in the VEN 600 mg + FLAI arm), of whom 1 patient achieved CR. Five patients were able to directly proceed to HSCT (2 in the VEN 400 mg + FLAI arm, and 3 in the VEN 600 mg + FLAI arm); 3 started maintenance with VEN; all of the other 37 patients received center-specific consolidation therapies containing intermediate- or high-dose Ara-C, anthracycline, and fludarabine, as specified in Table 3. VEN was restarted at count recovery after consolidation.
Drugs administered during center-specific consolidation after induction
| Consolidation course . | Overall, N = 37 n (%) . |
|---|---|
| Intermediate-dose Ara-C (≤10 g/m2 total dose) | 27 (73) |
| High-dose Ara-C (>10 g/m2 total dose) | 8 (22) |
| Intermediate-dose anthracycline (≤8 mg/m2 of idarubicin or equivalent total dose) | 20 (54) |
| High-dose anthracycline (>8 mg/m2 of idarubicin or equivalent total dose) | 7 (19) |
| Fludarabine as third agent | 21 (57) |
| Amsacrine | 2 (5) |
| High-dose Ara-C and/or high-dose anthracycline | 13 (35) |
| Consolidation course . | Overall, N = 37 n (%) . |
|---|---|
| Intermediate-dose Ara-C (≤10 g/m2 total dose) | 27 (73) |
| High-dose Ara-C (>10 g/m2 total dose) | 8 (22) |
| Intermediate-dose anthracycline (≤8 mg/m2 of idarubicin or equivalent total dose) | 20 (54) |
| High-dose anthracycline (>8 mg/m2 of idarubicin or equivalent total dose) | 7 (19) |
| Fludarabine as third agent | 21 (57) |
| Amsacrine | 2 (5) |
| High-dose Ara-C and/or high-dose anthracycline | 13 (35) |
Overall, 31 patients (55%) received a subsequent HSCT; 2 of them were in partial response, the remaining in CR at the time of transplant, and 20 of 30 (67%) in MRD-negative CR. Transplant was performed from matched related donors in 8 of 30 (27%), matched unrelated donors in 13 of 30 patients (43%), and a haploidentical donor in 9 of 30 (30%) patients. Thirteen of 31 patients (41%) received reduced intensity conditioning. Donor, source, and conditioning regimen were balanced between the 2 cohorts (data not shown).
Safety
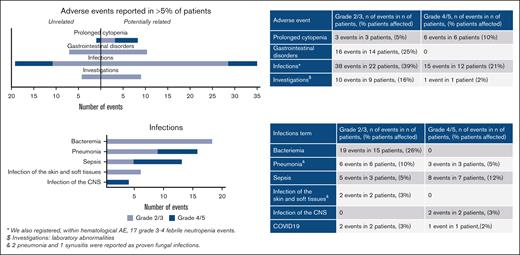
With a median exposure to VEN of 22 days, its addition to FLAI was generally well tolerated. No DLTs were observed in SRI-C1 or SRI-C2. Overall, infections were the most frequently registered NCI-CTCAE grade ≥3 adverse events in this study; adverse events are summarized in Figure 2. Of note, 4 patients (7%) had severe acute respiratory syndrome coronavirus 2 infection during treatment.
Adverse events grade 2+ reported in >5% of patients, classified according to NCI-CTCAE version 5.0. Adverse events are presented as related or not related according to the investigator’s judgment. Infections are presented in detail. CNS, central nervous system; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events.
Adverse events grade 2+ reported in >5% of patients, classified according to NCI-CTCAE version 5.0. Adverse events are presented as related or not related according to the investigator’s judgment. Infections are presented in detail. CNS, central nervous system; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events.
