Key Points
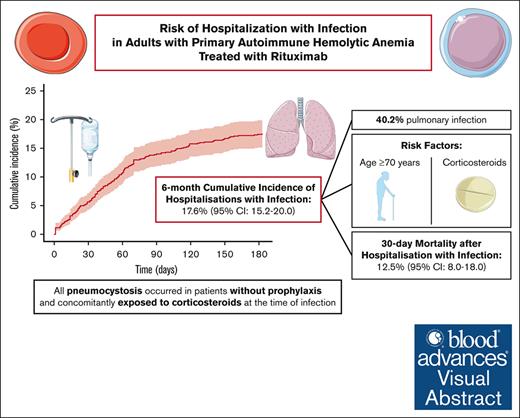
The 6-month cumulative incidence of hospitalization with infection was 18%; after this event, the 30-day mortality was 13%.
All pneumocystosis occurred in patients without prophylaxis and concomitantly exposed to corticosteroids at the time of infection.
Visual Abstract
Autoimmune hemolytic anemia (AIHA) is a rare and sometimes life-threatening disease. Infections are frequent and often severe during the course of AIHA. Rituximab is commonly used to treat patients with AIHA. This study aimed to assess the risk of hospitalization with infection after rituximab in patients with primary AIHA. We selected all adult patients newly diagnosed for primary AIHA and treated with rituximab between 2012 and 2018 in the French national health database. Patients were considered exposed to rituximab within 6 months after the first infusion. The main outcome was hospitalization with infection, identified by a discharge diagnosis of infection during the rituximab exposure. The cohort consisted of 959 patients (mean age of 67 years, standard deviation of 17.8 years; 60.5% of women). The 6-month cumulative incidence of hospitalization with infection was 17.6% (95% confidence interval [CI], 15.2-20.0). The most frequently characterized infections were pulmonary (40.2%). Opportunistic infections were observed in 28 (16.6%) patients, including 11 cases of pneumocystosis. All cases of pneumocystosis occurred in patients concomitantly exposed to corticosteroids, none of them had prophylaxis and all but 2 were aged ≥70 years. Overall, the main factors associated with hospitalization with infection were an age ≥70 years and the exposure to corticosteroids. The 30-day overall mortality after hospitalization with infection was 12.5% (95% CI, 8.0-18.0). In conclusion, the incidence of hospitalizations with infection, including opportunistic infections, as well as the subsequent mortality, are high in adult patients with primary AIHA treated with rituximab. Pneumocystosis prophylaxis should be encouraged in older patients exposed to corticosteroids.
Introduction
Autoimmune hemolytic anemia (AIHA) is a rare autoimmune disease caused by the production of warm or cold autoantibodies directed against red blood cells.1,2 Recently, the incidence of AIHA has been estimated at 2.44 per 100 000 person-years (95% confidence interval [CI], 2.39-2.48) in the AHEAD (Autoimmune Hemolytic Anemia: a population-based study) cohort built using the French national health database.3 AIHA is defined as secondary when associated with an underlying disease such as lymphoma, systemic autoimmune diseases, infection, or primary immune deficiency. Conversely, AIHA is isolated and defined as primary in >50% of cases.2 Patients with primary AIHA are at higher risk of infection in comparison with the general population. In the AHEAD cohort, the 1-year cumulative incidence of hospitalization for infection was 20.9% (95% CI, 19.8-22.0).3 In an Italian multicenter retrospective cohort of 308 adult patients with primary AIHA and followed-up for a median of 33 months, 26 patients had a serious infection, including 4 pneumocystosis and 1 herpes zoster infection.4 In Denmark, 1490 patients with primary AIHA were identified between 1980 and 2016 using the Danish National Patient Register. The 1-year cumulative infection-specific mortality was 1.5% (95% CI, 0.9-2.2) with an adjusted cause-specific hazard-ratio (csHR) of 9.3 (95% CI, 4.8-18.2) in comparison with the general population.5
Rituximab is commonly used off-label as a second-line treatment for warm AIHA (wAIHA), with a 1-year overall response rate of 75%.2,6 It is also the backbone of treatments for cold agglutinin disease (CAD).2 Exposure to rituximab is suspected to be a potential risk factor for infection in this setting, especially for patients concomitantly exposed to corticosteroids. Opportunistic infections may occur in patients exposed to rituximab because B lymphocytes are antigen-presenting cells. However, in patients with primary AIHA treated with rituximab, the risk of infection and notably of opportunistic infections is not known in the real world.
Consequently, this study aimed to: (i) measure the incidence of hospitalization with a diagnosis of infection during the rituximab exposure period in adult patients with primary AIHA; (ii) describe the types of infections; (iii) describe the cases of pneumocystosis; (iv) determine the factors significantly associated with hospitalization with infection; and (v) measure the mortality after hospitalization with infection.
Methods
Data source
The data source was the French national health database (Système national des données de santé [SNDS]). The SNDS is a data source frequently used in pharmacoepidemiology.7,8 This database covers virtually the entire French population (67 million individuals). It links anonymous and individualized data. The SNDS contains sociodemographic data as the date of birth, sex, and the place of residency. It also contains data from the national hospital discharge database for both public and private hospitals, including the dates of hospital stays, all discharge diagnoses coded using the International Classification of Diseases, 10th version (ICD-10), the dispensing of expensive drugs such as rituximab, and procedures such as red blood cell transfusion and splenectomy. Out-of-hospital data include drug dispensing in community pharmacies (coded using the Anatomical Therapeutic Chemical classification) and long-term disease diagnoses recorded by general practitioners and coded using the ICD-10, resulting in a full reimbursement of health care. Finally, the SNDS contains the dates and the causes of death from the national death registry. Detailed medical records, biological data, and nonexpensive drugs dispensed during hospital stays are not registered in the SNDS.
