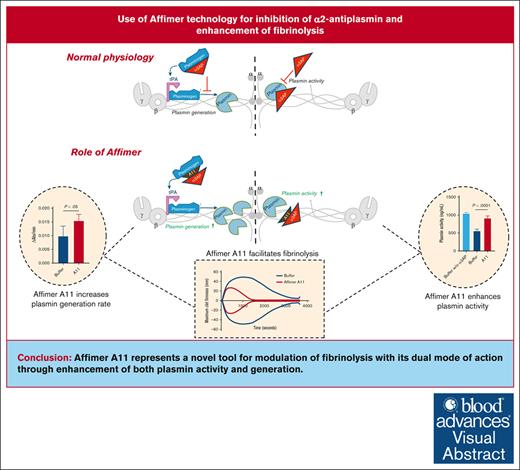
Key Points
Function of antifibrinolytic protein α2AP can be modulated using Affimers, facilitating lysis of clots from plasma and whole blood samples.
α2AP-specific Affimer enhanced clot lysis through a dual mode of action by increasing both plasmin activity and generation.
Visual Abstract
Hypofibrinolysis is a documented abnormality in conditions with high risk of vascular occlusion. A key inhibitor of fibrinolysis is α2-antiplasmin (α2AP), and we hypothesize that the Affimer technology, comprising small conformational proteins with 2 9-amino-acid variable regions, can be used to modulate α2AP activity and facilitate fibrinolysis. Using a phage display system, a library of Affimers was screened against α2AP. A total of 28 α2AP-specific Affimers were isolated, of which 1, termed Affimer A11, inhibited protein function and enhanced fibrinolysis. Affimer A11 displayed a monomeric form and consistently reduced the lysis time of clots made from plasma samples of individuals with type 2 diabetes mellitus (n = 15; from 150.8 ± 100.9 to 109.8 ± 104.8 minutes) and those with cardiovascular disease (n = 15; 117.6 ± 40.6 to 79.7 ± 33.3 minutes; P < .01 for both groups). The effects of A11 on fibrinolysis were maintained when clots were made from whole blood samples. Mechanistic studies demonstrated that A11 did not affect clot structure or interfere with the incorporation of α2AP into fibrin networks but significantly enhanced plasmin activity and accelerated plasmin generation. Affimer A11 reduced α2AP binding to plasmin(ogen), whereas molecular modeling demonstrated interactions with α2AP in an area responsible for binding to plasminogen, explaining the effects on both plasmin activity and generation. Affimer A11, at 0.15 to 0.60 mg/mL, had the ability to bind 70% to 90% of plasma α2AP. In conclusion, we demonstrate that Affimers are viable tools for inhibiting α2AP function and facilitating fibrinolysis, making them potential future therapeutic agents to reduce thrombosis risk.
Introduction
The blood clot is composed of a mesh of fibrin fibers with cellular elements embedded within this network. The structure of the fibrin network and its resistance to fibrinolysis can determine the predisposition to cardiovascular disease (CVD).1 In particular, prolonged fibrin clot lysis is associated with increased vascular risk and is an independent predictor of adverse outcome after acute coronary syndrome.2 Therefore, modulation of the hypofibrinolytic environment represents a credible strategy to reduce residual thrombosis risk in individuals on antiplatelet therapies, consequently improving vascular outcome in high-risk populations.3
Fibrinolytic agents, such as tissue plasminogen activator, have been successfully used in acute vascular occlusions, but these therapies have practical disadvantages. First, the fibrinolytic effects can be difficult to control, leading to bleeding complications. This is related to the mode of action of these agents, which generally target the fibrin network rather than focusing on specific pathways that may offer a better safety profile. Second, the application of fibrinolytic therapies is limited to acute events and cannot be used as a long-term preventive strategy in high vascular risk patients.4-6
A key regulator of fibrinolysis is α2-antiplasmin (α2AP), which is crosslinked into fibrin clots after fibrin network formation.7 Studies have shown increased incorporation of this protein into clots from high vascular risk individuals.8,9 Fibrinolysis inhibition by α2AP results from the direct inhibition of plasmin, although others have also suggested that its interaction with plasminogen may result in reduced plasmin generation, representing an additional mechanism of action.10 Therefore, the inhibition of α2AP has the potential to facilitate fibrinolysis, particularly in conditions associated with increased incorporation of the protein into fibrin clots.7 Indeed, monoclonal antibodies against α2AP have been developed that modulate protein function, and these showed early promise.11 However, the immune response generated in animals for the production of monoclonal antibodies will be directed against immunogenic domains, which do not necessarily represent the key functional areas of the protein. An alternative to antibodies is the Affimer technology that has the ability to pinpoint protein-protein interaction hot “spots.”12-14 The Affimer structure has 2 variable loop regions with 9 amino acid sequences with large diversity (>3 × 1010 randomized peptide library). In addition to the great diversity, Affimers are characterized by high stability, low immunogenicity, and ease of production in large quantities.15 Crucially, the isolation of target protein-specific Affimers does not require animal work and relies on the well-established phage display system. We have previously shown that fibrinogen-specific Affimers can stabilize the fibrin network by reducing susceptibility to lysis, with the potential use of such agents in bleeding disorders.14 However, it is unclear whether the same technology can be used to target less abundant plasma coagulation factors and whether this approach translates into an effect on fibrin clot characteristics.
Given the key role of α2AP in clot lysis and the association between hypofibrinolysis and vascular risk, we hypothesized that the Affimer technology can be used to isolate α2AP-specific Affimers that modulate protein function and enhance fibrinolysis. Our data describe the isolation of an α2AP-specific Affimer that modulates protein function and consequently normalizes the hypofibrinolytic environment in high vascular risk states. We also describe the mode of action of this Affimer and potential interaction sites on α2AP, which may help with novel drug development.