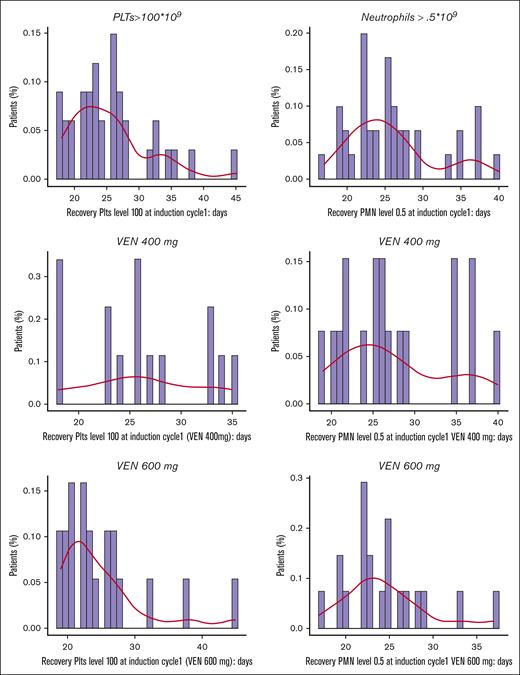
During induction therapy, median platelet recovery (>100 × 109/L) time was 24 days (interquartile range, 21-28), and median neutrophils recovery (> 0.5 × 109/L) time was 25 days (interquartile range, 22-28; Figure 3). As a safety finding, a prolonged time in aplasia was observed in patients receiving consolidation regimens with high doses of Ara-C and anthracycline, with an excess of patients without marrow recovery at day 42 during consolidation (12/37 patients [32.4%] did not reach full platelet and neutrophil recovery, and 9/37 patients [24.3%] did not reach full platelet recovery). Of note, per protocol, patients were not receiving VEN during consolidations. Because of this evidence, after patient number 28, we suggested to perform consolidation only in patients that cannot receive a transplant in a timely manner, choosing between an intermediate dose of Ara-C or 3 days of fludarabine and Ara-C plus a single dose of idarubicin at 8 mg/m2.
Time for platelet and neutrophil recovery after induction in VEN 400 mg + FLAI and VEN 600 mg + FLAI arms. PMN, polymorphonucleated cells; PLT, platelets.
Time for platelet and neutrophil recovery after induction in VEN 400 mg + FLAI and VEN 600 mg + FLAI arms. PMN, polymorphonucleated cells; PLT, platelets.
No unexpected safety signal was observed during or after transplant, and incidence of severe graft-versus-host disease was reported in only 1 patient (acute, grade 3).
At database lock, 22 patients had died, 15 (68%) of whom after disease persistence or relapse. In a multicenter setting, 30-day and 60-day mortality rates were 1.8% and 5.3%, respectively (supplemental Figure 4); nonrelapse mortality was observed in 7 patients; 4 after first consolidation (1 for central nervous system bleeding, 1 due to invasive fungal infection of the lung, 1 due to pneumonia sustained by multiresistant Klebsiella pneumoniae, and 1 due to persistent pancytopenia and sepsis) and 3 after transplant (1 due to severe pneumonia, 1 due to secondary tumor, and 1 due to encephalitis and graft-versus-host disease). Of note, a further patient developed a severe fungal infection of the frontal sinus and the central nervous system during consolidation, which delayed subsequent therapies and prevented administration of rescue therapies at AML relapse that happen 45 days after consolidation 1.
No significant differences in terms of safety, or time of recovery after induction were observed between VEN 400 mg + FLAI and VEN 600 mg + FLAI arms. With the adjustment of consolidation strategy, we were able to ameliorate hematological toxicities (unplanned analysis, supplemental Table 1).
Survival
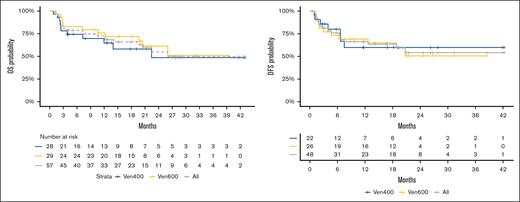
With a median follow-up of 20.6 months, median OS was reached at 26 months; probability of 12-month OS was 71% (95% CI, 59-84), 65% (95% CI, 48-87) for VEN 400 mg, and 76% (95% CI, 62-93) for VEN 600 mg, P = .53. Median DFS was not reached; probability of 12-month DFS was 66% (95% CI, 54-82), 60% (95% CI, 40-89) for VEN 400 mg and 69% (95% CI, 54-90) for VEN 600 mg, P = .88; survival data are shown in Figure 4. No significant differences in terms of CR rate or survival were observed between the VEN 400 mg + FLAI and the VEN 600 mg + FLAI arms. The incidence of relapse was 24% (95% CI, 12-37) at 1 year, and 29% (95% CI, 14-45) at 2 years (supplemental Figure 4). In a landmark analysis involving only patients who received HSCT, median OS at 1 year was 83.7% (95% CI, 70.2-99.8).
