Key Points
PTCy dosing can be safely reduced for bone marrow transplant, maintaining excellent control of acute GVHD.
Clinical benefits of PTCy dose reduction may include faster engraftment and T-cell recovery and less severe BK virus cystitis/urethritis and mucositis.
Visual Abstract
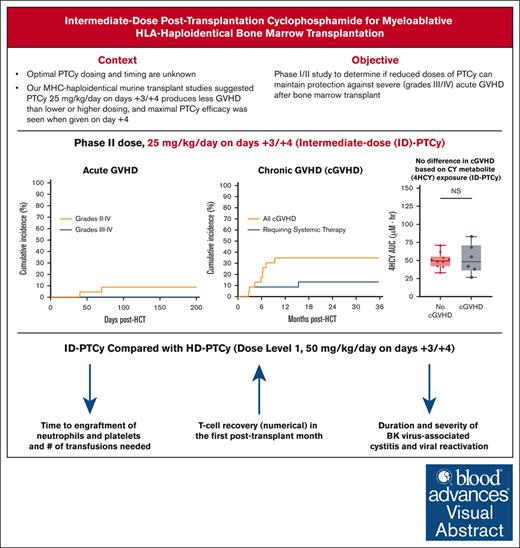
High-dose posttransplantation cyclophosphamide (HD-PTCy), given at 50 mg/kg/day on days +3/+4, is a standard-of-care graft-versus-host disease (GVHD) prophylaxis for allogeneic hematopoietic cell transplantation (HCT). Our murine MHC–haploidentical HCT studies suggested intermediate-dose PTCy produces superior GVHD control compared with HD-PTCy and PTCy is maximally effective on day +4. We conducted a single-institutional prospective phase 1/2 trial to reduce PTCy dosing to 25 mg/kg/day on days +3/+4 or on day +4 only for myeloablative HLA–haploidentical bone marrow HCT using PTCy, sirolimus, and mycophenolate mofetil. Among 35 patients, 89% were ethnic/racial minorities, 46% had high/very-high-risk disease, and median comorbidity score was 3. The phase 1 dose-limiting-toxicity, grade III-IV acute GVHD, was not observed after either reduced-PTCy dose level. PTCy 25 mg/kg/day on days +3/+4 (intermediate-dose (ID)-PTCy; n = 23), the phase 2 dose, resulted in no grade II-IV acute GVHD; 2-year cumulative incidences of chronic GVHD requiring systemic immunosuppression, nonrelapse mortality, and relapse were 13%, 17%, and 22%, and 2-year overall survival, disease-free survival, and GVHD-free/relapse-free survival were 61%, 61%, and 52%. In exploratory analysis compared with HD-PTCy (n = 5), ID-PTCy resulted in significantly faster engraftment and T-cell reconstitution, fewer transfusions, less mucositis, and reduced severity of BK-virus–associated cystitis/urethritis; area-under-the-curve exposure of 4-hydroxycyclophosphamide (4HCY), a key cyclophosphamide metabolite, correlated with these outcomes but not with chronic GVHD occurrence. Ideal-body-weight–based PTCy dosing best approximated 4HCY exposure. ID-PTCy is effective and has apparent clinical benefits compared with HD-PTCy. Before broader implementation, further studies are needed to confirm these findings and define optimal PTCy dosing across various donor/graft types. This trial was registered at www.clinicaltrials.gov as #NCT03983850.
Introduction
High-dose posttransplantation cyclophosphamide (HD-PTCy) reduces the incidences of severe acute (aGVHD) and chronic graft-versus-host disease (cGVHD) and facilitates engraftment after human leukocyte antigen (HLA)–partially mismatched allogeneic hematopoietic cell transplantation (HCT), overcoming historical barriers resultant from the strong bidirectional alloreactivity of HLA-mismatching.1-5 Consequently, because >50% of individuals from racial and ethnic minority backgrounds in the United States lack HLA-matched donors,6 HD-PTCy has greatly expanded access to HCT. Although ex vivo T-cell depletion and in vivo T-cell depletion also have overcome these historical barriers,1 HD-PTCy has become the dominant GVHD prophylaxis for HLA-partially-mismatched HCT,7 including HLA-haploidentical HCT, because of its favorable clinical outcomes paired with its low cost, universal availability, and ease of administration.1 HD-PTCy also recently became a standard-of-care GVHD prophylaxis for HLA-matched donor HCT.8,9 Despite these promising results, HD-PTCy has drawbacks, including delaying engraftment10 and increasing transplant-associated toxicities11 and posttransplant infections.12-18
Even with PTCy’s broad acceptance, the optimal dosing and timing for PTCy administration remain unknown. PTCy’s clinical implementation was largely extrapolated from murine major histocompatibility complex (MHC)-matched skin allografting studies, in which a very-high 200 mg/kg dose was given on day +2 or +3 after cell infusion, followed by success of this dosing for MHC-mismatched murine HCT.2,19-21 For the first clinical trial,4 50 mg/kg was chosen as it was near the maximally tolerated dose in humans and leveraged prior experience in aplastic anemia treatment.22 Administration on day +3 was chosen to decrease toxicity by spacing the cyclophosphamide further from conditioning. The phase 2 study enrolled two cohorts with one institution using 50 mg/kg on day +3 and the other institution empirically adding a second 50 mg/kg dose on day +4; the two-day (day +3/+4) dosing regimen appeared to have less extensive cGVHD compared with the one-day dosing regimen,3 thus creating the current standard dosing schedule.1-3,8,9,23
Our preclinical studies in murine MHC-haploidentical HCT models showed that 25 mg/kg per day PTCy given on days +3/+4 produces less clinical and histopathologic GVHD than high-dose (50-100 mg/kg per day) or low-dose (1-10 mg/kg per day) PTCy.24 Moreover, we found that PTCy was maximally effective when given on day +4.25 Compared with other two-day dosing schedules, 25 mg/kg per day PTCy on days +3/+4 effectively reduced alloreactive T-cell proliferation and maximally promoted preferential regulatory T-cell recovery, both of which appear critical to the mechanisms by which PTCy prevents GVHD.2,24-28 Given these findings and the potential drawbacks of HD-PTCy, we initiated a phase 1/2 study evaluating the safety of reducing the dose and number of days of administration of PTCy after myeloablatively conditioned HLA-haploidentical bone marrow HCT.
Methods
Study design
This was a phase 1/2, nonrandomized, single-institutional study (ClinicalTrials.gov identifier: NCT03983850), designed to determine whether reduced-dose PTCy, 25 mg/kg per day on days +3 and +4 (dose level 2 [DL2]) or on day +4 only (DL3), adequately protects against grade III-IV aGVHD after myeloablatively conditioned HLA-haploidentical bone marrow transplantation. The study was approved by the National Institutes of Health institutional review board, and all participants provided written informed consent before treatment.
The study began with a pilot phase of 5 patients treated with HD-PTCy (50 mg/kg per day on days +3/+4; DL1) to collect data for comparative analysis. Phase 1 then proceeded with a 3+3 two-level dose deescalation using grade III-IV aGVHD by day +60 as the dose-limiting toxicity (DLT). The DL considered safe (≤1 of 6 DLT events) with the least toxicity was taken to phase 2. Phase 2 was conducted using a Simon optimal two-stage trial design with a target grade III-IV aGVHD rate of 10% (goal of 90% avoiding DLT), aiming to rule out DLT of 30% with a type-1 error of 0.1 and type-2 error of 0.2. The first stage consisted of 6 patients in phase 1; if there were 0 to 1 of 6 with DLT, the trial would enroll 14 additional evaluable patients in the second stage. Seventeen or more patients without DLT among the 20 evaluable patients treated at the phase 2 dose would be considered a success. Patients without grade III-IV aGVHD, but with death, relapse, or graft failure, or receiving additional cell infusion before day +60 were not considered evaluable for the primary endpoint and were replaced. The study contained stopping rules for the rate of grade III-IV aGVHD at any time during phase 2 and the rate of primary graft failure during phase 1.
Endpoints
Because our preclinical studies supporting PTCy dose reduction were models of aGVHD,24,25 the DLT and primary endpoint for this trial was grade III-IV aGVHD. Secondary endpoints included cumulative incidences of grades II-IV and III-IV aGVHD at days 100 and 200, cumulative incidences of cGVHD and relapse at 1 year, cumulative incidences of nonrelapse mortality (NRM) at 100 days and 1 year, overall survival (OS) and disease-free survival (DFS) at 1 year, and engraftment kinetics. Exploratory endpoints included pharmacokinetics of cyclophosphamide and its metabolites, immune reconstitution, and infection. Patients were followed on protocol for up to 5 years.
aGVHD and cGVHD were defined per the 1994 Keystone29 and 2014 National Institutes of Health consensus criteria,30 respectively. Standardized cGVHD assessments included pulmonary function testing and evaluations by transplant, ophthalmology, dental, and gynecology (females only) at posttransplant days +100, +180, and +270; and years 1, 1.5, 2, 3, 4, and 5; and additionally as clinically indicated. Relapse was defined as any disease detectable after transplant and did not require morphologic relapse. OS was defined as the time from HCT to death or censored at last follow-up. DFS was defined as the time from HCT to relapse or death. GVHD-free relapse-free survival (GRFS) was as standardly defined,31 with events being grade III-IV aGVHD, cGVHD requiring systemic immunosuppression, relapse, or death.
Competing risks for aGVHD and cGVHD included relapse, graft failure, additional cell therapy, or death, and cGVHD also was a competing risk for aGVHD. Relapse and NRM were competing risks for each other. Neutrophil and platelet engraftment,32 engraftment syndrome,33 refined disease-risk index (DRI),34 and HCT-specific Comorbidity Index (HCT-CI)35 determinations were per standard criteria.