Methods
Isolation of α2AP-specific Affimer
Highly purified human α2AP (Athens Research & Technology, Athens, GA) was biotinylated, and functional studies were performed to ensure the α2AP protein does not lose activity after biotinylation. Phage selections against the target were then performed using an Affimer phage display library based on a human Stefin A–derived scaffold and a screening protocol, as previously described.15,16
After 3 rounds of enrichment, clones with unique sequences were subsequently subcloned into Escherichia coli expression vector (pD861) in BL21 DE3 cells (Thermo Fisher Scientific, Loughborough, United Kingdom). His-tagged Affimers were purified using Ni-NTA agarose (Generon, Slough, United Kingdom), followed by size exclusion to confirm the monomeric nature of the Affimers of interest. Enzyme-linked immunosorbent binding assays were performed to confirm the binding of purified Affimers to α2AP, and a negative control protein was used to confirm the selectivity of the lead clones.
Size exclusion chromatography
Size exclusion chromatography was performed using an ÄKTA pure purification system (GE Healthcare Life Sciences, Little Chalfont, United Kingdom) with a HiLoad 16/600 Superdex column (GE Healthcare Life Sciences). After sample loading and column equilibration, absorbance was monitored at 220, 260, and 280 nm. Eluted fractions with just 1 band corresponding to the Affimer’s molecular weight (therefore monomeric form) were combined and dialyzed overnight at 4°C into 100-mM NaCl, 50-mM Tris, pH 7.4 using Pur-A-Lyzer dialysis tubes (Sigma-Aldrich, Dorset, United Kingdom).
Turbidimetric analysis
ROTEM blood clot analysis
Whole blood clotting analysis was performed as previously described.14 Briefly, citrated blood samples were mixed with Affimer protein in saline solution (0.9% NaCl) and 2.5-nM tissue plasminogen activator (tPA), recalcified by adding Star-tem reagent, and clot formation was initiated by adding Ex-tem reagent. Tests were performed on a rotational thromboelastometry (ROTEM) delta machine (Werfen, Warrington, United Kingdom). Lysis time was determined as the time from maximum clot firmness to the time when maximum clot firmness was reduced by 50%. All blood samples were collected after informed consent, with appropriate ethical approval in place (granted by the University of Leeds Medical School Ethical Committee).
Platelet activation assay
Platelet activation was measured by whole blood flow cytometry as previously described.14 Whole blood samples were incubated with buffer only (phosphate-buffered saline), scaffold-only Affimer control, or A11 (Affimer-to-α2AP molar ratio of 40:1), stimulated with 5-μM thrombin, and stained with platelet marker CD42b-allophycocyanin and activation marker CD62PPE (BD Biosciences, CA) for 20 minutes. Samples were run on a Beckman Coulter CytoFLEX RUO Flow Cytometer and analyzed on FlowJo (v10). Automatic compensation was performed with BD CompBeads (BD Biosciences).
LSCM
Laser scanning confocal microscopy (LSCM) was used to study the potential effects of the Affimer of interest on overall clot structure and the incorporation of α2AP into the clot.
Clot structure studies
LSCM was performed on fully hydrated clots in the presence of increasing concentrations of Affimer proteins and visualized as previously described14 (details in supplemental Methods).
α2AP crosslinking studies
To investigate α2AP incorporation into fibrin clots, the protein was fluorescently labeled using a DyLight Microscale Antibody labeling kit (Thermo Fisher Scientific). Plasma samples (diluted 1:4) were mixed with Affimer A11, scaffold-only Affimer control, or buffer (used at 40:1 Affimer-to-α2AP molar ratio), along with fluorescently labeled fibrinogen (0.09 μΜ; AlexaFluor 594) and fluorescently labeled α2AP (1.6 μΜ; AlexaFluor 488), followed by activation mix (5-mM CaCl2 and 0.05 U/mL thrombin). The mixture was loaded onto an uncoated μ-slide ibidi VI 0.4 (Ibidi GmbH), with all samples prepared in duplicate and visualized using the LSM880 inverted microscope. To quantify α2AP incorporation into plasma clots in the presence of Affimer A11, scaffold-only Affimer control, or buffer, images were split into green (AlexaFluor 488–labeled α2AP) and red (AlexaFluor 594–labeled fibrinogen) channels. The background noise signal of the green image was subtracted before merging the green and red images. The incorporation of α2AP into the clot was determined by measuring the distribution of pixel intensity within fibrin fibers. Areas of clot fiber were superimposed on the green channel (α2AP), and fluorescence intensity from each pixel was measured using color thresholding function on the yellow range (hue 35°-50°). Data were displayed as histograms showing mean pixel intensity. The software package ImageJ (National Institutes of Health, Bethesda, MD) was used in all LSCM analyses.
Incorporation of α2AP into fibrin clots
This was studied by performing a plate-based assay following a modified protocol by Duval et al18 (full details in supplemental Methods).
Scanning electron microscopy
Scanning electron microscopy was used to analyze the potential effect of the Affimer of interest on the fibrin network ultrastructure. Plasma clots were prepared and visualized as previously described14 (details in supplemental Methods).
SPR
To study the binding characteristics of the Affimer of interest, biotinylated α2AP (14 μM in 100-mM NaCl, 50-mM Tris, pH 7.4) was immobilized onto a streptavidin chip using a Biacore 3000 (GE Healthcare Life Sciences). For binding tests, running buffer used was 100-mM NaCl, 50-mM Tris, 0.05% (volume-to-volume ratio [v/v]) Tween 20, pH 7.4, and the Affimer protein was injected for 120 seconds at 50 μL/min (Affimer concentrations 12.5-3200 nM). The surface was regenerated by flowing regeneration buffer (0.1-M sodium carbonate and 0.05% Tween 20) for 15 minutes, sufficient for the response units to return to baseline. All experiments were repeated on 3 different occasions. Data were processed by subtracting sensorgrams from the reference flow cell and a buffer injection over the derivatized surface. Affinities and rate constants for association and dissociation were analyzed using a 1:1 Langmuir binding model with BIAevaluation 3.1 software. Using data from the surface plasmon resonance (SPR) experiments, we calculated binding between the Affimer and α2AP in plasma using a validated online model (binding.streamlit.app).