According to the French law, authorizations regarding the AHEAD cohort were obtained from the Comité d’Expertise pour les Recherches, les Études et les Évaluations dans le domaine de la Santé on 11 July 2019 (no. TPS 532004) and the Commission Nationale de l’Informatique et des Libertés on 2 August 2019 (DR-2019-229). The SNDS data are fully anonymized and are not publicly available. The patients cannot be identified in the database. Consequently, according to the French law, informed consent of patients about studies conducted in the SNDS is not possible and therefore not required.
Study population
Patients were selected from the AHEAD cohort, namely a cohort of adult patients with an incident (ie, newly diagnosed) AIHA between 1 January 2012 and 1 July 2018.3 Patients with AIHA were identified using D59.1 (“Autoimmune Hemolytic Anemia”) ICD-10 code as hospital discharge or long-term disease diagnosis. Incident cases were selected using a prior observation period of at least 2 years without the D59.1 diagnosis code. This code yielded a positive predictive value of 90.0% (95% CI, 79.5-96.2) in a previous validation study using clinical data.9 We restricted the study population to the patients exposed to rituximab. Patients were considered exposed to rituximab within 6 months after the first infusion (index date). Patients with hospital discharge or long-term disease diagnoses that cause secondary AIHA between the year before diagnosis of AIHA and the index date were excluded (ICD-10 codes listed in supplemental Table 1).
Outcomes
Hospitalizations with infection were identified through hospital discharge diagnosis codes within the 6 months after the index date (ICD-10 codes listed in supplemental Table 2). These codes yielded a positive predictive value of 97% (95% CI, 93-100) for primary diagnosis codes and 70% (95% CI, 61-71) for related diagnosis codes in a previous validation study using clinical data.10 Hospitalizations with pneumocystosis were identified using the ICD-10 B48.5 and B59 codes.
The occurrence of death was searched within the 30 days after the first occurrence of hospitalization with infection.
Factors associated with infection
Several potential risk factors of infection were considered: age (categorized as 18-69 and ≥70 years), sex, Charlson Comorbidity Index (categorized as <1 and ≥1), red blood cell transfusions before the index date (categorized as <1 and ≥1) as marker of AIHA severity, exposure to corticosteroids, exposure to other immunosuppressive drugs, and splenectomy. Pneumococcal and influenza vaccinations before the index date were also considered.
The Charlson Comorbidity Index was built using the algorithm adapted for the SNDS by Bannay et al.11 Red blood cell transfusion and splenectomy were identified using the French health insurance classification (codes listed in supplemental Table 3). Drug exposure was searched in out-of-hospital drug dispensing data using Anatomical Therapeutic Chemical codes (listed in supplemental Table 4). Only the vaccines dispensed before the index date were considered because vaccinations during the exposure to rituximab have a huge decreased in efficacy.12,13 Patients were considered exposed for 5 years after pneumococcal vaccination and for 1 year after influenza vaccination.
Statistical analysis
Patients were followed-up from the first infusion of rituximab (index date) up to the end of follow-up, that is, the occurrence of hospitalization with infection, death, at the end of the 6-month rituximab exposure period, or 31 December 2018.
The qualitative variables were expressed using numbers and percentages. The quantitative variables were expressed using mean and standard deviation or median and first and third quartiles (Q1 and Q3, respectively) depending on their distribution.
We assessed the cumulative incidence of hospitalizations with infection within 6 months after the index date. The competitive risk of death was included in the calculation. The types of infection related to the first hospitalization were described as well as all cases of pneumocystosis.
The association of the aforementioned factors and the occurrence of the main outcome (hospitalization with infection) over time was assessed using survival analysis. We computed csHR for each variable using Cox regression models. Drug exposure and splenectomy were time-dependent variables. Each day was considered as unit in the counting process approach.14 The assumption of proportional risk was tested for non–time-dependent variables. Sensitivity analyses were conducted by calculating subdistribution hazard ratios using the Fine-Gray model15 with death as competitive risk because of the high mortality rate in patients with AIHA.3,5 Competitive risk of death was considered within 6 months after the index date. We also conducted sensitivity analyses with other hospital stays as time-dependent covariable because previous hospitalizations may affect the risk of infection.
In patients hospitalized with infection, we assessed the cumulative incidence of all-cause death within the 30 days after the date of the admission.
Results
Study population
Between 2012 and 2018, we identified 959 adult patients with an incident primary AIHA treated with rituximab (Figure 1). The total follow-up time was 145 984 person-days (median of 183 days). Patient characteristics are described in Table 1. The mean age was 67 years (standard deviation of 17.8 years), and 581 (60.5%) patients were women. The median time between the diagnosis of AIHA and the first rituximab infusion (index date) was 27 days (Q1, 5 days; Q3, 129 days). Before the index date, 230 (24.0%) patients were vaccinated against pneumococcus within 5 years, and 354 (36.9%) against influenza within 1 year. The mean number of red blood cell transfusions between the diagnosis of AIHA and the index date was 0.9 (minimum of 0; maximum of 43). During the rituximab exposure, 619 (64.5%) patients were exposed at least once to corticosteroids, and 43 (4.5%) to other immunosuppressants. Overall, 87 patients died within the 6 months after the index date, with a cumulative incidence of all-cause death of 9.1% (95% CI, 7.4-11.0). In deceased patients, the median time between the index date and death was 49 days (Q1, 22 days; Q3, 84 days).