Eligibility
Eligible patients were aged 15 to 65 years with adequate end-organ function and hematologic malignancy with standard indication for allogeneic HCT (supplemental Methods). The presence of donor-specific antibodies was not exclusionary although no such patients were included in the study.
Treatment regimen
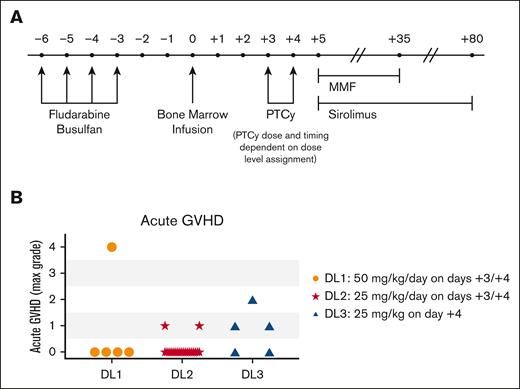
The treatment schema, described in detail in Figure 1A, included myeloablative conditioning with intravenous daily busulfan and fludarabine. Busulfan was pharmacokinetically adjusted based on a test dose for a target daily area-under-the-curve (AUC) of 4600 μMol-min/L (18.9 mg × h/L). Fludarabine 40 mg/m2 per day was administered immediately before busulfan. T-cell–replete bone marrow from an HLA-haploidentical donor was administered fresh, with the graft dose based on recipient ideal body weight (IBW). No graft manipulation was performed other than red blood cell reduction for major ABO incompatibility and universal plasma removal, per institutional standards. Day +3 and +4 intravenous PTCy was started between 70 to 74 hours and 94 to 98 hours, respectively, after the start of bone marrow infusion on day 0. PTCy dose was based on the patient’s IBW with the following exceptions: (1) if actual body weight (ABW) was <IBW, ABW was used; (2) if ABW > 120% IBW, adjusted IBW was used, computed as IBW + (0.25 × [ABW – IBW]). Mesna was administered at 100% of the cyclophosphamide dose as a continuous infusion over 48 hours starting with cyclophosphamide initiation; peri-PTCy oral hydration was standard.
Treatment schema and aGVHD. (A) Treatment schema. (B) Maximal grade of aGVHD among evaluable patients (n = 5 each for DL1 and DL3; n = 20 for DL2). Patients without aGVHD but with death, graft failure, or relapse before day +60 were not included, as per the protocol these patients were considered inevaluable for the primary endpoint.
Treatment schema and aGVHD. (A) Treatment schema. (B) Maximal grade of aGVHD among evaluable patients (n = 5 each for DL1 and DL3; n = 20 for DL2). Patients without aGVHD but with death, graft failure, or relapse before day +60 were not included, as per the protocol these patients were considered inevaluable for the primary endpoint.
Sirolimus was given orally with a one-time loading dose on day +5, followed by once-daily maintenance dosing to maintain a trough of 5 to 12 ng/mL thereafter until day +80, when it was discontinued without taper. An undetectable or very low sirolimus level on day +6 prompted a second full or partial loading dose. Mycophenolate mofetil (MMF) was administered at 15 mg/kg per dose (maximum 1000 mg per dose) 3 times daily from day +5 through day +35. Other supportive care measures included granulocyte colony-stimulating factor (5 μg/kg per dose rounded to vial size) starting on day +5 through engraftment; ursodiol until at least day +30; letermovir starting on day +5 for recipients with cytomegalovirus (CMV) seropositivity, and continued until approximately day +70; and antifungal prophylaxis until day +100. Letermovir, azoles, and other medications with potential interactions with cyclophosphamide being administered before transplant were stopped prior to cyclophosphamide with sufficient washout period and were resumed on the morning of day +5, as needed. No prophylactic antibacterials were used during posttransplant neutropenia.
Cyclophosphamide and 4HCY pharmacokinetics
Samples were collected before and after each cyclophosphamide dose at 2 (end of infusion), 4, 8, 16, 20, and 24 hours from the start of infusion. Concentrations of cyclophosphamide and 4-hydroxycyclophosphamide (4HCY) were quantitated as previously reported,36 using liquid chromatography tandem mass spectrometry. The area-under-the-plasma-concentration-time curve from time 0 to 48 hours was estimated using the trapezoidal numerical estimation function from the PKNCA package in R (R Foundation, Vienna, Austria).
Statistical analysis
Analyses of OS, DFS, and GRFS were performed using the Kaplan-Meier methods, with the curves compared by log-rank test. Cumulative incidence curves for aGVHD, cGVHD, relapse, and NRM incorporated competing risks, and differences were calculated via the Gray method. Comparisons between dose levels were performed via the Mann-Whitney U test for continuous variables and the Fisher exact test for binary outcomes. Correlations between parameters were determined using Spearman correlation coefficients. The database was locked as of 1 August 2023, and data were analyzed using R, SAS version 9.4 (SAS Institute, Cary NC), and GraphPad Prism (GraphPad Software, Boston, MA).
Results
Patient, donor, and transplant characteristics
Thirty-five patients underwent HCT between July 2019 and January 2022 (Table 1). Overall, patients were young (median age, 28 years) but heavily pretreated (median 3 lines of prior therapy) and medically infirm (median HCT-CI, 3), and predominantly ethnic and/or racial minorities (only 11% White Non-Hispanic). Most patients had advanced hematologic malignancies (46% high/very-high risk by DRI), with the most frequent indication being acute leukemia (69%). Median follow-up of survivors was 2.4 years for all patients, and 2.3 years for DL2 patients.
Patient, donor, and graft characteristics
| Characteristic . | HD-PTCy . | ID-PTCy . | . | All patients . |
|---|---|---|---|---|
| DL1 . | DL2 . | DL3 . | ||
| 50 mg/kg per day . | 25 mg/kg per day . | 25 mg/kg . | ||
| Days +3/+4 . | Days +3/+4 . | Day +4 . | ||
| (n = 5) . | (n = 23) . | (n = 7) . | (N = 35) . | |
| Age, median (range), y | ||||
| Recipient | 25 (21-38) | 34 (18-57) | 23 (20-30) | 28 (18-57) |
| Donor | 42 (23-49) | 31 (17-54) | 43 (29-52) | 38 (17-54) |
| Male sex, n (%) | ||||
| Recipient | 3 (60) | 14 (61) | 3 (43) | 20 (57) |
| Donor | 2 (40) | 17 (74) | 3 (43) | 22 (63) |
| Race or ethnic group, n (%) | ||||
| White, Non-Hispanic | 0 (0) | 3 (13) | 1 (14) | 4 (11) |
| White, Hispanic | 5 (100) | 15 (65) | 2 (29) | 22 (63) |
| African American | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| Asian | 0 (0) | 0 (0) | 1 (14) | 1 (3) |
| American Indian/Alaskan Native | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| Multiracial | 0 (0) | 3 (13) | 1 (14) | 4 (11) |
| Karnofsky performance status, n (%) | ||||
| 100% | 2 (40) | 5 (22) | 1 (14) | 8 (23) |
| 90% | 1 (20) | 14 (61) | 4 (57) | 19 (54) |
| <90% | 2 (40) | 4 (17) | 2 (29) | 8 (23) |
| Comorbidity index (HCT-CI), n (%) | ||||
| 0 | 0 (0) | 2 (9) | 2 (29) | 4 (11) |
| 1-2 | 0 (0) | 8 (35) | 1 (14) | 9 (26) |
| 3-4 | 2 (40) | 8 (35) | 1 (14) | 11 (31) |
| ≥5 | 3 (60) | 5 (22) | 3 (43) | 11 (31) |
| Disease, n (%) | ||||
| Acute myeloid leukemia | 1 (20) | 5 (22) | 3 (43) | 9 (26) |
| Acute lymphoblastic leukemia | 3 (60) | 10 (43) | 2 (29) | 15 (43) |
| Myelodysplastic syndrome | 0 (0) | 0 (0) | 1 (14) | 1 (3) |
| Chronic myelogenous leukemia | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Primary myelofibrosis | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| Non-Hodgkin lymphoma | 1 (20) | 3 (13) | 0 (0) | 4 (11) |
| Hodgkin lymphoma | 0 (0) | 2 (9) | 1 (14) | 3 (9) |
| Diagnosis to transplant, median (range), y | 1.0 (0.4-8.5) | 2.0 (0.4-14.7) | 1.1 (0.2-2.6) | 1.7 (0.2-14.7) |
| Prior lines of therapy, median (range), n | 3 (2-8) | 3 (1-10) | 3 (1-6) | 3 (1-10) |
| Disease status at transplant, n (%) | ||||
| CR1, MRD (−) | 2 (40) | 7 (30) | 4 (57) | 13 (37) |
| CR1, MRD (+) | 1 (20) | 1 (4) | 0 (0) | 2 (6) |
| CR ≥ 2, MRD (−) | 1 (20) | 8 (35) | 2 (29) | 11 (31) |
| CR ≥ 2, MRD (+) | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| SD | 0 (0) | 2 (9) | 1 (14) | 3 (9) |
| PD | 1 (20) | 3 (13) | 0 (0) | 4 (11) |
| Disease Risk Index (refined), n (%) | ||||