Competitive inhibition assay
To understand the effects of the Affimer of interest on α2AP-plasmin(ogen) binding, human α2AP at 1 μg/mL in Tris-buffered saline (TBS; 50-mM Tris, 150-mM NaCl, pH 7.4) was coated in Nunc-Immuno MicroWell 96-well plate (Thermo Fisher Scientific) and incubated at 4°C overnight. Wells were washed with TBS containing 0.1% (v/v) Tween 20 and blocked with TBS containing 0.1% (v/v) Tween 20 containing 3% (weight-to-volume ratio) bovine serum albumin for 1 hour at 37°C. A dilution series of Affimer protein (0-5000 nm) was added and incubated with plasminogen (2.5 μg/mL) for 1 hour at 37°C. After being washed, wells were incubated with goat anti–plasminogen-horseradish peroxidase antibody (Enzyme Research Laboratories) for 1 hour at 37°C and then further washed. The reaction was stopped with 1.5-M H2SO4, and the absorbance at 490 nm was read on a Multiscan Go plate reader (Thermo Fisher Scientific).
Plasmin activity and plasmin generation assay
To investigate the direct effect of Affimer on plasmin activity, a commercial plasmin activity assay kit (ab204728; Abcam, Cambridge, MA) was used. Following the manufacturer’s protocol, a mixture of Affimer + α2AP (40:1 Affimer-to-α2AP molar ratio) was preincubated for 20 minutes at room temperature, followed by the addition of plasmin (20 nM) and further incubation for 5 minutes at room temperature (50 μL total volume of sample). A reaction mix containing 49.5-μL plasmin assay buffer and 0.5 μL of plasmin substrate was added to the 50-μL sample mixture, and the output was measured on a fluorescent microplate reader at excitation/emission of 360/450 nm, every 50 seconds, for 1 hour at 37°C, protected from light. Plasmin activity (based on the release of substrate-fluorophore 7-amino-4-methylcoumarin from the peptide substrate- fluorophore 7-amino-4-methylcoumarin complex by plasmin) in samples was interpolated from plasmin standard curve, using 5, 10, 20, 30, 40, and 50 ng/well. Plasmin activity in the test was calculated as the amount of plasmin (ng) per original sample volume (mL).
Molecular modeling
A homology model of Affimer A11 was created using the online homology modeling server Iterative Threading ASSEmbly Refinement.20 Because the Affimer structure is based on the stefin A structure (PBD ID 3KFQ), this crystal structure was chosen as a template. Iterative Threading ASSEmbly Refinement was also used to produce a prediction of the human α2AP protein structure, using the structure of mouse α2AP (protein data bank ID 2R9Y) as a template. Prediction of the binding mode of A11 with the homology model of human α2AP was performed using AutoDock 4.2.21 A total of 100 docking iterations were calculated for each predicted site using a Lamarkian Genetic Algorithm. The resulting poses were clustered, based on a 2 Å root mean squared deviation. The cluster poses with the lowest energy and also the most populated cluster pose (magenta ribbons, Figure 6D) were further examined using PyMOL (PyMOL Molecular Graphics System, Version 2.0; Schrödinger, LLC).
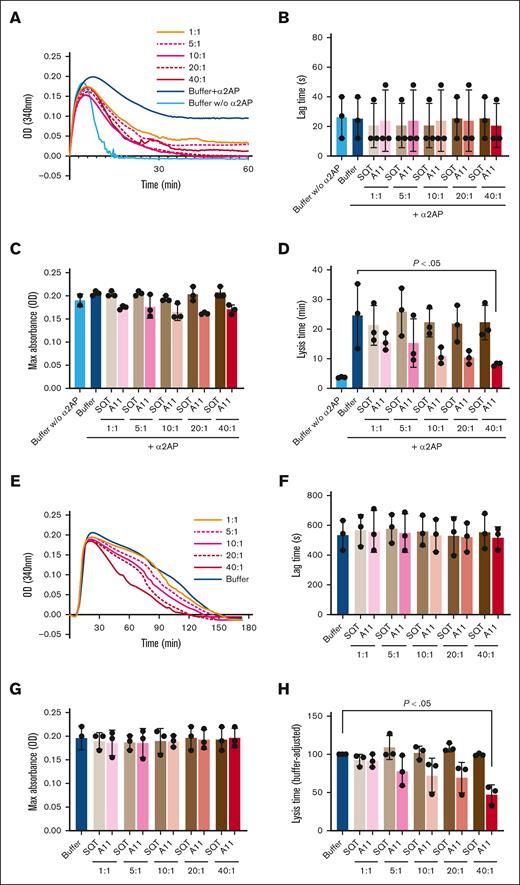
The effect of Affimer A11 on fibrin clot formation and lysis tested in turbidimetric assays. (A) Representative turbidity and lysis curves in a purified system. (B-D) The effect of Affimer A11 and control SQT on lag time (B) and maximum absorbance (C) and clot lysis time (D) of clots made from purified fibrinogen in the presence of α2AP and factor XIII. Numbers depict Affimer-to-α2AP molar ratios. (E) Representative turbidity and lysis curves in plasma. (F-H) The effect of Affimer A11 and SQT on lag time (F), maximum absorbance (G), and lysis time (H) of clots made from pooled human plasma. Data are presented as the mean ± standard deviation (SD) of 3 independent experiments performed in duplicate. One-way analysis of variance (ANOVA) test was used for statistical analysis to compare A11 with buffer only control. Max, maximum; OD, optical density.