Flowchart of the selection of adult patients with incident primary AIHA treated with rituximab.
Flowchart of the selection of adult patients with incident primary AIHA treated with rituximab.
Characteristics of study population: adult patients with incident primary AIHA treated with rituximab
| Variables . | Total population . | Patients hospitalized with infection . | Patients not hospitalized with infection . |
|---|---|---|---|
| (n = 959) . | (n = 169) . | (n = 790) . | |
| Mean age, y (SD) | 67.5 (17.6) | 73.4 (16.5) | 66.3 (17.6) |
| Male sex, n (%) | 378 (39.4) | 70 (41.4) | 308 (39.0) |
| Mean Charlson Comorbidity Index score∗ (SD) | 1.5 (2.5) | 2.2 (2.8) | 1.3 (2.4) |
| Comorbidities, n (%) | |||
| Myocardial infarction | 76 (7.9) | 30 (17.8) | 46 (5.8) |
| Congestive heart failure | 123 (12.8) | 35 (20.7) | 88 (11.1) |
| Peripheral vascular disease | 87 (9.1) | 24 (14.2) | 63 (8.0) |
| Cerebrovascular disease | 81 (8.4) | 23 (13.6) | 58 (7.3) |
| Hemiplegia | 35 (3.6) | 14 (8.3) | 21 (2.7) |
| Dementia | 34 (3.5) | 11 (6.5) | 24 (2.9) |
| Chronic pulmonary disease | 84 (8.6) | 22 (13.0) | 62 (7.8) |
| Diabetes | 180 (18.8) | 46 (27.2) | 134 (17.0) |
| Diabetes with end-organ damage† | 71 (7.4) | 23 (13.6) | 48 (6.1) |
| Moderate or severe renal disease | 96 (10.0) | 24 (14.2) | 72 (9.1) |
| Mild liver disease | 47 (4.9) | 8 (4.7) | 39 (4.9) |
| Moderate or severe liver disease | 22 (2.3) | 6 (3.6) | 16 (2.0) |
| Ulcer disease | 23 (2.4) | 8 (4.7) | 15 (1.9) |
| Tumor | 143 (14.9) | 32 (18.9) | 111 (14.1) |
| Metastatic solid tumor | 21 (2.2) | 5 (3.0) | 16 (2.0) |
| Splenectomy, n (%) | 29 (3.0) | 5 (3.0) | 24 (2.9) |
| Mean red blood cell transfusions‡ (SD) | 0.9 (2.5) | 1.5 (3.9) | 0.8 (2.0) |
| All-cause hospitalization§ | |||
| Mean (SD) | 3.5 (3.6) | 4.8 (3.3) | 3.3 (3.7) |
| Mean cumulative duration, d (SD) | 6.1 (9.0) | 5.6 (8.0) | 6.2 (9.3) |
| All-cause deaths,§ n (%) | 87 (9.1) | 39 (23.1) | 48 (6.1) |
| Drug exposures, n (%) | |||
| Corticosteroids|| | 619 (64.5) | 112 (66.3) | 507 (64.2) |
| Other immunosuppressants|| | 43 (4.5) | 5 (3.0) | 38 (4.8) |
| Pneumococcal vaccination¶ | 230 (24.0) | 30 (17.8) | 200 (25.3) |
| Influenza vaccination¶ | 354 (36.9) | 73 (43.2) | 281 (35.6) |
| Variables . | Total population . | Patients hospitalized with infection . | Patients not hospitalized with infection . |
|---|---|---|---|
| (n = 959) . | (n = 169) . | (n = 790) . | |
| Mean age, y (SD) | 67.5 (17.6) | 73.4 (16.5) | 66.3 (17.6) |
| Male sex, n (%) | 378 (39.4) | 70 (41.4) | 308 (39.0) |
| Mean Charlson Comorbidity Index score∗ (SD) | 1.5 (2.5) | 2.2 (2.8) | 1.3 (2.4) |
| Comorbidities, n (%) | |||
| Myocardial infarction | 76 (7.9) | 30 (17.8) | 46 (5.8) |
| Congestive heart failure | 123 (12.8) | 35 (20.7) | 88 (11.1) |
| Peripheral vascular disease | 87 (9.1) | 24 (14.2) | 63 (8.0) |
| Cerebrovascular disease | 81 (8.4) | 23 (13.6) | 58 (7.3) |
| Hemiplegia | 35 (3.6) | 14 (8.3) | 21 (2.7) |
| Dementia | 34 (3.5) | 11 (6.5) | 24 (2.9) |
| Chronic pulmonary disease | 84 (8.6) | 22 (13.0) | 62 (7.8) |
| Diabetes | 180 (18.8) | 46 (27.2) | 134 (17.0) |
| Diabetes with end-organ damage† | 71 (7.4) | 23 (13.6) | 48 (6.1) |
| Moderate or severe renal disease | 96 (10.0) | 24 (14.2) | 72 (9.1) |
| Mild liver disease | 47 (4.9) | 8 (4.7) | 39 (4.9) |
| Moderate or severe liver disease | 22 (2.3) | 6 (3.6) | 16 (2.0) |
| Ulcer disease | 23 (2.4) | 8 (4.7) | 15 (1.9) |
| Tumor | 143 (14.9) | 32 (18.9) | 111 (14.1) |
| Metastatic solid tumor | 21 (2.2) | 5 (3.0) | 16 (2.0) |
| Splenectomy, n (%) | 29 (3.0) | 5 (3.0) | 24 (2.9) |
| Mean red blood cell transfusions‡ (SD) | 0.9 (2.5) | 1.5 (3.9) | 0.8 (2.0) |
| All-cause hospitalization§ | |||
| Mean (SD) | 3.5 (3.6) | 4.8 (3.3) | 3.3 (3.7) |
| Mean cumulative duration, d (SD) | 6.1 (9.0) | 5.6 (8.0) | 6.2 (9.3) |
| All-cause deaths,§ n (%) | 87 (9.1) | 39 (23.1) | 48 (6.1) |
| Drug exposures, n (%) | |||
| Corticosteroids|| | 619 (64.5) | 112 (66.3) | 507 (64.2) |
| Other immunosuppressants|| | 43 (4.5) | 5 (3.0) | 38 (4.8) |
| Pneumococcal vaccination¶ | 230 (24.0) | 30 (17.8) | 200 (25.3) |
| Influenza vaccination¶ | 354 (36.9) | 73 (43.2) | 281 (35.6) |
SD, standard deviation.