| Low | 0 (0) | 2 (9) | 1 (14) | 3 (9) |
| Intermediate | 3 (60) | 11 (48) | 2 (29) | 16 (46) |
| High/very high | 2 (40) | 10 (43) | 4 (57) | 16 (46) |
| CMV seropositive, n (%) | ||||
| Recipient | 4 (80) | 17 (74) | 6 (86) | 27 (77) |
| Donor | 5 (100) | 18 (78) | 7 (100) | 30 (86) |
| ABO matching, n (%) | ||||
| Matched | 4 (80) | 16 (70) | 6 (86) | 26 (74) |
| Minor mismatch | 1 (20) | 4 (17) | 0 (0) | 5 (14) |
| Major mismatch | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| Bidirectional mismatch | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| GVH HLA matching, n (%) | ||||
| 5/10 | 3 (60) | 12 (52) | 5 (71) | 20 (57) |
| 6/10 | 2 (40) | 6 (26) | 2 (29) | 10 (29) |
| 7/10 | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| 8/10 | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| 10/10 | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| HVG HLA matching, n (%) | ||||
| 5/10 | 2 (40) | 14 (61) | 4 (57) | 20 (57) |
| 6/10 | 2 (40) | 4 (17) | 2 (29) | 8 (23) |
| 7/10 | 1 (20) | 3 (13) | 1 (14) | 5 (14) |
| 8/10 | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| Female into male, n (%) | 2 (40) | 3 (13) | 2 (29) | 7 (20) |
| Donor relation, n (%) | ||||
| Father | 2 (40) | 2 (9) | 2 (29) | 6 (17) |
| Mother | 1 (20) | 2 (9) | 2 (29) | 5 (14) |
| Brother | 0 (0) | 7 (30) | 0 (0) | 7 (20) |
| Sister | 2 (40) | 2 (9) | 2 (29) | 6 (17) |
| Son | 0 (0) | 6 (26) | 0 (0) | 6 (17) |
| Daughter | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Cousin (male) | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| Cousin (female) | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Nephew | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Infused cell dose, median (range) | ||||
| Total nucleated cells (108/kg) | 5.6 (3.6-6.6) | 4.7 (1.8-9.5) | 4.3 (2.8-6.6) | 4.7 (1.8-9.5) |
| CD34+ cells (106/kg) | 7.4 (2.8-8.2) | 5.1 (2.6-11.3) | 3.9 (3.0-7.2) | 4.8 (2.6-11.3) |
| CD3+ cells (107/kg) | 3.9 (3.1-4.0) | 3.3 (1.9-6.7) | 3.7 (2.3-4.9) | 3.4 (1.9-6.7) |
| Characteristic . | HD-PTCy . | ID-PTCy . | . | All patients . |
|---|---|---|---|---|
| DL1 . | DL2 . | DL3 . | ||
| 50 mg/kg per day . | 25 mg/kg per day . | 25 mg/kg . | ||
| Days +3/+4 . | Days +3/+4 . | Day +4 . | ||
| (n = 5) . | (n = 23) . | (n = 7) . | (N = 35) . | |
| Age, median (range), y | ||||
| Recipient | 25 (21-38) | 34 (18-57) | 23 (20-30) | 28 (18-57) |
| Donor | 42 (23-49) | 31 (17-54) | 43 (29-52) | 38 (17-54) |
| Male sex, n (%) | ||||
| Recipient | 3 (60) | 14 (61) | 3 (43) | 20 (57) |
| Donor | 2 (40) | 17 (74) | 3 (43) | 22 (63) |
| Race or ethnic group, n (%) | ||||
| White, Non-Hispanic | 0 (0) | 3 (13) | 1 (14) | 4 (11) |
| White, Hispanic | 5 (100) | 15 (65) | 2 (29) | 22 (63) |
| African American | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| Asian | 0 (0) | 0 (0) | 1 (14) | 1 (3) |
| American Indian/Alaskan Native | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| Multiracial | 0 (0) | 3 (13) | 1 (14) | 4 (11) |
| Karnofsky performance status, n (%) | ||||
| 100% | 2 (40) | 5 (22) | 1 (14) | 8 (23) |
| 90% | 1 (20) | 14 (61) | 4 (57) | 19 (54) |
| <90% | 2 (40) | 4 (17) | 2 (29) | 8 (23) |
| Comorbidity index (HCT-CI), n (%) | ||||
| 0 | 0 (0) | 2 (9) | 2 (29) | 4 (11) |
| 1-2 | 0 (0) | 8 (35) | 1 (14) | 9 (26) |
| 3-4 | 2 (40) | 8 (35) | 1 (14) | 11 (31) |
| ≥5 | 3 (60) | 5 (22) | 3 (43) | 11 (31) |
| Disease, n (%) | ||||
| Acute myeloid leukemia | 1 (20) | 5 (22) | 3 (43) | 9 (26) |
| Acute lymphoblastic leukemia | 3 (60) | 10 (43) | 2 (29) | 15 (43) |
| Myelodysplastic syndrome | 0 (0) | 0 (0) | 1 (14) | 1 (3) |
| Chronic myelogenous leukemia | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Primary myelofibrosis | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| Non-Hodgkin lymphoma | 1 (20) | 3 (13) | 0 (0) | 4 (11) |
| Hodgkin lymphoma | 0 (0) | 2 (9) | 1 (14) | 3 (9) |
| Diagnosis to transplant, median (range), y | 1.0 (0.4-8.5) | 2.0 (0.4-14.7) | 1.1 (0.2-2.6) | 1.7 (0.2-14.7) |
| Prior lines of therapy, median (range), n | 3 (2-8) | 3 (1-10) | 3 (1-6) | 3 (1-10) |
| Disease status at transplant, n (%) | ||||
| CR1, MRD (−) | 2 (40) | 7 (30) | 4 (57) | 13 (37) |
| CR1, MRD (+) | 1 (20) | 1 (4) | 0 (0) | 2 (6) |
| CR ≥ 2, MRD (−) | 1 (20) | 8 (35) | 2 (29) | 11 (31) |
| CR ≥ 2, MRD (+) | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| SD | 0 (0) | 2 (9) | 1 (14) | 3 (9) |
| PD | 1 (20) | 3 (13) | 0 (0) | 4 (11) |
| Disease Risk Index (refined), n (%) | ||||
| Low | 0 (0) | 2 (9) | 1 (14) | 3 (9) |
| Intermediate | 3 (60) | 11 (48) | 2 (29) | 16 (46) |
| High/very high | 2 (40) | 10 (43) | 4 (57) | 16 (46) |
| CMV seropositive, n (%) | ||||
| Recipient | 4 (80) | 17 (74) | 6 (86) | 27 (77) |
| Donor | 5 (100) | 18 (78) | 7 (100) | 30 (86) |
| ABO matching, n (%) | ||||
| Matched | 4 (80) | 16 (70) | 6 (86) | 26 (74) |
| Minor mismatch | 1 (20) | 4 (17) | 0 (0) | 5 (14) |
| Major mismatch | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| Bidirectional mismatch | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| GVH HLA matching, n (%) | ||||
| 5/10 | 3 (60) | 12 (52) | 5 (71) | 20 (57) |
| 6/10 | 2 (40) | 6 (26) | 2 (29) | 10 (29) |
| 7/10 | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| 8/10 | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| 10/10 | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| HVG HLA matching, n (%) | ||||
| 5/10 | 2 (40) | 14 (61) | 4 (57) | 20 (57) |
| 6/10 | 2 (40) | 4 (17) | 2 (29) | 8 (23) |
| 7/10 | 1 (20) | 3 (13) | 1 (14) | 5 (14) |
| 8/10 | 0 (0) | 2 (9) | 0 (0) | 2 (6) |
| Female into male, n (%) | 2 (40) | 3 (13) | 2 (29) | 7 (20) |
| Donor relation, n (%) | ||||
| Father | 2 (40) | 2 (9) | 2 (29) | 6 (17) |
| Mother | 1 (20) | 2 (9) | 2 (29) | 5 (14) |
| Brother | 0 (0) | 7 (30) | 0 (0) | 7 (20) |
| Sister | 2 (40) | 2 (9) | 2 (29) | 6 (17) |
| Son | 0 (0) | 6 (26) | 0 (0) | 6 (17) |
| Daughter | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Cousin (male) | 0 (0) | 1 (4) | 1 (14) | 2 (6) |
| Cousin (female) | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Nephew | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Infused cell dose, median (range) | ||||
| Total nucleated cells (108/kg) | 5.6 (3.6-6.6) | 4.7 (1.8-9.5) | 4.3 (2.8-6.6) | 4.7 (1.8-9.5) |
| CD34+ cells (106/kg) | 7.4 (2.8-8.2) | 5.1 (2.6-11.3) | 3.9 (3.0-7.2) | 4.8 (2.6-11.3) |
| CD3+ cells (107/kg) | 3.9 (3.1-4.0) | 3.3 (1.9-6.7) | 3.7 (2.3-4.9) | 3.4 (1.9-6.7) |
Patient demographics by PTCy DL. DL2 includes patients in both phase 1 and 2. Infused cell doses were based on recipient IBW.
CR, complete remission; HVG, host-versus-graft; MRD, measurable residual disease; PD, progressive disease; SD, stable disease.
Phase 1: PTCy dose deescalation
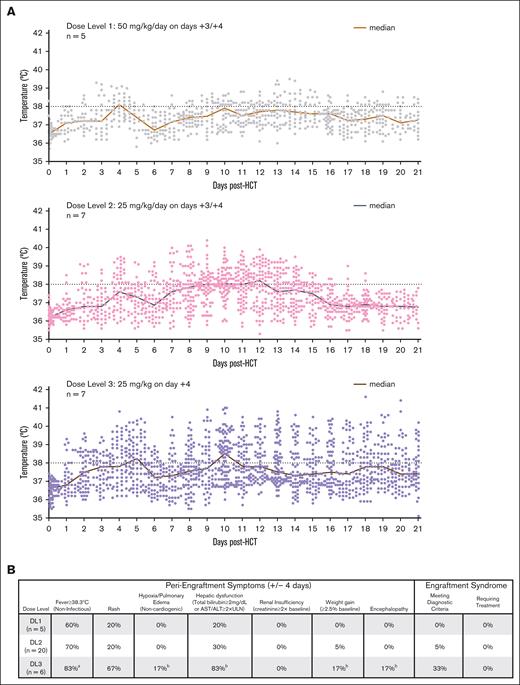
No grade III-IV aGVHD events were observed at either reduced-PTCy dose level but did occur in 1 of 5 patients treated with HD-PTCy (Figure 1B; Table 2). Early posttransplant fevers were exceedingly common across all 3 dose levels but were higher and more protracted at DL3 (Figure 2A-B). Because of high fevers and rapid rise in transaminases on day +10, 1 patient in DL3 received tocilizumab due to concern for cytokine release syndrome, but he was subsequently found to have rapidly progressive sinusoidal obstructive syndrome. No other patients were given immunosuppressive treatment for cytokine release syndrome from haplo-fevers or engraftment (Figure 2B). Despite both reduced-PTCy dose levels protecting against grade III-IV aGVHD, given the prolonged fevers and less reliably early engraftment with DL3 (see hereafter), DL2 (PTCy 25mg/kg per day on days +3/+4, subsequently referred to as intermediate-dose PTCy [ID-PTCy]) was taken to phase 2.