The effect of Affimer A11 on fibrin clot formation and lysis tested in turbidimetric assays. (A) Representative turbidity and lysis curves in a purified system. (B-D) The effect of Affimer A11 and control SQT on lag time (B) and maximum absorbance (C) and clot lysis time (D) of clots made from purified fibrinogen in the presence of α2AP and factor XIII. Numbers depict Affimer-to-α2AP molar ratios. (E) Representative turbidity and lysis curves in plasma. (F-H) The effect of Affimer A11 and SQT on lag time (F), maximum absorbance (G), and lysis time (H) of clots made from pooled human plasma. Data are presented as the mean ± standard deviation (SD) of 3 independent experiments performed in duplicate. One-way analysis of variance (ANOVA) test was used for statistical analysis to compare A11 with buffer only control. Max, maximum; OD, optical density.
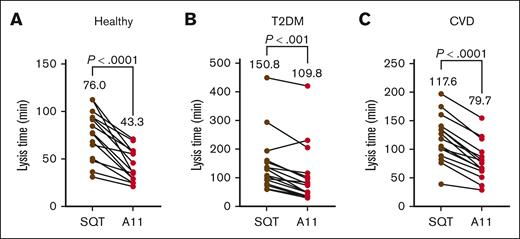
The effect of Affimer A11 on fibrin clot formation and lysis in plasma from different patient populations tested in turbidimetric assays. Clot lysis in healthy controls (A) and individual with T2DM (B) and CVD (C; n = 15 for each group). A 2-tailed, paired Student t test was performed for comparisons of the effect of Affimer A11 with scaffold-only Affimer control protein (SQT). T2DM, type 2 diabetes mellitus.
The effect of Affimer A11 on fibrin clot formation and lysis in plasma from different patient populations tested in turbidimetric assays. Clot lysis in healthy controls (A) and individual with T2DM (B) and CVD (C; n = 15 for each group). A 2-tailed, paired Student t test was performed for comparisons of the effect of Affimer A11 with scaffold-only Affimer control protein (SQT). T2DM, type 2 diabetes mellitus.
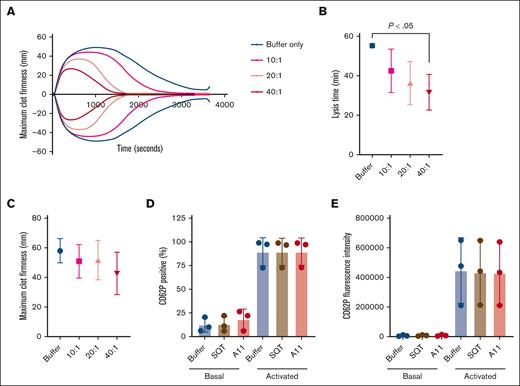
The effect of Affimer A11 on clot formation and lysis in whole blood from 3 healthy individuals using ROTEM assay and on platelet activation. (A-C) Representative trace (A), lysis time (calculated from the time of maximum clot firmness to the time of 50% reduction in maximum clot firmness; (B), and maximum clot firmness (C) from ROTEM experiments. To test the effect of Affimer A11 on platelet activation, blood was incubated with buffer, SQT, or Affimer A11 and then stimulated with thrombin. (D-E) CD62P (P-selectin) was used as platelet activation marker and presented as CD62P expression (percentage of positivity; D) and CD62P mean fluorescence intensity (E). Data are presented as the mean ± SD of 3 independent experiments. One-way ANOVA test was used for statistical analysis to compare A11 with buffer only control.
The effect of Affimer A11 on clot formation and lysis in whole blood from 3 healthy individuals using ROTEM assay and on platelet activation. (A-C) Representative trace (A), lysis time (calculated from the time of maximum clot firmness to the time of 50% reduction in maximum clot firmness; (B), and maximum clot firmness (C) from ROTEM experiments. To test the effect of Affimer A11 on platelet activation, blood was incubated with buffer, SQT, or Affimer A11 and then stimulated with thrombin. (D-E) CD62P (P-selectin) was used as platelet activation marker and presented as CD62P expression (percentage of positivity; D) and CD62P mean fluorescence intensity (E). Data are presented as the mean ± SD of 3 independent experiments. One-way ANOVA test was used for statistical analysis to compare A11 with buffer only control.
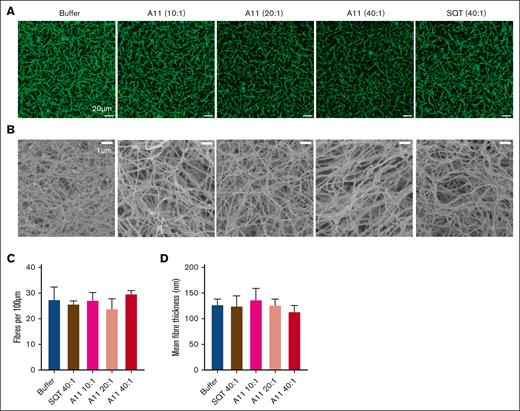
The effect of Affimer A11 in fibrin clot structure. Plasma clots in the presence of Affimer A11 at 10:1, 20:1, and 40:1 Affimer-to-α2AP molar ratio (scaffold-only Affimer and buffer with no Affimer were used as controls) were visualized using LSCM and scanning electron microscopy (SEM). (A) Z stacks (20.30 μm; 30 slices) were compiled to form 3-dimensional (3D) images that are presented here (scale bar, 20 μm). (B) SEM images (scale bar, 1 μm). (C) Fiber density was determined by counting the number of fibers using ImageJ software and presented as the average number of fibers per 100 μm. Two clots were made for each condition, and 3 images were taken in different areas of each clot. (D) Mean fiber thickness of fibers calculated in SEM images using ImageJ software. Two clots were made for each condition, and images were taken in 5 different areas of each clot; 20 fibers were measured in each image. Data presented as mean ± SD. Statistical analysis was performed using 1-way ANOVA; ∗P value < .05 represents difference from buffer control.