Adapted for the French national health database by Bannay et al.11
All patients in this “end-organ damage” subgroup were also included in the “diabetes” subgroup.
Number of transfusions between the diagnosis of AIHA and the index date (first infusion of rituximab).
During the 6-month rituximab exposure period after the index date (first infusion of rituximab).
Exposed at least once during the 6-month rituximab exposure period after the index date (first infusion of rituximab).
Before the index date (first infusion of rituximab).
Cumulative incidence of hospitalization with infection
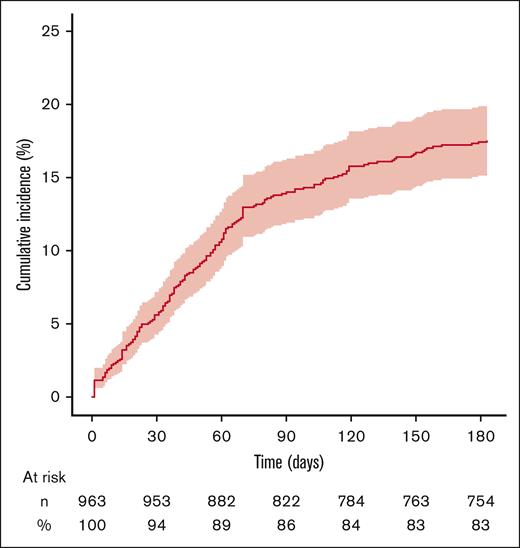
In total, 169 patients were hospitalized with infection. The 6-month cumulative incidence of hospitalization with infection was 17.6% (95% CI, 15.2-20.0; Figure 2). The median time between the index date and the first hospitalization with infection was 43 days (Q1, 19 days; Q3, 70 days). The median duration of this hospital stay was 10 days (Q1, 5 days; Q3, 19 days).
Six-month cumulative incidence of hospitalization with a discharge diagnosis of infection in adult patients with incident primary AIHA treated with rituximab. The vertical axis represents cumulative incidence. The horizontal axis represents time. The colored area represents the 95% CI. Numbers and percentages of patients at risk for the event are indicated below the graph.
Six-month cumulative incidence of hospitalization with a discharge diagnosis of infection in adult patients with incident primary AIHA treated with rituximab. The vertical axis represents cumulative incidence. The horizontal axis represents time. The colored area represents the 95% CI. Numbers and percentages of patients at risk for the event are indicated below the graph.
Types of infection
The sites of first infections are described in Table 2. The most frequently characterized sites were pulmonary (40.2%), urogenital (16.0%), and gastrointestinal (12.4%). Pulmonary infections were more frequent in men, and urogenital infections in women. Opportunistic infections were observed in 28 (16.6%) patients: 11 pneumocystosis, 6 candidiasis (2 sepsis and 4 stomatitis), 5 aspergillosis (3 pulmonary and 2 unspecified), 2 cytomegalovirus, 2 tuberculosis, 2 varicella-zoster virus infections, and 1 nocardiosis. The site of infection could not be characterized using ICD-10 codes for 34 (20.1%) hospitalizations.