Individual patient outcomes
| Dose level . | Age, y . | Sex . | Diagnosis . | Disease status at BMT . | MRD status . | Refined DRI . | Prior lines of therapy . | HCT-CI . | Evaluable for primary endpoint (event) . | aGVHD∗ . | Engraftment syndrome . | SOS . | TMA . | Persistent host T-cell chimerism . | cGVHD† . | NRM . | NRM (cause) . | Relapse (day) . | Alive (day of death) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No . | Max. grade . | Yes/No . | Max severity . | Systemic immune suppression . | |||||||||||||||||||
| DL1 PTCy 50 mg/kg per day on days +3/+4 | 23 | F | ALL | CR3 | Negative | High | 3 | 4 | Yes | No | - | No | No | No | No | No | - | - | No | - | Yes (187) | No (389) | |
| 25 | M | ALL | CR1 | Negative | Intermediate | 2 | 7 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 21 | M | ALL | CR1 | Negative | Intermediate | 2 | 4 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 27 | M | AML | CR1 | Positive | Intermediate | 3 | 6 | Yes | Yes | 4 | No | No | No | No | No | - | - | Yes | aGVHD, viral/bacterial pneumonia | No | No (165) | ||
| 38 | F | DLBCL | PD | N/A | Very high | 8 | 7 | Yes | No | - | No | Yes | No | No | Yes | Mild | No | No | - | No | Yes | ||
| DL2 PTCy 25 mg/kg per day on days +3/+4 | Phase 1 | 28 | F | ALL | CR3 | Negative | High | 3 | 3 | Yes | No | - | No | No | No | Yes | No | - | - | No | - | Yes (335) | No (417) |
| 38 | F | ALL | CR1 | Negative | Intermediate | 3 | 5 | Yes | Yes | 1 | No | Yes | No | No | Yes | Severe | Yes | No | - | No | Yes | ||
| 43 | M | AML | CR2 | Positive | High | 2 | 4 | Yes | No | - | No | No | No | No | Yes | Mild | No | No | - | Yes (312) | No (542) | ||
| 28 | F | ALL | CR1 | Negative | Intermediate | 2 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 26 | F | AML | CR1 | Negative | Intermediate | 1 | 6 | No (graft failure) | N/A‡ | - | N/A‡ | No | No | N/A | N/A‡ | - | - | No | - | No | Yes | ||
| 47 | F | ALL | CR1 | Positive | Intermediate | 1 | 1 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 27 | M | HL | PD | N/A | High | 3 | 2 | Yes | No | - | No | No | No | No | Yes | Moderate | Yes | Yes | Interstitial lung disease (chemotherapy) | No | No (237) | ||
| Phase 2 | 57 | M | DLBCL | PD | N/A | Very high | 8 | 7 | Yes | No | - | No | No | No | No | No | - | - | Yes | Bacterial pneumonia | No | No (112) | |
| 42 | M | ALL | CR5 | Negative | High | 6 | 3 | Yes | No | - | No | No | No | Yes | No | - | - | No | - | Yes (286) | No (339) | ||
| 30 | M | ALL | CR1 | Negative | Intermediate | 2 | 4 | Yes | No | - | No | No | No | No | Yes | Moderate | No | No | - | No | Yes | ||
| 31 | M | ALL | CR3 | Negative | High | 3 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 47 | F | DLBCL | PD | N/A | Very high | 10 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | Yes (217) | No (333) | ||
| 28 | M | ALL | CR1 | Negative | Intermediate | 2 | 4 | Yes | Yes | 1 | No | No | Yes§ | No | Yes | Moderate | Yes | No | - | No | Yes | ||
| 50 | F | AML | CR1 | Negative | High | 3 | 1 | Yes | No | - | No | No | No | No | No | - | - | Yes | Interstitial lung disease (chemo vs infection) | No | No (357) | ||
| 53 | M | PMF | SD | N/A | Intermediate | 1 | 3 | Yes | No | - | No | No | No | No | Yes | Mild | No | No | - | No | Yes | ||
| 39 | M | HL | CR4 | Negative‖ | Low | 7 | 0 | Yes | No | - | Yes | No | No | No | Yes | Moderate | No | No | - | No | Yes | ||
| 40 | M | CML | CP | Positive | Intermediate | 3 | 4 | No (early relapse) | N/A¶ | - | No | No | No | N/A | N/A¶ | - | - | No | - | Yes (41) | No (161) | ||
| 30 | F | ALL | CR2 | Negative | High | 2 | 5 | Yes | No | - | No | No | No | Yes | No | - | - | No | - | No | Yes | ||
| 28 | M | DLBCL | CR3 | Negative‖ | Intermediate | 4 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 34 | M | ALL | CR5 | Negative | High | 8# | 1 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 21 | M | AML | CR2 | Negative | Low | 2 | 3 | Yes | No | - | No | No | No | Yes | No | - | - | Yes | ARDS/infection (toxo, AdV) after second HCT | No | No (161) | ||
| 52 | M | PMF | SD | N/A | Intermediate | 4 | 5 | No (graft failure) | N/A‡ | - | N/A‡ | No | No | N/A | N/A‡ | - | - | No | - | No | Yes | ||
| 18 | F | AML | CR1 | Negative | Intermediate | 3 | 0 | Yes | No | - | No | No | No | No | Yes | Mild | No | No | - | No | Yes | ||
| DL3 PTCy 25 mg/kg on day +4 | 28 | M | ALL | CR3 | Negative | High | 6 | 8 | No (NRM) | No | - | No | Yes | No | N/A | N/A∗∗ | - | - | Yes | SOS | No | No (33) | |
| 23 | F | HL | CR1 | Negative‖ | Low | 4 | 0 | Yes | Yes | 2 | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 30 | M | ALL | CR2 | Negative | High | 2 | 6 | Yes | Yes | 1 | No | No | No | No | No | - | - | Yes | CMV pneumonitis/enteritis | No | No (102) | ||
| 20 | M | AML | CR1 | Negative | Intermediate | 3 | 3 | Yes | Yes | 1 | Yes | No | No | No | Yes | Moderate | Yes | Yes | Unknown†† | No | No (545) | ||
| 22 | F | AML | CR1 | Negative | Intermediate | 1 | 0 | Yes | No | - | No | No | No | Yes | No | - | - | Yes | Bacterial and fungal pneumonia | No | No (502) | ||
| 26 | F | AML | CR1 | Negative | High | 1‡‡ | 6 | No (Early relapse) | N/A§§ | - | N/A§§ | No | No | N/A | N/A§§ | - | - | No | - | Yes (24) | No (300) | ||
| 21 | F | MDS | SD | N/A | High | 4 | 2 | Yes | No | - | Yes | No | No | No | Yes | Severe | Yes | No | - | Yes (369) | No (394) | ||
| Dose level . | Age, y . | Sex . | Diagnosis . | Disease status at BMT . | MRD status . | Refined DRI . | Prior lines of therapy . | HCT-CI . | Evaluable for primary endpoint (event) . | aGVHD∗ . | Engraftment syndrome . | SOS . | TMA . | Persistent host T-cell chimerism . | cGVHD† . | NRM . | NRM (cause) . | Relapse (day) . | Alive (day of death) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes/No . | Max. grade . | Yes/No . | Max severity . | Systemic immune suppression . | |||||||||||||||||||
| DL1 PTCy 50 mg/kg per day on days +3/+4 | 23 | F | ALL | CR3 | Negative | High | 3 | 4 | Yes | No | - | No | No | No | No | No | - | - | No | - | Yes (187) | No (389) | |
| 25 | M | ALL | CR1 | Negative | Intermediate | 2 | 7 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 21 | M | ALL | CR1 | Negative | Intermediate | 2 | 4 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 27 | M | AML | CR1 | Positive | Intermediate | 3 | 6 | Yes | Yes | 4 | No | No | No | No | No | - | - | Yes | aGVHD, viral/bacterial pneumonia | No | No (165) | ||
| 38 | F | DLBCL | PD | N/A | Very high | 8 | 7 | Yes | No | - | No | Yes | No | No | Yes | Mild | No | No | - | No | Yes | ||
| DL2 PTCy 25 mg/kg per day on days +3/+4 | Phase 1 | 28 | F | ALL | CR3 | Negative | High | 3 | 3 | Yes | No | - | No | No | No | Yes | No | - | - | No | - | Yes (335) | No (417) |
| 38 | F | ALL | CR1 | Negative | Intermediate | 3 | 5 | Yes | Yes | 1 | No | Yes | No | No | Yes | Severe | Yes | No | - | No | Yes | ||
| 43 | M | AML | CR2 | Positive | High | 2 | 4 | Yes | No | - | No | No | No | No | Yes | Mild | No | No | - | Yes (312) | No (542) | ||
| 28 | F | ALL | CR1 | Negative | Intermediate | 2 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 26 | F | AML | CR1 | Negative | Intermediate | 1 | 6 | No (graft failure) | N/A‡ | - | N/A‡ | No | No | N/A | N/A‡ | - | - | No | - | No | Yes | ||
| 47 | F | ALL | CR1 | Positive | Intermediate | 1 | 1 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 27 | M | HL | PD | N/A | High | 3 | 2 | Yes | No | - | No | No | No | No | Yes | Moderate | Yes | Yes | Interstitial lung disease (chemotherapy) | No | No (237) | ||
| Phase 2 | 57 | M | DLBCL | PD | N/A | Very high | 8 | 7 | Yes | No | - | No | No | No | No | No | - | - | Yes | Bacterial pneumonia | No | No (112) | |