The effect of Affimer A11 in fibrin clot structure. Plasma clots in the presence of Affimer A11 at 10:1, 20:1, and 40:1 Affimer-to-α2AP molar ratio (scaffold-only Affimer and buffer with no Affimer were used as controls) were visualized using LSCM and scanning electron microscopy (SEM). (A) Z stacks (20.30 μm; 30 slices) were compiled to form 3-dimensional (3D) images that are presented here (scale bar, 20 μm). (B) SEM images (scale bar, 1 μm). (C) Fiber density was determined by counting the number of fibers using ImageJ software and presented as the average number of fibers per 100 μm. Two clots were made for each condition, and 3 images were taken in different areas of each clot. (D) Mean fiber thickness of fibers calculated in SEM images using ImageJ software. Two clots were made for each condition, and images were taken in 5 different areas of each clot; 20 fibers were measured in each image. Data presented as mean ± SD. Statistical analysis was performed using 1-way ANOVA; ∗P value < .05 represents difference from buffer control.
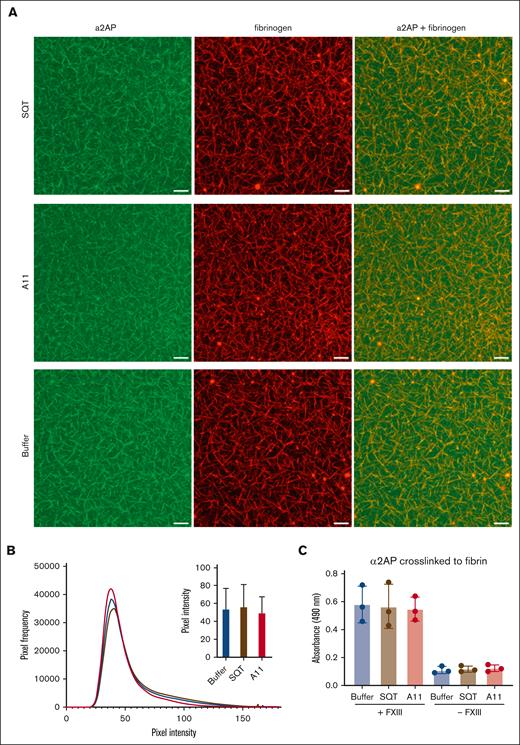
Incorporation of α2AP into the fibrin clot. (A) 3D images of compiled Z stacks of plasma clots made using AlexaFluor 594–labeled fibrinogen (red) and AlexaFluor 488–labeled α2AP (green; scale bar, 20 μm). (B) Histograms of pixel intensity within fibrin fibers demonstrating α2AP incorporation into the clot in the presence of A11, scaffold-only Affimer, or buffer only. (C) α2AP crosslinked to fibrin with or without factor XIII (FXIII) in the presence of Affimer A11, scaffold-only Affimer control protein, or buffer only studied using an enzyme-linked immunosorbent assay. Data presented as the mean ± SD of 3 independent experiments. One-way ANOVA test was used for statistical analysis.
Incorporation of α2AP into the fibrin clot. (A) 3D images of compiled Z stacks of plasma clots made using AlexaFluor 594–labeled fibrinogen (red) and AlexaFluor 488–labeled α2AP (green; scale bar, 20 μm). (B) Histograms of pixel intensity within fibrin fibers demonstrating α2AP incorporation into the clot in the presence of A11, scaffold-only Affimer, or buffer only. (C) α2AP crosslinked to fibrin with or without factor XIII (FXIII) in the presence of Affimer A11, scaffold-only Affimer control protein, or buffer only studied using an enzyme-linked immunosorbent assay. Data presented as the mean ± SD of 3 independent experiments. One-way ANOVA test was used for statistical analysis.
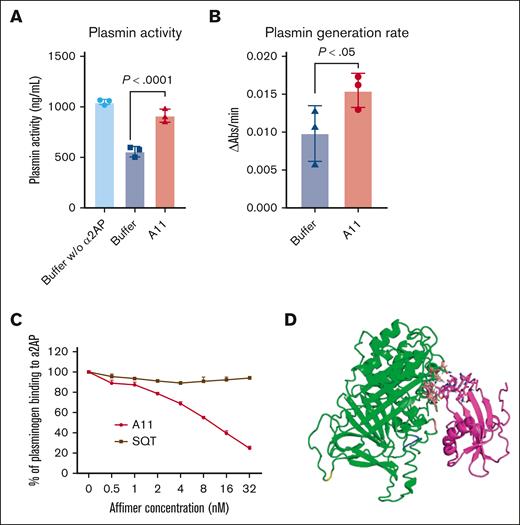
The effect of Affimer A11 in plasmin activity and plasmin generation rate and interaction with plasminogen. (A) Fluorometric plasmin activity assay investigating the effects of α2AP on plasmin activity in the absence and presence of Affimer A11. Data are presented as the mean ± SD of 3 independent experiments. One-way ANOVA test was used for statistical analysis to compare A11 with buffer only control. (B) Chromogenic substrate S2251 hydrolysis assay analyzing plasminogen conversion to plasmin in the absence and presence of Affimer A11. Data are presented as the mean ± SD of 3 independent experiments. One-tailed Mann Whitney test was used for statistical analysis to compare A11 with buffer only control. (C) Competitive inhibition of plasminogen binding to α2AP in the presence of increasing concentrations of Affimer. The absorbance units were converted to the percentage of α2AP-bound plasminogen, with results normalized to the binding of plasminogen in the absence of Affimer. Data are presented as the mean ± SD of 3 independent experiments. (D) Predicted binding pose of Affimer A11 (magenta) with α2AP (green). The 2 loops of A11 are highlighted as sticks; the residues of α2AP interacting with the Affimer are shown as coral sticks, and RGD domain is highlighted blue. Figure produced using PyMOL version 2.5.2.