Sites of infections among the first hospital stay with a discharge diagnosis of infection within 6 months after rituximab in adult patients with incident primary AIHA
| Sites of infections, n (%) . | Total . | Age groups . | Sex . | Corticosteroids . | |||
|---|---|---|---|---|---|---|---|
| 18-69 y . | ≥70 y . | Female . | Male . | Exposed∗ . | Not exposed . | ||
| Total | 169 (100.0) | 43 (100.0) | 126 (100.0) | 99 (100.0) | 70 (100.0) | 112 (100.0) | 57 (100.0) |
| Pulmonary | 68 (40.2) | 14 (32.6) | 54 (42.9) | 35 (35.4) | 33 (47.1) | 50 (44.6) | 18 (31.6) |
| Urogenital | 27 (16.0) | 4 (9.3) | 23 (18.3) | 23 (23.2) | 4 (5.7) | 19 (17.0) | 8 (14.0) |
| Gastrointestinal | 21 (12.4) | 6 (14.0) | 15 (11.9) | 11 (11.1) | 10 (14.3) | 13 (11.6) | 8 (14.0) |
| Dermatological | 13 (7.7) | 6 (14.0) | 7 (5.6) | 8 (8.1) | 5 (7.1) | 9 (8.0) | 4 (7.0) |
| Equipment | 4 (2.4) | 1 (2.3) | 3 (2.4) | 2 (2.0) | 2 (2.9) | 3 (2.7) | 1 (1.8) |
| Cardiovascular | 3 (1.8) | 1 (2.3) | 2 (1.6) | 1 (1.0) | 2 (2.9) | 1 (0.9) | 2 (3.5) |
| Musculoskeletal | 2 (1.2) | 1 (2.3) | 1 (0.8) | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (3.5) |
| Upper respiratory tract | 2 (1.2) | 0 (0.0) | 2 (1.6) | 1 (1.0) | 1 (1.4) | 1 (0.9) | 1 (1.8) |
| Nervous system | 1 (0.6) | 0 (0.0) | 1 (0.8) | 1 (1.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) |
| Unspecified site† | 34 (20.1) | 10 (23.3) | 24 (19.0) | 20 (20.2) | 14 (20.0) | 21 (18.8) | 13 (22.8) |
| Sites of infections, n (%) . | Total . | Age groups . | Sex . | Corticosteroids . | |||
|---|---|---|---|---|---|---|---|
| 18-69 y . | ≥70 y . | Female . | Male . | Exposed∗ . | Not exposed . | ||
| Total | 169 (100.0) | 43 (100.0) | 126 (100.0) | 99 (100.0) | 70 (100.0) | 112 (100.0) | 57 (100.0) |
| Pulmonary | 68 (40.2) | 14 (32.6) | 54 (42.9) | 35 (35.4) | 33 (47.1) | 50 (44.6) | 18 (31.6) |
| Urogenital | 27 (16.0) | 4 (9.3) | 23 (18.3) | 23 (23.2) | 4 (5.7) | 19 (17.0) | 8 (14.0) |
| Gastrointestinal | 21 (12.4) | 6 (14.0) | 15 (11.9) | 11 (11.1) | 10 (14.3) | 13 (11.6) | 8 (14.0) |
| Dermatological | 13 (7.7) | 6 (14.0) | 7 (5.6) | 8 (8.1) | 5 (7.1) | 9 (8.0) | 4 (7.0) |
| Equipment | 4 (2.4) | 1 (2.3) | 3 (2.4) | 2 (2.0) | 2 (2.9) | 3 (2.7) | 1 (1.8) |
| Cardiovascular | 3 (1.8) | 1 (2.3) | 2 (1.6) | 1 (1.0) | 2 (2.9) | 1 (0.9) | 2 (3.5) |
| Musculoskeletal | 2 (1.2) | 1 (2.3) | 1 (0.8) | 2 (2.0) | 0 (0.0) | 0 (0.0) | 2 (3.5) |
| Upper respiratory tract | 2 (1.2) | 0 (0.0) | 2 (1.6) | 1 (1.0) | 1 (1.4) | 1 (0.9) | 1 (1.8) |
| Nervous system | 1 (0.6) | 0 (0.0) | 1 (0.8) | 1 (1.0) | 0 (0.0) | 1 (0.9) | 0 (0.0) |
| Unspecified site† | 34 (20.1) | 10 (23.3) | 24 (19.0) | 20 (20.2) | 14 (20.0) | 21 (18.8) | 13 (22.8) |
Exposed at least once during the 6-month rituximab exposure period after the index date (first infusion of rituximab).
Hospital discharge diagnosis codes that do not specify the site of infection.
Of note, within the 6 months after the index date, 33 patients had 2 hospitalizations with infection, 12 had 3, 6 had 4, and 1 had 7. The pattern of sites was similar to that of the first hospitalization.
Patients with pneumocystosis
In total, within the 6 months after the index date, 14 (1.4%) patients were hospitalized with pneumocystosis. Patient characteristics are described in Table 3. All cases occurred in patients concomitantly exposed to corticosteroids at the time of infection and without prophylaxis during rituximab exposure. Twelve (85.7%) patients were aged ≥70 years, and 10 (71.4%) were women. The median time between the index date and admission at hospital was 60 days (Q1, 53 days; Q3,107 days). The 30-day cumulative incidence of all-cause death after the admission was 28.6% (95% CI, 8.2-53.4). The median time between hospital admission and death was 11 days (Q1, 10 days; Q3, 13 days).