| 42 | M | ALL | CR5 | Negative | High | 6 | 3 | Yes | No | - | No | No | No | Yes | No | - | - | No | - | Yes (286) | No (339) | ||
| 30 | M | ALL | CR1 | Negative | Intermediate | 2 | 4 | Yes | No | - | No | No | No | No | Yes | Moderate | No | No | - | No | Yes | ||
| 31 | M | ALL | CR3 | Negative | High | 3 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 47 | F | DLBCL | PD | N/A | Very high | 10 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | Yes (217) | No (333) | ||
| 28 | M | ALL | CR1 | Negative | Intermediate | 2 | 4 | Yes | Yes | 1 | No | No | Yes§ | No | Yes | Moderate | Yes | No | - | No | Yes | ||
| 50 | F | AML | CR1 | Negative | High | 3 | 1 | Yes | No | - | No | No | No | No | No | - | - | Yes | Interstitial lung disease (chemo vs infection) | No | No (357) | ||
| 53 | M | PMF | SD | N/A | Intermediate | 1 | 3 | Yes | No | - | No | No | No | No | Yes | Mild | No | No | - | No | Yes | ||
| 39 | M | HL | CR4 | Negative‖ | Low | 7 | 0 | Yes | No | - | Yes | No | No | No | Yes | Moderate | No | No | - | No | Yes | ||
| 40 | M | CML | CP | Positive | Intermediate | 3 | 4 | No (early relapse) | N/A¶ | - | No | No | No | N/A | N/A¶ | - | - | No | - | Yes (41) | No (161) | ||
| 30 | F | ALL | CR2 | Negative | High | 2 | 5 | Yes | No | - | No | No | No | Yes | No | - | - | No | - | No | Yes | ||
| 28 | M | DLBCL | CR3 | Negative‖ | Intermediate | 4 | 2 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 34 | M | ALL | CR5 | Negative | High | 8# | 1 | Yes | No | - | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 21 | M | AML | CR2 | Negative | Low | 2 | 3 | Yes | No | - | No | No | No | Yes | No | - | - | Yes | ARDS/infection (toxo, AdV) after second HCT | No | No (161) | ||
| 52 | M | PMF | SD | N/A | Intermediate | 4 | 5 | No (graft failure) | N/A‡ | - | N/A‡ | No | No | N/A | N/A‡ | - | - | No | - | No | Yes | ||
| 18 | F | AML | CR1 | Negative | Intermediate | 3 | 0 | Yes | No | - | No | No | No | No | Yes | Mild | No | No | - | No | Yes | ||
| DL3 PTCy 25 mg/kg on day +4 | 28 | M | ALL | CR3 | Negative | High | 6 | 8 | No (NRM) | No | - | No | Yes | No | N/A | N/A∗∗ | - | - | Yes | SOS | No | No (33) | |
| 23 | F | HL | CR1 | Negative‖ | Low | 4 | 0 | Yes | Yes | 2 | No | No | No | No | No | - | - | No | - | No | Yes | ||
| 30 | M | ALL | CR2 | Negative | High | 2 | 6 | Yes | Yes | 1 | No | No | No | No | No | - | - | Yes | CMV pneumonitis/enteritis | No | No (102) | ||
| 20 | M | AML | CR1 | Negative | Intermediate | 3 | 3 | Yes | Yes | 1 | Yes | No | No | No | Yes | Moderate | Yes | Yes | Unknown†† | No | No (545) | ||
| 22 | F | AML | CR1 | Negative | Intermediate | 1 | 0 | Yes | No | - | No | No | No | Yes | No | - | - | Yes | Bacterial and fungal pneumonia | No | No (502) | ||
| 26 | F | AML | CR1 | Negative | High | 1‡‡ | 6 | No (Early relapse) | N/A§§ | - | N/A§§ | No | No | N/A | N/A§§ | - | - | No | - | Yes (24) | No (300) | ||
| 21 | F | MDS | SD | N/A | High | 4 | 2 | Yes | No | - | Yes | No | No | No | Yes | Severe | Yes | No | - | Yes (369) | No (394) | ||
AdV, adenovirus; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ARDS, acute respiratory distress syndrome; BMT, bone marrow transplantation; CML, chronic myelogenous leukemia; CP, chronic phase; CR1/2/3/4/5, first/second/third/fourth/fifth complete remission; DLBCL, diffuse large B-cell lymphoma; F, female; HCT-CI, HCT-specific comorbidity index; HL, Hodgkin lymphoma; M, male; MDS, myelodysplastic syndrome; MRD, measurable residual disease; N/A, not applicable; PD, progressive disease; PMF, primary myelofibrosis; SD, stable disease; SOS, sinusoidal obstructive syndrome; TMA, thrombotic microangiopathy; Toxo, toxoplasmosis.
Diagnosed and graded per Keystone criteria.
Diagnosed and staged per National Institutes of Health (2014) consensus criteria.
Primary graft failure requiring second transplant.
No clinical sequelae other than anemia and thrombocytopenia that resolved with discontinuation of sirolimus.
Based on negative positron emission tomography imaging (HL and DLBCL), negative bone marrow (HL and DLBCL), and cerebrospinal fluid analysis by flow cytometry (DLBCL).
Loss of graft because of disease relapse.
Includes prior allogeneic HCT for primary disease.
Follow-up <100 days because of NRM.
International patient with NRM due to meningitis per verbal family report only; no confirmatory documentation available.
Also had prior allogeneic HCT for sickle cell disease.
Disease relapse resulting in engraftment failure.
Temperature curves and peri-engraftment symptoms. (A) All temperature readings over the course of the first 21 days for all phase 1 patients are shown. Fevers were more common for patients in DL3 (PTCy 25 mg/kg on day +4 only) and were noted to be higher and more prolonged beyond the time of engraftment. (B) Fevers, rash, and transaminitis were common during the peri-engraftment period for all 3 dose levels, but engraftment syndrome criteria were met only by 3 patients: 1 at DL2, and 2 at DL3 (all 3 with fever, rash, and transaminitis); none of these patients required treatment for engraftment syndrome and the symptoms quickly improved without intervention. aIncludes 1 patient with prolonged engraftment time and fevers early in course of neutrophil recovery (>4 days from day of engraftment); b1 patient with symptoms that were attributable to sinusoidal obstructive syndrome. This patient was the first DL3 patient and had severe and rapid increase in transaminases along with concomitant fever on day +10 and received 1 dose of tocilizumab before it was determined that he was having sinusoidal obstruction syndrome during this peri-engraftment period as the cause of the transaminase elevation. This was the only patient with immunosuppressive treatment before day +21 beyond the intended PTCy, sirolimus, and mycophenolate mofetil.
Temperature curves and peri-engraftment symptoms. (A) All temperature readings over the course of the first 21 days for all phase 1 patients are shown. Fevers were more common for patients in DL3 (PTCy 25 mg/kg on day +4 only) and were noted to be higher and more prolonged beyond the time of engraftment. (B) Fevers, rash, and transaminitis were common during the peri-engraftment period for all 3 dose levels, but engraftment syndrome criteria were met only by 3 patients: 1 at DL2, and 2 at DL3 (all 3 with fever, rash, and transaminitis); none of these patients required treatment for engraftment syndrome and the symptoms quickly improved without intervention. aIncludes 1 patient with prolonged engraftment time and fevers early in course of neutrophil recovery (>4 days from day of engraftment); b1 patient with symptoms that were attributable to sinusoidal obstructive syndrome. This patient was the first DL3 patient and had severe and rapid increase in transaminases along with concomitant fever on day +10 and received 1 dose of tocilizumab before it was determined that he was having sinusoidal obstruction syndrome during this peri-engraftment period as the cause of the transaminase elevation. This was the only patient with immunosuppressive treatment before day +21 beyond the intended PTCy, sirolimus, and mycophenolate mofetil.
GVHD and immunosuppressive requirements of ID-PTCy–treated patients
Overall, 50% of ID-PTCy–treated patients discontinued sirolimus earlier than the planned day +80 stop date (median discontinuation at day +76, earliest was at day +55) because of hematologic toxicity, which was related to thrombocytopenia in 80% of such patients (supplemental Tables 1 and 2); in such cases, the sirolimus was not replaced with another immunosuppressive agent.