The effect of Affimer A11 in plasmin activity and plasmin generation rate and interaction with plasminogen. (A) Fluorometric plasmin activity assay investigating the effects of α2AP on plasmin activity in the absence and presence of Affimer A11. Data are presented as the mean ± SD of 3 independent experiments. One-way ANOVA test was used for statistical analysis to compare A11 with buffer only control. (B) Chromogenic substrate S2251 hydrolysis assay analyzing plasminogen conversion to plasmin in the absence and presence of Affimer A11. Data are presented as the mean ± SD of 3 independent experiments. One-tailed Mann Whitney test was used for statistical analysis to compare A11 with buffer only control. (C) Competitive inhibition of plasminogen binding to α2AP in the presence of increasing concentrations of Affimer. The absorbance units were converted to the percentage of α2AP-bound plasminogen, with results normalized to the binding of plasminogen in the absence of Affimer. Data are presented as the mean ± SD of 3 independent experiments. (D) Predicted binding pose of Affimer A11 (magenta) with α2AP (green). The 2 loops of A11 are highlighted as sticks; the residues of α2AP interacting with the Affimer are shown as coral sticks, and RGD domain is highlighted blue. Figure produced using PyMOL version 2.5.2.
Results
Effects of α2AP-specific Affimer on fibrinolysis
Biotinylation of α2AP was confirmed using immunoblotting, enzyme-linked immunosorbent and pull-down assays (data not shown). Importantly, biotinylated protein remained functional, inhibiting fibrinolysis when clots were made from purified fibrinogen (supplemental Figure 1). Upon screening an Affimer-based phage display library, a total of 120 Affimer clones were picked, of which 28 had distinct sequences, and these were subcloned into an expression plasmid for large-scale bacterial expression and purification. Based on the monomeric nature of the α2AP-specific Affimers, selectivity for binding to the target protein, and degree of α2AP inhibitory activity, these 28 Affimers were rank ordered.
One candidate Affimer, termed A11, fulfilled all the above criteria by showing a highly pure monomeric form (supplemental Figure 2), high selectivity to α2AP, and significant inhibition of α2AP protein activity (detailed below).
Affimer A11 enhances fibrin clot lysis using purified proteins and pooled human plasma
To confirm α2AP specificity, clots were made from purified fibrinogen in the presence of α2AP and factor XIII, with and without Affimer A11, which showed a dose-dependent reduction in lysis time without affecting clotting lag time or maximum absorbance (Figure 1A-D). The same range of concentrations of control SQT (scaffold-only Affimer protein without any variable region inserts) showed no effect on clot lysis, maximum absorbance, or lag time in plasma or purified conditions (Figure 1). A11 reduced clot lysis time in pooled human plasma without affecting lag time or maximum absorbance (Figure 1E-H). Furthermore, turbidity and lysis assays were performed, in which the Affimer was not present during clot formation. Affimer A11 marginally reduced the time to 75% lysis when a lysis mix containing plasminogen and tPA was added to preformed clots, despite α2AP being crosslinked into the clot (supplemental Figure 3). Of note, Affimer A11 was tested in murine and bovine plasma and showed no effect on fibrinolysis, indicating high specificity to human protein (supplemental Figure 4).
Interindividual variability in the fibrinolytic effects of Affimer A11
The effect of Affimer A11 was consistent in individual samples from healthy controls (n = 15), significantly enhancing fibrinolysis (Figure 2A) without affecting lag time or maximum absorbance (supplemental Figure 5A,D). Given the potential for clinical application of targeting fibrinolysis to reduce thrombosis, particularly in a hypofibrinolytic environment, the effect of Affimer A11 on clot lysis was investigated in individuals with type 2 diabetes mellitus and individuals with CVD (n = 15 in each group). Affimer A11 consistently decreased fibrin clot lysis time in both patient populations (Figure 2B-C) without significantly affecting lag time or maximum absorbance (supplemental Figure 5B-C,E-F).
Affimer A11 reduces clot lysis using whole blood samples
The effect of A11 on clot lysis was also investigated in human whole blood from 3 healthy donors using ROTEM to confirm that the cellular components do not interfere with the fibrinolytic activity of A11. Affimer A11 decreased lysis time in a dose-dependent manner without causing significant changes in maximum clot firmness (Figure 3A-C). Furthermore, the effect of Affimer A11 on platelet activation was tested using whole blood, and it was shown that Affimer A11 did not affect platelet CD62P expression in basal (resting) or thrombin-activated platelets (Figure 3D-E).
Mechanisms for Affimer A11–induced inhibition of α2AP
Affimer A11 does not alter clot structure
Confocal microscopy was used to study potential A11-induced changes in hydrated clots, whereas electron microscopy was used to investigate clot ultrastructure (Figure 4A-B). Affimer A11 did not affect clot fiber density (Figure 4C) or fiber thickness (Figure 4D), indicating that the addition of A11 to the clot does not result in significant changes to clot structure.