Characteristics of patients hospitalized with a discharge diagnosis of pneumocystosis within 6 months after rituximab in adult patients with incident primary AIHA
| Patients . | Age, y . | Sex . | Comorbidities . | Red blood cell transfusions,† n . | Corticosteroids exposure . | Pneumocystosis prophylaxis‡ . | Time from rituximab to infection, d . | 30-d all-cause death . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Charlson Comorbidity Index score∗ . | Chronic pulmonary disease . | On the day of infection . | Cumulative, d . | |||||||
| 1 | 49 | F | 0 | No | 0 | Yes | 55 | No | 63 | No |
| 2 | 61 | F | 2 | No | 0 | Yes | 54 | No | 53 | No |
| 3 | 71 | F | 0 | No | 0 | Yes | 42 | No | 56 | No |
| 4 | 72 | F | 0 | No | 0 | Yes | 56 | No | 62 | No |
| 5 | 74 | M | 0 | No | 8 | Yes | 39 | No | 38 | No |
| 6 | 77 | F | 1 | No | 0 | Yes | 53 | No | 59 | No |
| 7 | 78 | F | 0 | No | 0 | Yes | 32 | No | 49 | No |
| 8 | 78 | M | 0 | No | 0 | Yes | 65 | No | 158 | No |
| 9 | 78 | F | 2 | Yes | 2 | Yes | 119 | No | 118 | Yes |
| 10 | 84 | M | 5 | Yes | 8 | Yes | 61 | No | 91 | Yes |
| 11 | 84 | F | 0 | No | 0 | Yes | 95 | No | 119 | No |
| 12 | 86 | M | 0 | No | 0 | Yes | 46 | No | 47 | No |
| 13 | 87 | F | 1 | No | 0 | Yes | 49 | No | 56 | Yes |
| 14 | 87 | F | 1 | No | 0 | Yes | 103 | No | 107 | Yes |
| Patients . | Age, y . | Sex . | Comorbidities . | Red blood cell transfusions,† n . | Corticosteroids exposure . | Pneumocystosis prophylaxis‡ . | Time from rituximab to infection, d . | 30-d all-cause death . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Charlson Comorbidity Index score∗ . | Chronic pulmonary disease . | On the day of infection . | Cumulative, d . | |||||||
| 1 | 49 | F | 0 | No | 0 | Yes | 55 | No | 63 | No |
| 2 | 61 | F | 2 | No | 0 | Yes | 54 | No | 53 | No |
| 3 | 71 | F | 0 | No | 0 | Yes | 42 | No | 56 | No |
| 4 | 72 | F | 0 | No | 0 | Yes | 56 | No | 62 | No |
| 5 | 74 | M | 0 | No | 8 | Yes | 39 | No | 38 | No |
| 6 | 77 | F | 1 | No | 0 | Yes | 53 | No | 59 | No |
| 7 | 78 | F | 0 | No | 0 | Yes | 32 | No | 49 | No |
| 8 | 78 | M | 0 | No | 0 | Yes | 65 | No | 158 | No |
| 9 | 78 | F | 2 | Yes | 2 | Yes | 119 | No | 118 | Yes |
| 10 | 84 | M | 5 | Yes | 8 | Yes | 61 | No | 91 | Yes |
| 11 | 84 | F | 0 | No | 0 | Yes | 95 | No | 119 | No |
| 12 | 86 | M | 0 | No | 0 | Yes | 46 | No | 47 | No |
| 13 | 87 | F | 1 | No | 0 | Yes | 49 | No | 56 | Yes |
| 14 | 87 | F | 1 | No | 0 | Yes | 103 | No | 107 | Yes |
F, female; M, male.
Adapted for the French national health database by Bannay et al.11
Number of transfusions between the diagnosis of AIHA and the index date (first infusion of rituximab).
Exposed at least once during the 6 months after the index date (first infusion of rituximab).
Of note, the characteristics of patients hospitalized with invasive fungal infection are described in supplemental Table 5. Five (71.4%) patients were aged ≥70 years.
Factors associated with hospitalization with infection
In multivariable analyses, an age of ≥70 years (adjusted csHR, 2.74; 95% CI, 1.64-4.58) and a concomitant exposure to corticosteroids (adjusted csHR, 1.59; 95% CI, 1.17-2.17) were the only 2 factors significantly associated with hospitalization with infection (Table 4). Sensitivity analysis led to similar results (supplemental Table 6).
Factors associated with hospitalizations with a discharge diagnosis of infection within 6 months after rituximab in adult patients with incident primary AIHA
| Variables . | Patients, n . | Events, n . | 6-mo cumulative incidence, % (95% CI) . | Univariable Cox model . | Multivariable Cox model . | Multivariable Fine-Gray model . | |||
|---|---|---|---|---|---|---|---|---|---|
| csHR . | 95% CI . | csHR . | 95% CI . | sHR . | 95% CI . | ||||
| Total | 959 | 169 | 18 (15-20) | - | - | - | - | - | - |
| Age, ≥70 y | 530 | 126 | 24 (20-27) | 2.90 | 1.85-4.54 | 2.74 | 1.64-4.58 | 2.12 | 1.10-4.09 |
| Male sex | 378 | 70 | 18 (15-22) | 1.31 | 0.89-1.93 | 1.25 | 0.83-1.89 | 1.19 | 0.69-2.06 |
| Charlson Comorbidity Index score∗ of ≥1 | 438 | 104 | 24 (20-28) | 1.98 | 1.33-2.94 | 1.49 | 0.95-2.33 | 0.80 | 0.46-1.42 |
| Splenectomy | 29 | 5 | 17 (6-33) | 0.73 | 0.19-2.81 | 0.89 | 0.23-3.43 | 1.34 | 0.34-5.39 |
| Red blood cell transfusions,† ≥1 | 297 | 72 | 24 (19-29) | 1.66 | 1.12-2.46 | 1.46 | 0.99-2.16 | 1.06 | 0.62-1.80 |
| Corticosteroids | 619 | 112 | 18 (15-21) | 1.49 | 1.10-2.03 | 1.59 | 1.17-2.17 | 1.52 | 1.00-2.33 |
| Other immunosuppressants‡ | 43 | 5 | 12 (4-23) | 1.21 | 0.36-4.05 | 1.35 | 0.41-4.49 | 2.63 | 0.80-8.61 |
| Pneumococcal vaccination | 230 | 30 | 13 (9-18) | 0.65 | 0.39-1.08 | 0.74 | 0.44-1.23 | 0.86 | 0.45-1.66 |
| Influenzae vaccination | 354 | 73 | 21 (16-25) | 1.32 | 0.89-1.94 | 0.87 | 0.58-1.31 | 0.76 | 0.45-1.29 |