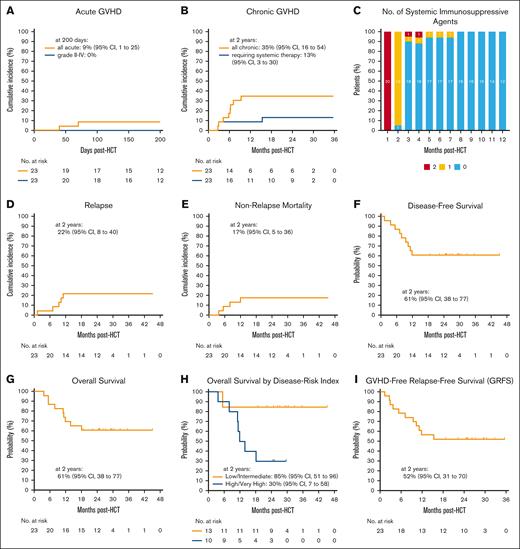
No ID-PTCy–treated patients had grade II-IV aGVHD (Figure 3A). Two-year cumulative incidences of all cGVHD and cGVHD requiring systemic immunosuppression for ID-PTCy–treated patients were 35% and 13%, respectively (Figure 3B; supplemental Figure 1). Because of minimal aGVHD and cGVHD requiring systemic immunosuppression, 90% and 100% of patients were off systemic immunosuppression for GVHD prophylaxis or treatment by 3 and 8 months, respectively (Figure 3C).
GVHD outcomes and survival probabilities for ID-PTCy–treated patients. (A-B) Cumulative incidences of (A) all aGVHD and grade II-IV aGVHD and (B) all cGVHD and cGVHD requiring systemic therapy. (C) Prophylactic and therapeutic systemic immunosuppression use for GVHD in the first posttransplant year. Patients with graft failure were excluded. Patients were counted as being on immunosuppression if alive and still taking immunosuppression on the last day of each month. The number of patients in each group at each time point are shown. (D-E) Cumulative incidences of (D) relapse and (E) nonrelapse mortality. (F-I) Kaplan-Meier estimates of (F) disease-free survival, (G) overall survival, (H) overall survival stratified by the DRI, and (I) GVHD-free, relapse-free survival (GRFS). CI, confidence interval.
GVHD outcomes and survival probabilities for ID-PTCy–treated patients. (A-B) Cumulative incidences of (A) all aGVHD and grade II-IV aGVHD and (B) all cGVHD and cGVHD requiring systemic therapy. (C) Prophylactic and therapeutic systemic immunosuppression use for GVHD in the first posttransplant year. Patients with graft failure were excluded. Patients were counted as being on immunosuppression if alive and still taking immunosuppression on the last day of each month. The number of patients in each group at each time point are shown. (D-E) Cumulative incidences of (D) relapse and (E) nonrelapse mortality. (F-I) Kaplan-Meier estimates of (F) disease-free survival, (G) overall survival, (H) overall survival stratified by the DRI, and (I) GVHD-free, relapse-free survival (GRFS). CI, confidence interval.
Relapse and survival outcomes for ID-PTCy–treated patients
The cumulative incidence of relapse at 1 and 2 years was 22% among ID-PTCy–treated patients (Figure 3D) and was higher for patients with high/very-high DRI compared with low/intermediate DRI (40% vs 8%, P = .09; supplemental Figure 2; supplemental Table 3). The cumulative incidences of NRM at 100 days and 1 year were 0% and 17%, respectively (Figure 3E; supplemental Table 3).
One-year and 2-year DFS was 61% (Figure 3F). One-year and 2-year OS probabilities were 70% and 61% (Figure 3G). Two-year OS was significantly worse for patients with high/very-high DRI than for those with low/intermediate DRI (30% vs 85%, P = .01; Figure 3H; supplemental Table 3). One-year and 2-year GRFS probabilities were 57% and 52%, respectively (Figure 3I; supplemental Figure 2).
Engraftment, transfusions, mucositis, and donor chimerism
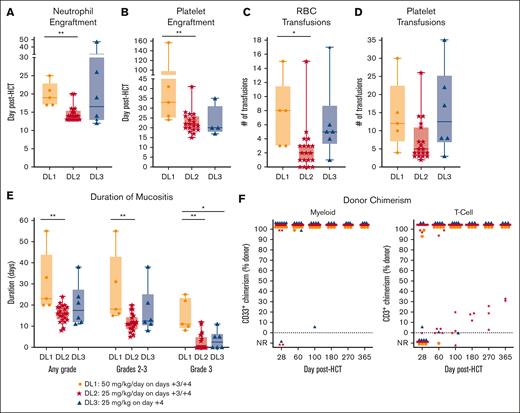
ID-PTCy resulted in significantly faster neutrophil (median, 14 vs 19 days; P = .0004) and platelet (median, 22 vs 33 days; P = .0097) engraftment than HD-PTCy (Figure 4A-B). Consequently, ID-PTCy resulted in significantly fewer red blood cell transfusions (median, 2 vs 8 units; P = .015; Figure 4C-D) and less severe mucositis than HD-PTCy (Figure 4E).
Faster engraftment, less transfusions, and less severe mucositis with ID-PTCy than with HD-PTCy. (A-E) Results by treatment group are shown. (A-B) Day of neutrophil and platelet engraftment. (C-D) Numbers of red blood cell (RBC) and platelet transfusions in the first 30 days after HCT. Of note, transfusion thresholds for patients were 7 g/dL for hemoglobin and 10 × 103/μL for platelets unless clinical circumstances required higher thresholds (eg, fever or bleeding). (E) Duration of mucositis (including oral, pharyngeal, and rectal) as graded by common terminology criteria for adverse events version 5.0. Patients who did not engraft were not included for panels A-E. (F) Donor myeloid (CD33+) and T-cell (CD3+) chimerism results for all patients. One patient in DL2 with early malignancy relapse had insufficient cells at day +28 for CD33+ bead-sorted chimerism but flow cytometrically sorted chimerism of CD14+ cells at day +22 had showed 99% donor chimerism, and that result is included for the day +28 timepoint. Otherwise, patients with peripheral blood counts insufficient to perform chimerism analyses were categorized as not reported (NR). ∗P<.05; ∗∗P<.01.
Faster engraftment, less transfusions, and less severe mucositis with ID-PTCy than with HD-PTCy. (A-E) Results by treatment group are shown. (A-B) Day of neutrophil and platelet engraftment. (C-D) Numbers of red blood cell (RBC) and platelet transfusions in the first 30 days after HCT. Of note, transfusion thresholds for patients were 7 g/dL for hemoglobin and 10 × 103/μL for platelets unless clinical circumstances required higher thresholds (eg, fever or bleeding). (E) Duration of mucositis (including oral, pharyngeal, and rectal) as graded by common terminology criteria for adverse events version 5.0. Patients who did not engraft were not included for panels A-E. (F) Donor myeloid (CD33+) and T-cell (CD3+) chimerism results for all patients. One patient in DL2 with early malignancy relapse had insufficient cells at day +28 for CD33+ bead-sorted chimerism but flow cytometrically sorted chimerism of CD14+ cells at day +22 had showed 99% donor chimerism, and that result is included for the day +28 timepoint. Otherwise, patients with peripheral blood counts insufficient to perform chimerism analyses were categorized as not reported (NR). ∗P<.05; ∗∗P<.01.
Most of the ID-PTCy–treated patients achieved full donor myeloid and T-cell chimerism (Figure 4F). Two patients treated with ID-PTCy had primary graft failure, 1 of whom had primary myelofibrosis with massive splenomegaly. Four engrafting ID-PTCy–treated patients had mixed T-cell chimerism (Figure 4F); 3 were stable off immunosuppression, but 1 had declining but persistent donor myeloid chimerism and low counts and so underwent repeat HCT from the same donor. None of the 5 HD-PTCy–treated patients had mixed T-cell chimerism, but 1 had poor graft function. Notably, in most patients, myeloablative busulfan/fludarabine conditioning was not highly lymphodepleting, and high-level host T-cell persistence was noted on day +3 (supplemental Table 4).
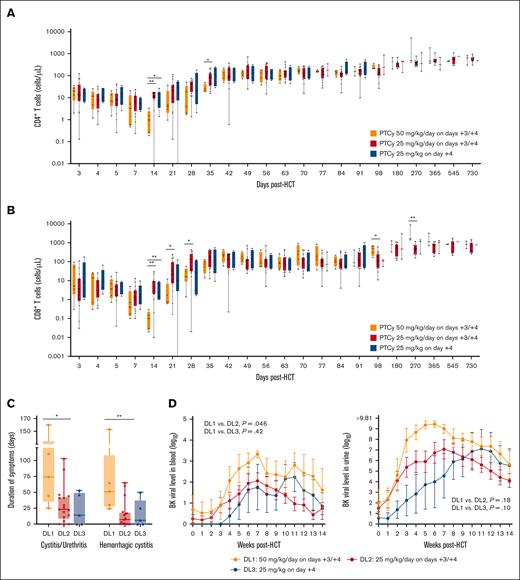
T-cell reconstitution and infections
T-cell reconstitution kinetics were significantly more rapid in engrafting ID-PTCy–treated patients than HD-PTCy–treated patients (Figure 5A-B; supplemental Figure 3). Correspondingly, CMV reactivation requiring preemptive therapy, a negative effect of HD-PTCy,13 was seen in all 4 (100%) recipients treated with HD-PTCy who were CMV seropositive, but occurred in 7 of 14 (50%) evaluable recipients who were CMV seropositive receiving ID-PTCy (P = .058). Of note, the 1 CMV-seronegative recipient treated with HD-PTCy had a CMV-seropositive donor and also had CMV reactivation requiring preemptive therapy. Among ID-PTCy–treated patients, CMV primary infection of the pharynx at day +6 and later gastrointestinal disease occurred in 1 patient who was CMV seronegative but had a CMV-seropositive donor, and another patient developed human herpesvirus 6–related encephalitis; both fully recovered. There were no cases of Epstein-Barr virus–related post-transplant lymphoproliferative disorder for any DL.