Affimer A11 does not alter α2AP incorporation into the fibrin clot
Plasma clots were visualized in the presence of Affimer A11, scaffold-only Affimer, or buffer using fluorescent-labeled fibrinogen (red) and α2AP (green) to investigate the incorporation of α2AP into the fibrin network (Figure 5A). Measurement of pixel intensity of incorporated α2AP into the fibrin fibers in clots made in the presence of A11 showed no difference compared with control clots (scaffold-only Affimer or buffer; Figure 5B). An additional plate-based α2AP incorporation assay was performed to confirm these findings.18 The amount of α2AP incorporated into the fibrin network (in fibrinogen-coated plates) was measured in the presence of A11, with scaffold-only protein or buffer used as negative controls. The activation mix contained α2AP, thrombin, CaCl2, and factor XIII, whereas α2AP was detected using anti-human α2AP peroxidase-conjugated antibody. In agreement with our findings from confocal experiments, this assay demonstrated no difference in α2AP incorporation when A11 was present compared with scaffold-only Affimer or buffer controls (Figure 5C). These results indicate that the inhibition of α2AP incorporation into the clot is not the mechanism by which Affimer A11 enhances clot lysis.
Effects of Affimer A11 on plasmin activity and generation
AFFIMER A11 ENHANCES PLASMIN ACTIVITY
Because Affimer A11 did not affect α2AP incorporation into the plasma clots, the effect of Affimer A11 on plasmin activity was investigated using a fluorometric assay. We demonstrated that Affimer A11 significantly increased plasmin activity from 556.8 ± 51.53 AU in control samples (buffer) to 913.3 ± 65.94 AU (P <. 0001) at 40:1 Affimer-to-α2AP molar ratio (Figure 6A), whereas the scaffold-only Affimer control protein did not have any effect (data not shown).
AFFIMER A11 ENHANCES THE RATE OF PLASMIN GENERATION
Moreover, the effect of Affimer A11 on plasminogen conversion to plasmin was studied using the chromogenic substrate S2251 assay. This showed that A11 enhanced plasmin generation compared with buffer control (Figure 6B), indicating plasmin generation as an additional mechanism by which A11 enhances fibrinolysis.
AFFIMER A11 INTERFERES WITH PLASMINOGEN-α2AP INTERACTION
Given that Affimer A11 causes an increase in plasmin generation, the interaction between α2AP and plasminogen in the presence of Affimer A11 was investigated. Competitive binding assays showed that increasing concentrations of Affimer A11 reduced the binding of plasminogen to α2AP by up to 80%, compared with scaffold-only Affimer (Figure 6C). Taken together, our data indicate that A11 enhances fibrinolysis not only by enhancing plasmin activity but also by preventing the interaction of plasminogen with α2AP, thereby increasing plasmin generation, which in turn facilitates fibrinolysis.
Molecular modeling to elucidate α2AP-Affimer A11 interaction sites
Examination of the predicted binding poses of A11 with α2AP showed both the lowest energy pose and the most populated pose are in the same region. Moreover, the Affimer loops are predicted to interact with α2AP close to its arginine-glycine-aspartic acid (RGD) domain located at the C-terminal of α2AP, which also contains the lysine-binding sites of plasminogen/plasmin (Figure 6D).
Binding properties of Affimer A11
To further investigate α2AP binding to Affimer A11, SPR studies were performed to determine association/dissociation rates and binding affinity (supplemental Figure 2). The binding affinity for the interaction of 3800 ± 250 nM was then used to determine the fraction of α2AP protein in Affimer ligand-bound state using the binding.streamlit.app. At an Affimer-to-α2AP molar ratio of 40:1 (≈0.6 mg/mL of Affimer), 90% of α2AP is bound to the Affimer (supplemental Figure 6). Even when the Affimer is used at a lower concentration of ≈0.15 mg/mL, >70% of α2AP is bound.
Discussion
Our work demonstrates that the Affimer technology can be successfully used to modulate α2AP function and facilitate fibrinolysis. Inhibition of α2AP by Affimer A11 enhances fibrin clot lysis, and therefore, such a targeted approach may become useful in conditions characterized by hypofibrinolysis and adverse clinical outcome.22,23 However, preclinical in vivo animal work is required before Affimer-based α2AP inhibition is to be considered in future human studies.
Our selection criteria dictated that our Affimer of interest should show α2AP specificity, display significant protein inhibition, and exist in a monomeric form, which makes it suitable as a therapeutic in the future. Affimer A11 complied with these criteria, showing inhibition of α2AP in a system using purified proteins. Importantly, neither plasma proteins nor blood cells affected the enhancement of fibrin clot lysis by Affimer A11. Moreover, this Affimer showed consistency across a large range of plasma samples, indicating that such an approach can be clinically viable. In particular, Affimer A11 reduced clot lysis by 30% to 40% in high-risk populations, including individuals with diabetes and CVD. It should be noted that each 50% increase in clot lysis time is associated with a 36% to 38% increased risk of mortality in the first year after myocardial infarction in individuals on aggressive antiplatelet therapies. This finding is consistent regardless of diabetes status,22,23 and therefore, the effects of Affimer A11 on fibrinolysis are clinically relevant.