| Variables . | Patients, n . | Events, n . | 6-mo cumulative incidence, % (95% CI) . | Univariable Cox model . | Multivariable Cox model . | Multivariable Fine-Gray model . | |||
|---|---|---|---|---|---|---|---|---|---|
| csHR . | 95% CI . | csHR . | 95% CI . | sHR . | 95% CI . | ||||
| Total | 959 | 169 | 18 (15-20) | - | - | - | - | - | - |
| Age, ≥70 y | 530 | 126 | 24 (20-27) | 2.90 | 1.85-4.54 | 2.74 | 1.64-4.58 | 2.12 | 1.10-4.09 |
| Male sex | 378 | 70 | 18 (15-22) | 1.31 | 0.89-1.93 | 1.25 | 0.83-1.89 | 1.19 | 0.69-2.06 |
| Charlson Comorbidity Index score∗ of ≥1 | 438 | 104 | 24 (20-28) | 1.98 | 1.33-2.94 | 1.49 | 0.95-2.33 | 0.80 | 0.46-1.42 |
| Splenectomy | 29 | 5 | 17 (6-33) | 0.73 | 0.19-2.81 | 0.89 | 0.23-3.43 | 1.34 | 0.34-5.39 |
| Red blood cell transfusions,† ≥1 | 297 | 72 | 24 (19-29) | 1.66 | 1.12-2.46 | 1.46 | 0.99-2.16 | 1.06 | 0.62-1.80 |
| Corticosteroids | 619 | 112 | 18 (15-21) | 1.49 | 1.10-2.03 | 1.59 | 1.17-2.17 | 1.52 | 1.00-2.33 |
| Other immunosuppressants‡ | 43 | 5 | 12 (4-23) | 1.21 | 0.36-4.05 | 1.35 | 0.41-4.49 | 2.63 | 0.80-8.61 |
| Pneumococcal vaccination | 230 | 30 | 13 (9-18) | 0.65 | 0.39-1.08 | 0.74 | 0.44-1.23 | 0.86 | 0.45-1.66 |
| Influenzae vaccination | 354 | 73 | 21 (16-25) | 1.32 | 0.89-1.94 | 0.87 | 0.58-1.31 | 0.76 | 0.45-1.29 |
sHR, subdistribution hazard ratio.
Adapted for the French national health database by Bannay et al.11
Number of transfusions between the diagnosis of AIHA and the index date (first infusion of rituximab).
Azathioprine, cyclosporin, and mycophenolate.
Overall mortality after hospitalization with infection
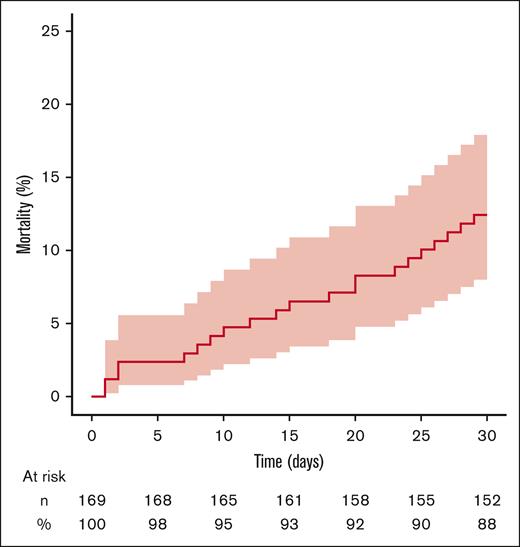
Among the 169 patients hospitalized with infection, the 30-day overall mortality after the first hospitalization with infection was 12.5% (95% CI, 8.0-18.0) (Figure 3). The median time between the hospital admission and death was 15 days (Q1, 8 days; Q3, 24 days).
Thirty-day cumulative incidence of all-cause deaths after hospitalization with a discharge diagnosis of infection in adult patients with incident primary AIHA treated with rituximab. The vertical axis represents cumulative incidence. The horizontal axis represents time. The colored area represents the 95% CI. Numbers and percentages of patients at risk for the event are indicated below the graph.
Thirty-day cumulative incidence of all-cause deaths after hospitalization with a discharge diagnosis of infection in adult patients with incident primary AIHA treated with rituximab. The vertical axis represents cumulative incidence. The horizontal axis represents time. The colored area represents the 95% CI. Numbers and percentages of patients at risk for the event are indicated below the graph.
Discussion
In this nationwide population-based study focused on adult patients with primary AIHA treated with rituximab between 2012 and 2018, we found a 6-month cumulative incidence of hospitalization with infection of 17.6% (95% CI, 15.2-20.0). Among the 169 patients hospitalized with infection, pulmonary infections were by far the most frequent (40.2%) and opportunistic infections were observed in 28 (16.6%) patients. Patients with pneumocystosis were concomitantly exposed to corticosteroids without prophylaxis. Overall, an age of ≥70 years and concomitant exposure to corticosteroids were the main factors associated with hospitalization with infection. The 30-day overall mortality after hospitalization with infection was 12.5% (95% CI, 8.0-18.0).
The median time between the AIHA diagnosis and the first rituximab infusion was <1 month. This is consistent with French clinical practice, which recommends considering rituximab as second line after 2 weeks of corticosteroids in case of insufficient response in patient with wAIHA.16 In this setting, the early used of rituximab was more explained by refractory AIHA or high level corticodependence than an AIHA relapse.
The incidence of serious infection after rituximab was higher in our real-world study than previously reported. In a meta-analysis conducted to assess the effectiveness and safety of rituximab in patients with wAIHA between 2001 and 2013 (21 studies; 409 patients), 18 serious infections were reported, including 1 pneumocystosis.17 The risk of serious infection was also higher in our study than in prospective clinical trials. In the RAIHA randomized trial, 2 serious infections occurred among the 32 patients with AIHA treated with rituximab.6 In a Norwegian multicenter phase 2 trial, 37 courses of rituximab were administered to 27 patients with CAD, and only 1 nonserious infection was observed.18 In a Danish multicenter phase 2 trial, 20 patients with CAD were treated with rituximab, and no infection was reported.19 This difference can be explained by the overselected populations and control for concomitant drug exposures as compared with the real world.