Historically, the risk for BK virus-associated cystitis/urethritis (BK-C) appears to be increased in HD-PTCy–treated patients.12 The duration of symptomatic BK-C, including hemorrhagic cystitis (BK-HC), was significantly shorter among ID-PTCy–treated than HD-PTCy–treated patients (median, 7 vs 51 days for BK-HC; P = .006; Figure 5C). Additionally, the incidence of grades 2 to 4 BK-HC per Bedi criteria37 was 100% for HD-PTCy; whereas it was 59% for ID-PTCy (supplemental Figure 4); no patients receiving ID-PTCy required instrumentation or bladder irrigation. Although the reduction in BK-C and BK-HC symptom severity with ID-PTCy may, in part, be because of less acrolein exposure from lower-dose chemotherapy, there also were significantly lower BK virus levels on serial weekly monitoring until 100 days after transplant, especially in the blood, suggesting better immunologic control of BK virus (Figure 5D).
Faster T-cell reconstitution and less severe BK virus–associated cystitis/urethritis with ID-PTCy than with HD-PTCy. (A) CD4+ and (B) CD8+ T-cell recovery over 2 years after HCT for each dose level. Both CD4+ and CD8+ T-cell counts were significantly higher for patients in DL2 and DL3 than those treated with HD-PTCy at day +14 and also at other early post-transplant time periods, before counts numerically normalized by day +42. T-cell counts were evaluated before PTCy administration on days +3 and +4, before starting sirolimus and mycophenolate mofetil on day +5, on day +7, weekly for 100 days, and then at days +180, +270, +365, +545 (1.5 years), and +730 (2 years) after transplant. Up to day +21, samples were collected on the day labeled. Windows for subsequent samples are as follows: days +28 to +99 ± 3 days; days +180 and +270 ± 14 days; and all following time points ± 30 days. Patients who did not engraft and those with persistent mixed T-cell chimerism were not included. (C) Duration of symptomatic BK virus–associated cystitis/urethritis and hemorrhagic cystitis. BK cystitis symptoms were defined by the presence of dysuria, urinary urgency, urinary frequency, bladder spasms, or macroscopic hematuria occurring within the first 180 days after HCT in patients with BK viruria. (D) BK virus levels in urine and blood on serial monitoring in the first 100 days after transplant. Mean values with standard error of the means are shown. Statistical comparisons shown are of the AUC of weekly testing over the first 100 days. For panels C-D, patients included are known to be at risk for BK cystitis, defined by BK virus detection in the urine or blood at any point on serial monitoring; 2 patients in DL2 without symptoms but also without any detectable BK virus in urine or blood at any timepoint were excluded in case they were unexposed and not at risk for reactivation. Patients who did not engraft, those with follow-up of <100 days because of relapse or NRM (unrelated to BK virus), or with BK virus–associated cystitis/urethritis symptoms at the time of initiation of conditioning for HCT also were excluded. ∗P < .05; ∗∗P < .01.
Faster T-cell reconstitution and less severe BK virus–associated cystitis/urethritis with ID-PTCy than with HD-PTCy. (A) CD4+ and (B) CD8+ T-cell recovery over 2 years after HCT for each dose level. Both CD4+ and CD8+ T-cell counts were significantly higher for patients in DL2 and DL3 than those treated with HD-PTCy at day +14 and also at other early post-transplant time periods, before counts numerically normalized by day +42. T-cell counts were evaluated before PTCy administration on days +3 and +4, before starting sirolimus and mycophenolate mofetil on day +5, on day +7, weekly for 100 days, and then at days +180, +270, +365, +545 (1.5 years), and +730 (2 years) after transplant. Up to day +21, samples were collected on the day labeled. Windows for subsequent samples are as follows: days +28 to +99 ± 3 days; days +180 and +270 ± 14 days; and all following time points ± 30 days. Patients who did not engraft and those with persistent mixed T-cell chimerism were not included. (C) Duration of symptomatic BK virus–associated cystitis/urethritis and hemorrhagic cystitis. BK cystitis symptoms were defined by the presence of dysuria, urinary urgency, urinary frequency, bladder spasms, or macroscopic hematuria occurring within the first 180 days after HCT in patients with BK viruria. (D) BK virus levels in urine and blood on serial monitoring in the first 100 days after transplant. Mean values with standard error of the means are shown. Statistical comparisons shown are of the AUC of weekly testing over the first 100 days. For panels C-D, patients included are known to be at risk for BK cystitis, defined by BK virus detection in the urine or blood at any point on serial monitoring; 2 patients in DL2 without symptoms but also without any detectable BK virus in urine or blood at any timepoint were excluded in case they were unexposed and not at risk for reactivation. Patients who did not engraft, those with follow-up of <100 days because of relapse or NRM (unrelated to BK virus), or with BK virus–associated cystitis/urethritis symptoms at the time of initiation of conditioning for HCT also were excluded. ∗P < .05; ∗∗P < .01.
ID-PTCy–treated patients also had fewer bacterial bloodstream infections with a cumulative incidence at day +100 of 35% compared to 60% for HD-PTCy (P = .15; supplemental Figure 5).
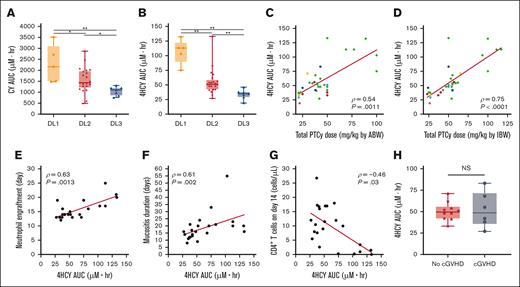
4HCY exposure correlates with the apparent clinical benefits of reduced-dose PTCy
Cyclophosphamide is a prodrug that is activated in the liver, and there may be substantial interpatient variability when using standard weight-based dosing.36 Therefore, we performed cyclophosphamide pharmacokinetics, including measurements of cyclophosphamide and its metabolite 4HCY, which is the principal precursor to cyclophosphamide's cytotoxic metabolite phosphoramide mustard. Generally, cyclophosphamide and 4HCY AUCs showed clear separation between the weight-based dose levels, particularly for 4HCY, but there were notable outliers (Figure 6A-B). Overall, 4HCY exposure appeared to correlate best with PTCy dosing based on IBW rather than ABW (Figure 6C-D; supplemental Table 5). Additionally, 4HCY exposure significantly correlated with the clinical endpoints that were apparent benefits of weight-based PTCy dose reduction, including time to neutrophil and platelet engraftment, duration of mucositis, and CD4+ and CD8+ T-cell recovery, but did not significantly correlate with symptom duration of BK-C (Figure 6E-G; supplemental Figures 6-8). Importantly, there were no differences in 4HCY exposure related to development of cGVHD or mixed T-cell chimerism/graft failure for ID-PTCy–treated patients (Figure 6H; supplemental Figure 9).
Pharmacokinetics of 4HCY correlate with IBW PTCy dosing and the apparent clinical benefits of reduced-dose PTCy. (A-B) The area-under-the-curve (AUC) of cyclophosphamide (CY) and its active metabolite 4HCY were measured for patients in all DLs although 2 DL2 patients had missing samples precluding AUC calculations. ∗P < .05; ∗∗P < .01. (C-D) For each patient, the relationship between PTCy weight-based dose and 4HCY AUC were compared using either the (C) ABW or (D) IBW for the PTCy dose calculation. Spearman correlation coefficients (ρ) and associated P values are shown. Colors reflect the race/ethnicity of individual patients. Green = White/Hispanic; red = White/Non-Hispanic; yellow = African-American; orange = Asian; light blue = American Indian/Alaskan Native; dark blue = multiracial. (E-H) Measured 4HCY exposures significantly correlated with clinical outcomes that appeared superior for ID-PTCy compared with HD-PTCy, including (E) time to neutrophil engraftment, (F) duration of mucositis (oral, pharyngeal, and/or rectal), and (G) absolute number of CD4+ T cells in the blood on day +14. Patients with graft failure, mixed T-cell chimerism, or those receiving tocilizumab (n = 1) were excluded from panels E-G. (H) No difference was seen in 4HCY exposure among evaluable ID-PTCy–treated patients with regard to the development of cGVHD.
Pharmacokinetics of 4HCY correlate with IBW PTCy dosing and the apparent clinical benefits of reduced-dose PTCy. (A-B) The area-under-the-curve (AUC) of cyclophosphamide (CY) and its active metabolite 4HCY were measured for patients in all DLs although 2 DL2 patients had missing samples precluding AUC calculations. ∗P < .05; ∗∗P < .01. (C-D) For each patient, the relationship between PTCy weight-based dose and 4HCY AUC were compared using either the (C) ABW or (D) IBW for the PTCy dose calculation. Spearman correlation coefficients (ρ) and associated P values are shown. Colors reflect the race/ethnicity of individual patients. Green = White/Hispanic; red = White/Non-Hispanic; yellow = African-American; orange = Asian; light blue = American Indian/Alaskan Native; dark blue = multiracial. (E-H) Measured 4HCY exposures significantly correlated with clinical outcomes that appeared superior for ID-PTCy compared with HD-PTCy, including (E) time to neutrophil engraftment, (F) duration of mucositis (oral, pharyngeal, and/or rectal), and (G) absolute number of CD4+ T cells in the blood on day +14. Patients with graft failure, mixed T-cell chimerism, or those receiving tocilizumab (n = 1) were excluded from panels E-G. (H) No difference was seen in 4HCY exposure among evaluable ID-PTCy–treated patients with regard to the development of cGVHD.
Discussion
The broad acceptance of HD-PTCy has resulted from its ability to reduce both severe aGVHD and cGVHD without increasing the risk of relapse.1,8,9,23 This prospective phase 1/2 trial demonstrates that PTCy dose reduction is feasible and effective in patients undergoing myeloablative T-cell–replete HLA-haploidentical bone marrow HCT. In our study, ID-PTCy retained outstanding aGVHD control, and cGVHD rates were similar to those observed in recent studies of myeloablative bone marrow HCT using HD-PTCy.10,38 These two effects combined to result in low immunosuppressive requirements. Importantly, ID-PTCy maintained effective graft-versus-tumor immunity and pathogen-specific immune responses. Indeed, ID-PTCy resulted in lower relapse incidence than expected for the disease risk,34,39 and viral and bacterial infectious complications appeared reduced compared with those seen for our HD-PTCy–treated patients.