Our mechanistic studies demonstrated that Affimer A11 maintained physiological clot structure, further emphasizing the selective mode of action. This is particularly important because previous work from our group has shown that certain fibrinogen-specific Affimers can induce significant and nonphysiological changes in clot structure, making clinical applicability problematic.14
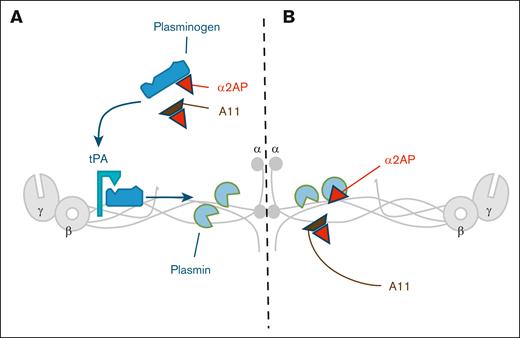
Affimer A11 had no effect on α2AP incorporation into the fibrin clot but directly modulated α2AP-induced inhibition of plasmin activity. An additional mode of action is related to Affimer A11’s ability to enhance plasmin generation. Early work has shown that α2AP can reduce plasmin generation by interfering with plasminogen binding to fibrin.24-26 Others suggested that reduced plasmin generation is related to an indirect process related to limiting the generation of C-terminal lysine residues (due to inhibition of plasmin), which in turn reduces plasminogen binding and plasmin generation.27 This additional mode of action of Affimer A11 is further supported by a competitive inhibition assay revealing that Affimer A11 reduces α2AP binding to plasminogen. Moreover, molecular modeling simulations predicted that Affimer A11 interacts with α2AP at the C-terminal of the protein close to RGD domain. Given that this domain contains the lysine-binding sites of plasminogen and plasmin, these findings are consistent with Affimer A11–induced inhibition of plasmin activity and generation.10 Taken together, our data indicate that Affimer A11 binding to α2AP reduces the ability of the protein to inhibit both plasmin activity and generation, thus making the fibrinolytic process more efficient as depicted in Figure 7.
Schematic representation of Affimer A11 dual mechanism. (A) Affimer A11 increases plasmin production by facilitating plasminogen binding to fibrinogen through modulation of α2AP-plasminogen interaction. (B) Affimer A11 interferes with the suppression of plasmin activity by α2AP.
Schematic representation of Affimer A11 dual mechanism. (A) Affimer A11 increases plasmin production by facilitating plasminogen binding to fibrinogen through modulation of α2AP-plasminogen interaction. (B) Affimer A11 interferes with the suppression of plasmin activity by α2AP.
Our study has a number of strengths, including the highly stringent criteria used to isolate Affimer A11. Moreover, Affimer A11 showed specific α2AP inhibition in clot lysis experiments conducted using purified protein, while also having consistent effects in different plasma and whole blood samples. In addition, this work provides clear mechanistic explanations for the enhancement of fibrin clot lysis by Affimer A11, which reduces the inhibitory effects of α2AP on plasmin activity and generation. However, there are a number of potential criticisms to be acknowledged. It can be argued that the binding affinity of the Affimer is relatively low, but this is not unprecedented28 and is within the range of affinities observed for monoclonal antibodies,29 as well as known physiological interactions.30 Moreover, we show that >90% of plasma α2AP binds Affimer A11 when used at 40 μM (≈0.6 mg/mL), and this, together with the consistent facilitation of fibrinolysis in high vascular risk samples, makes the case for Affimer A11 as an effective modulator of fibrinolysis. The importance of a trade-off between affinity and specificity has been recently elegantly demonstrated,31 whereas others further emphasized the importance of additional trade-offs with solubility and stability, and therefore, very high affinity is not always critically important when attempting to modulate protein activity.32 Another potential criticism is the absence of data to demonstrate that Affimer 11 is effective at reducing thrombosis in vivo. However, Affimer A11 failed to show cross-reactivity with animal samples and was therefore unable to alter clot lysis in these species. Notably, our plasmin activity and generation data, as well as the results of molecular modeling, suggest that Affimer A11 interacts with α2AP close to the RGD domain, which is not conserved between human and mouse protein, consequently explaining the inability of the Affimer to alter fibrinolysis in mice. Therefore, in vivo testing will require humanized animal models, which is a major undertaking and reserved for a new piece of work.
In conclusion, our findings demonstrate that the Affimer technology can be used to inhibit α2AP and modulate fibrinolysis in human samples, particularly in conditions associated with increased thrombosis risk. This, in turn, will offer a novel therapeutic opportunity to improve the hypofibrinolytic environment in individuals at risk of vascular events. Importantly, given the highly targeted approach and the limited increase in endogenous plasmin generation, this strategy has the potential to keep the bleeding risk to a minimum. Further animal in vivo studies analyzing Affimer pharmacokinetics and investigating the antithrombotic effects are key steps for the development of Affimer A11 as a future novel therapeutic.
Acknowledgments
The authors thank Avacta Life Sciences Ltd for performing the screening of Affimers and providing advice and support to this project. The authors also thank Diabetes UK for supporting this work. Biacore experiments were performed in the Wellcome Trust–funded Biomolecular Interactions facility, University of Leeds. The authors thank Lih Cheah for her assistance with the platelet activation assay.
This work was supported by Diabetes UK (14/0005006) and Avacta Life Sciences Ltd.
Authorship
Contribution: N.P. and R.A.A. designed the experiments, interpreted the data, and wrote the manuscript; R.A.A. conceived the project and obtained funding; N.P. performed experiments, analyzed the data, prepared the figures, and drafted the manuscript; B.A. and F.P. performed experiments and analysis of data; K.S. performed molecular modeling; N.K. contributed in the analysis of results; M.A., T.G., N.K., S.P., C.D., R.A.S.A., C.T., and D.C.T. contributed to discussion and interpretation of results; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: D.C.T. is a coinventor of the Adhiron/Affimer technology; the Adhiron/Affimer patent (patent application number PCT/GB2014/050435) is owned by the University of Leeds and licensed to Avacta Life Sciences Ltd. The remaining authors declare no competing financial interests.
Correspondence: Ramzi A. Ajjan, Leeds Institute for Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, LS2 9JT, United Kingdom; email: R.Ajjan@leeds.ac.uk.
References
Author notes
Data are available on request from the corresponding author, Ramzi A. Ajjan (R.Ajjan@leeds.ac.uk).
The full-text version of this article contains a data supplement.