The vaccination coverage measured before rituximab was low in this study (24.0% for pneumococcal vaccination and 36.9% for influenza vaccination). Similar finding has been reported previously in patients with immune thrombocytopenia (ITP) treated with rituximab.20,21 In a retrospective study conducted in a French University Hospital, only 32.4% of 43 patients with ITP treated with rituximab between 1997 and 2010 were vaccinated against pneumococcus.20 In another French nationwide population-based study in the SNDS, 32.4% of 423 patients with primary ITP treated with rituximab between 2009 and 2011 were vaccinated against pneumococcus before rituximab.21 This may be explained, in part, by the relatively urgent need of rituximab for some patients with autoimmune cytopenia.
This study including only patients exposed to rituximab was mainly descriptive and had some limitations because of the data source. Firstly, the use of diagnosis codes for patient selection could lead to a potential selection bias. This risk appears minimal, given the use of codes previously validated on clinical data with an excellent positive predictive value for both AIHA and infection.9,10 In addition, the restriction to patients treated with rituximab makes the selection of other causes of hemolytic anemia very unlikely. Second, some patients with lymphoproliferative disorder or immunodeficiency may have not been identified using ICD-10 codes in the absence of clinical and biological data. This could result in an increased incidence of hospitalization with infection. However, this potential effect remains limited. Indeed, although some indolent lymphoproliferative disorders may not be recorded, hematological malignancies requiring treatment are well coded because they result in high health care expenses and full reimbursement for the patient. Furthermore, undiagnosed immunodeficiencies are rare in adults, especially in older patients (the majority of our study population). Indeed, clinical and biological data on severity of infection were not recorded. Moreover, infections causing hospitalizations and infections occurring during hospitalizations were indistinguishable. However, the mortality after the events was high, reflecting their severity. Potentially, patients with AIHA treated with rituximab have a severe disease, which may partly explain the high mortality. Third, because of the level of precision of ICD-10 codes in the SNDS and the absence of biological data, we could not conduct subgroup analyses by wAIHA vs CAD, for example. Fourth, because hospital dispensing of noncostly drugs are not recorded in the SNDS, some corticosteroid dispensing may have not been captured. However, the sensitivity analysis including hospital stays as time-varying covariable led to similar results. In addition, the mean cumulative duration of all-cause hospital stays was short (6 days) and it was similar between groups with and without the outcome. Fifth, the prescribed dose is not recorded in the SNDS. Consequently, we could not assess the risk function associated with corticosteroids by daily dose or cumulative dose received. However, an increased risk of infection has been reported at any dose with corticosteroids in other autoimmune diseases.22 Lastly, this study aimed to measure the incidence of hospitalization with infection among patients with primary AIHA treated with rituximab. We did not compare this incidence with patient with AIHA treated with other treatments nor with the general population. The incidence of hospitalization with infection has been previously assessed in France in the whole population of patients with AIHA compared with the general population.3 Pneumocystosis was only observed in patients concomitantly treated with corticosteroids. Although the incidence of pneumocystosis was not compared with the incidence in patients exposed to corticosteroids alone (ie, not concomitantly exposed to rituximab), this finding argues for the recommendation of use of prophylaxis in patients coexposed to rituximab and corticosteroids. Of note, previous studies encouraged prophylaxis against pneumocystosis in who do not have HIV exposed to rituximab or corticosteroids for other autoimmune diseases.23,24
In conclusion, although the efficacy of rituximab in the setting of AIHA and especially wAIHA is high and well established, the incidence of hospitalizations with infection and the subsequent mortality are high in adult patients with primary AIHA treated with rituximab. An advanced age and a concomitant exposure to corticosteroids are the main factors associated with hospitalization with infection. The risk of opportunistic infection is not anecdotal. Prior vaccination toward seasonal viral infections and pneumococcus should be strongly encouraged in patients with AIHA who require rituximab, as well as prophylaxis toward pneumocystosis in older patients concomitantly treated with prolonged corticosteroids courses.
Authorship
Contribution: Y.Z., M.L., M.M., M.L.-M., and G.M. designed the study and interpreted the results; Y.Z. conducted the data management and the statistical analyses; Y.Z., M.L., and G.M. wrote the article; and all authors reviewed the manuscript and gave final approval for publication.
Conflict-of-interest disclosure: G.M. received meeting attendance grants from Amgen, Grifols, and Novartis; is coordinator of research studies granted by Amgen, CSL Behring, Grifols, Novartis, and Sanofi; and participated in educational sessions funded by Amgen, Grifols, and Novartis, and on boards for Amgen, Argenx, Novartis, Sanofi, and Sobi. M.M. participated to educational sessions and boards for Amgen, Novartis, UCB, Argenx. The remaining authors declare no competing financial interests.
Correspondence: Yoann Zadro, Service de Médecine interne Le Tallec, Centre Hospitalier Universitaire de Toulouse Purpan, place du Dr Baylac, TSA40031, 31059 Toulouse Cedex 9, France; email: zadro.y@chu-toulouse.fr.
References
Author notes
The Système national des données de santé data may be obtained from a third party and are not publicly available. Importantly, data are fully anonymized. Data may be accessed by submitting a request to the Health Data Hub at https://www.health-data-hub.fr/depot. The data management and statistical analysis code is available on reasonable request from the corresponding author, Yoann Zadro (zadro.y@chu-toulouse.fr).
The full-text version of this article contains a data supplement.