For this study, severe aGVHD was chosen as the primary endpoint for two reasons: (1) our preclinical studies supporting PTCy dose reduction used aGVHD models24,25; (2) assessment for cGVHD requires 6-12 months of follow-up, at minimum, and if selected as the primary DLT would have taken several years just to complete the phase 1 dose-deescalation portion of the study. As a result, cGVHD was included as a key secondary endpoint in phase 2 with all patients receiving comprehensive cGVHD assessments, involving routine serial evaluations by multiple subspecialists. Although we cannot rule out a small increase in cGVHD incidence with ID-PTCy compared with HD-PTCy, some patients diagnosed with cGVHD were asymptomatic and most patients developing cGVHD after ID-PTCy did not require systemic immunosuppression. Additionally, no apparent relationship was identified between 4HCY AUCs and cGVHD occurrence (Figure 6H). Nevertheless, given the importance of HD-PTCy’s impact on reducing cGVHD incidence and severity, future comparative phase 2/3 studies should include cGVHD as a component of the primary endpoint.
Despite effective control of GVHD by ID-PTCy, 4 patients had mixed T-cell chimerism. Mixed T-cell chimerism is a known problem with busulfan/fludarabine myeloablative conditioning,40 likely because of incomplete host immunoablation as reflected in our day +3 chimerism results (supplemental Table 4), although it was not seen when combined with HD-PTCy for HLA-matched HCT.41 Notably, we did not observe differences in 4HCY exposure for patients developing graft failure/mixed T-cell chimerism (supplemental Figure 9), suggesting factors other than PTCy dosing contributed to those outcomes. Additionally, 2 of 4 patients with mixed T-cell chimerism and both patients with primary graft failure had T-cell doses in the bone marrow graft in the lowest tertile for the ID-PTCy cohort, which may have been a factor, especially in the setting of persistent host T cells. Encouragingly, interim results from another of our ongoing studies suggest that combining ID-PTCy, sirolimus, and MMF with more lymphodepleting, rather than myeloablative, chemotherapy for HLA-partially-mismatched bone marrow HCT seems to be associated with overall excellent engraftment, including only rare (2.4%) mixed T-cell chimerism, and should be further tested.42
Notably, this is not the first study to use reduced-dose PTCy. Most prior studies have added reduced-dose PTCy to antithymocyte globulin (ATG)-based GVHD prophylaxis after peripheral blood stem cell (PBSC) HCT.43-46 Two PBSC HCT studies without ATG have empirically reduced the dosing of PTCy from 50 mg/kg per day to 40 mg/kg per day on days +3/+4 to improve tolerability, with promising results.47,48 There have been very limited data using 25 mg/kg per day PTCy without ATG,49-51 restricted primarily to small cohorts of patients with diseases poorly tolerant of high-dose chemotherapy.49,50 To our knowledge, this study is novel for the following reasons: (1) it includes pharmacokinetic studies to relate PTCy metabolite exposure with effects of weight-based reduced-dose PTCy; (2) it is the most aggressive effort at PTCy dose reduction without ATG, including the only study to test 25 mg/kg on day +4 only, because our study design was guided by preclinical data showing that PTCy 25 mg/kg per day lessens toxicity and improves aGVHD control24,25; (3) it includes a prospective pilot phase treated identically except with HD-PTCy to provide directly comparative data rather than using historical controls; and (4) it combines reduced-dose PTCy with sirolimus and bone marrow allografts. Our prior work has demonstrated that PTCy’s efficacy is dependent on CD4+ regulatory T cells,2,24,27,28 which may be increased by sirolimus, whereas calcineurin inhibitors (CNI) are toxic to regulatory T cells.27,52 This theoretical consideration, as well as promising results from others53 and our own prior study54 combining HD-PTCy with sirolimus and MMF prompted our inclusion of sirolimus. Although either sirolimus or CNI use appears effective in combination with HD-PTCy,55 it is unclear whether our findings using ID-PTCy also are applicable when a CNI is used rather than sirolimus or when CNI/MMF are initiated before PTCy.1
The purpose of this study was to assess the feasibility of modulating both the timing and dosing of PTCy for HLA-haploidentical bone marrow HCT, but did not comprehensively assess a range of PTCy doses; it is possible that lower or higher dosing than 25 mg/kg per day may be optimal. At the lowest dose level tested in this study (DL3; PTCy 25 mg/kg on day +4 only), in some patients we observed either higher fevers that lasted long beyond engraftment or delays in engraftment, potentially reflective of less controlled bidirectional alloreactivity. These factors led us to proceed to phase 2 with DL2 (ID-PTCy). The small numbers in DL3 preclude definitive conclusions regarding the feasibility of PTCy dosing on day +4 only, but the overall results, including NRM, using this dosing schedule were not encouraging. Moreover, the optimal dosing of PTCy, even when given on days +3 and +4, likely will vary depending on the donor-type (eg, HLA-haploidentical vs HLA-matched) or graft source (eg, bone marrow vs PBSCs) because of differences in graft composition and kinetics of T-cell27,56 and other immune responses and should continue to be rigorously tested in multiple HCT platforms, as we (ClinicalTrials.gov identifier: NCT04959175 and NCT05436418) and others (eg, ClinicalTrials.gov identifier: NCT06001385) are doing in ongoing clinical trials. Given these outstanding questions and lack of randomized data, reduced-dose PTCy should continue to be reserved for use in clinical trials, except for patients with germ line DNA repair defects.49
Encouragingly, not only does PTCy weight-based dose reduction appear to have meaningful clinical benefits, but 4HCY plasma exposure correlated strongly with these apparent benefits. Although the weight-based PTCy dosing used in this study approximated 4HCY exposure for many patients, some patients had aberrant exposures, and these differences appear to have clinical consequences. Personalizing PTCy doses to a target 4HCY exposure is feasible and should be investigated further. But clinical implementation remains challenging because of the short-course of PTCy treatment, the fact that cyclophosphamide can modulate its own metabolism with multiple doses,57 and drug-drug interaction involving PTCy. Moreover, the metabolism of cyclophosphamide is complex, and further research on cyclophosphamide metabolites beyond 4HCY should be performed.57 Meanwhile, weight-based dosing will remain standard, but there is also uncertainty about whether ABW or IBW, the most common methods used for dose calculations, is preferable. Our data would suggest that dosing by IBW most strongly correlated with 4HCY exposure, and if confirmed in larger datasets, should be uniformly implemented.
Although our single-institutional study included a diverse patient population, which would seemingly improve generalizability, our study is limited by the small number of patients within each dose level, especially DL1 and DL3, and also our institution-specific referral patterns, which resulted in a younger and more heavily Latino/a patient population than the “average” HCT patient in the United States. Encouragingly, interim results of our recent two-institutional study using reduced-intensity conditioning with ID-PTCy, including older and medically infirm patients with a racial/ethnic composition closer to the general American populace, have also shown promising GVHD and survival outcomes with ID-PTCy, suggesting these results may be more generalizable.42
HD-PTCy has become a standard-of-care approach to GVHD prophylaxis across both HLA-matched and HLA-partially-mismatched HCT because of severe GVHD prevention without compromising relapse or survival outcomes.1,8,9,23 Yet, increasing data are revealing that HD-PTCy has drawbacks, including delayed engraftment,10 immune reconstitution defects,58 and increased infectious complications.12-18 The results of this study suggest that PTCy dosing can be optimized and that ID-PTCy may address many of these limitations while maintaining GVHD and relapse protection. Ultimately, our outcomes provide strong rationale to undertake prospective randomized studies to directly compare ID-PTCy with HD-PTCy. If our results are validated, ID-PTCy has the potential to lessen the overall burden of HCT on the healthcare system. Furthermore, our data highlight the potential to think beyond population-based improvements in outcomes: identification of an optimal PTCy pharmacokinetic exposure may facilitate dose personalization to maximize GVHD and survival outcomes for all patients.
Acknowledgments
The authors thank Jeremy Rose and other laboratory members who performed T-cell immunophenotyping.
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The pharmacokinetic studies were supported, in part, by a grant from the National Cancer Institute (U01CA239373).
Authorship
Contribution: C.G.K. designed the study; S.M.S. and J.A.K. contributed to the study design; M.A.H., A.D., M.J.M., F.A.F., and C.G.K. gathered and analyzed data; A.D., J.L.S., and A.C. provided research nurse support to ensure execution of the clinical protocol and compliance with regulatory oversight; J.S.M. designed the pharmacokinetic methods and supervised the quantitation and analysis of cyclophosphamide and 4-hydroxycyclophosphamide pharmacokinetics; F.A.F. performed T-cell immunophenotyping; M.A.H., D.D., R.S., M.J.M., C.M., T.E.H., S.N., A.S., J.S., K.R., M.P., J.C.-R., J.A.K., and C.G.K. provided care of patients and assessed endpoints; W.D.F. supervised processing of cyclophosphamide pharmacokinetic samples; H.C.-W. and S.M.S. performed statistical analyses; M.A.H. and C.G.K. prepared figures and wrote the manuscript; and all authors revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher G. Kanakry, Center for Immuno-Oncology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Building 10-CRC, Room 4-3142, 10 Center Dr, Bethesda, MD 20892; email: christopher.kanakry@nih.gov.
References
Author notes
The data sets generated during and/or analyzed during this study are available on reasonable request from the corresponding author, Christopher G. Kanakry (christopher.kanakry@nih.gov).
The full-text version of this article contains a data supplement.