OS and event-free survival for VEN 400 mg + FLAI and VEN 600 mg + FLAI combinations.
OS and event-free survival for VEN 400 mg + FLAI and VEN 600 mg + FLAI combinations.
Discussion
Effective induction regimens represent an important need for patients with AML, because treatment failure and relapse remain the main causes of mortality.29,30 Nowadays, in the population treated with curative intent, the main early objective is to maximize the possibility of achieving a good-quality CR, because the achievement of CR31 and MRD negativity9,32 translates into better survival.
In this study we present preliminary data from a multicenter experience on a novel combination regimen, V-FLAI, for intermediate- or high-risk young and fit patients with newly diagnosed AML.
The combination of FLAI with VEN 400 mg or VEN 600 mg resulted into a good safety profile during induction, with a rate of high-grade toxicity that does not exceed other high-intensity chemotherapies.2,3,33,34 Particularly, the higher dose of VEN did not seem to affect safety during induction, consolidation, or transitioning to transplant. It is important to point out that safety was demonstrated in 15 different centers at the national level. It is important to point out that our study optimized combination dosages exclusively in newly diagnosed AML, thus a decrease in infective risk compared with that previously reported is consistent with a healthier population, not previously exposed to chemotherapies.18,19,35,36
A delayed recovery after consolidation chemotherapy was already reported in patients receiving FLAG-Ida.3 In our study, it was described both for patients receiving VEN 400 mg + FLAI and VEN 600 mg + FLAI. In other VEN- chemotherapy combinations, it was suggested that late recovery was related with the rechallenge of VEN plus chemotherapy as consolidation course.15,20 Here, consolidation-related myelotoxicity was reported even if no patient received VEN in combination with consolidation courses. Even if this toxicity may be related to FLAI alone, we speculated that the off-target effect of V-FLAI on the long-term repopulating progenitors,37 that share leukemia stem cell–specific metabolism and mechanisms of survival,38,39 may worsen this aspect. In this respect, the nonaffected short-term repopulating cells spare induction-related toxicities, whereas long-term repopulating cells, that are more similar to leukemia stem cell, fail in full marrow recovery in subsequent courses. Another possible explanation can be found in the clonal mutations often found in nonleukemic “healthy” bone marrow counterparts in intermediate and high-risk AML,40-42 that can diminish repopulating fitness. The reduction of the myelosuppressive potential of consolidation courses provided a good solution for this issue and may designate the way to follow in this young and poor-prognosis population, with a very active induction followed by gentle consolidations and transplant as soon as possible. Clinical data of the combination of VEN with high intensity chemotherapy was previously reported only in a few low-risk patients19,20; in this population, toxicities are of relevance because the patients do not benefit from transplant in first CR. However, different biology can confer a better safety profile.
Of note, this study differs from others also in terms of VEN exposure. In the AML1718 study, VEN was administered for 21 days in most patients. Contrariwise, most of the experiences reported in the current literature are based on a duration of exposures between 7 and 14 days.18-20 Thus, we cannot exclude that augmented duration of VEN administration may be related to worst recoveries, even if our data on recovery seems to be comparable with data observed in protocols with shorter administration. Remarkably, also, our efficacy data do not document a large increase in MRD negativity, cCR, or survival compared with other VEN-chemotherapy combinations18-20; consequently, we are not able to demonstrate that prolonging VEN administration after day 14 may confer better outcomes.
The V-FLAI regimen holds the promise of a high rate of CR in patients with non–low-risk AML. Overall, 84% of patients achieved remission with a single course of chemotherapy; an MRD-negativity rate of 74% was observed within patients locally tested after CR. In a uniform, large, multicenter cohort of patients with newly diagnosed non–low-risk AML, these results confirm the excellent activity profile that was previous reported in pivotal experiences with comparable regimens.18-20 Interpretation of results is even more impressive whenever considering that the achievement of fast and deep CR translated into a high transplant rate (53%), and in a median survival was reached at 26 months. The lack of a predefined consolidation strategy is a weakness of the study and, unfortunately, definitive conclusions cannot be drawn on consolidation and transplant. However, the opportunity to administer HSCT as an immunological consolidation in the optimal setting of a good-quality response enhanced the probability of survival.43 Consistently, the relapse rate in the population stayed <25% at 1 and 2 years. The effectiveness seems consistent between VEN 400 mg and VEN 600 mg cohorts.
The AML1718 study enrolled 55 more patients in a confirmatory cohort (part 2). All patients were treated with VEN 400 mg + FLAI, the effective regiment with the lower VEN dose, as predefined. Long-term follow-up and central MRD evaluation are warranted for this cohort. Furthermore, even if VEN 600 mg did not show significant higher effectiveness, evaluation of higher VEN dosages with shorter ramp-up are warranted in future experiences because a plateau of effectiveness was never demonstrated in VEN-chemotherapy combinations.44 Finally, different durations of VEN exposure have been evaluated in different combinations, and an optimization of the schedule will be further explored.19,20 This combination has been prepared to be tested in a controlled study, especially because other promising therapies such as CPX-351 are showing activity in the young population that also represent the best target for V-FLAI.21,45,46
In AML1718 V-FLAI safety run-in and part 1 of the study we optimized the safety of the combination. Result of the part 2 of the study will eventually confirm effectiveness.
Acknowledgment
AbbVie provided drug and nonconditionate economic support for the clinical trial.
Authorship
Contribution: G. Marconi and G. Martinelli conceptualized the study; G. Marconi, G. Martinelli, and A.P. were responsible for the methodology; A.P., F.P., E.C., and E.l.S. were responsible for the data curation and formal analysis; G. Marconi, E.A., C.P., M.C., C.M., F.G., M.B., M.R., A.L., M.G.C., M.G.D.P., M.F., L.G., C.C., M.C.D.C., B.S., R.F., S.C., A.C., P.M., J.N., B.A.Z., G.S., and R.M.L. were responsible for the clinical investigation; G.S. and M.T.B. were responsible for the laboratory investigation; A.V., P.F., M.V., and G. Martinelli were responsible for the project administration; A.P., G. Marconi, F.P., E.C., and E.l.S. were responsible for the visualization; G. Marconi wrote the first draft of the manuscript; G. Martinelli, P.F., M.V., and A.V. were responsible for the funding acquisition; G. Martinelli supervised the study; and all authors contributed to writing the manuscript, reviewed the manuscript after accessing data, and approved the final draft.
Conflict-of-interest disclosure: G. Marconi acts as a consultant/speakers' bureau member of AbbVie, Astellas, AstraZeneca, Immunogen, Menarini/Stemline, Pfizer, Ryvu, Servier, Syros, and Takeda, and reports research support from AbbVie, Astellas, AstraZeneca, and Pfizer. E.A. received honoraria from AbbVie. C.P. served on an advisory board of, and/or received honoraria from, Amgen, Pfizer, Astellas, AbbVie, Blueprint, Novartis, Delbert Pharma, GlaxoSmithKline, Stemline, Incyte, Janssen, and Bristol Myers Squibb. F.G. acts as a consultant for Jazz and Astellas. M.C. received honoraria from AbbVie. G. Martinelli acts as a consultant/advisor/speakers' bureau member of Ariad/Incyte, Pfizer, Celgene/Bristol Myers Squibb, Amgen, Roche, AbbVie, GlaxoSmithKline, Astellas, Daiichi Sankyo, Takeda, and Janssen, and received research support from Pfizer, AbbVie, AstraZeneca, Daiichi Sankyo, Takeda, and Ariad/Incyte. The remaining authors declare no competing financial interests.
Correspondence: Giovanni Marconi, UOC Ematologia, Ospedale S. Maria delle Croci, 48121, Ravenna, Italy; email: giovanni.marconi@unibo.it; and Giovanni Martinelli, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli,” Bologna, Italy; email: giovanni.martinelli2@unibo.it.
References
Author notes
Presented data are interim results of an ongoing clinical trial. Data are available on request from the corresponding authors, Giovanni Marconi (giovanni.marconi@unibo.it) and Giovanni Martinelli (giovanni.martinelli2@unibo.it).
The full-text version of this article contains a data supplement.