Key Points
There is an identifiable cohort of adults with SCD who have clinical encounters with documented respiratory symptoms without asthma.
Intermittent respiratory symptom status is associated with significantly increased health care utilization.
Visual Abstract
Adults with sickle cell disease (SCD) and asthma have increased mortality and health care utilization; however, there are individuals with respiratory symptoms (including cough and wheeze) without asthma. These individuals may have similar patterns of increased mortality and health care utilization. To characterize the association between respiratory phenotype and health care utilization by adults with SCD. Cross-sectional study of adults with SCD presenting for emergency and inpatient hospital care from 2012 to 2014 in Florida, Iowa, and New York using state-level health care utilization databases. Outcomes of interest included all-cause, SCD-related acute, painful episode, and acute chest syndrome–related care. Respiratory phenotype was defined as SCD + asthma, SCD + respiratory symptoms, and SCD + none. We built multivariable logistic regression and negative binomial regression models to evaluate the association adjusting for demographics, social determinant of health proxies, year of care, and state. Of 29 952 identified individuals, 3.4% had intermittent respiratory symptoms, and a larger proportion (15.6%) had asthma. There was a high rate of inpatient hospitalizations (43%) and emergency department visits (60%). Individuals with asthma had a higher annual risk of inpatient hospitalizations (48% vs 37%) but lower annual risk of an emergency department visit (62% vs 86%) than individuals with intermittent respiratory symptoms. The pattern of increased health care utilization among individuals with intermittent respiratory symptoms was consistent across each utilization type. In this large cohort of adults with SCD, we identified some with intermittent respiratory symptoms who had significantly increased health care utilization. This warrants further evaluation to understand potential etiologies and interventions.
Introduction
Sickle cell disease (SCD) is a multisystemic disorder driven by an underlying pathophysiology of episodic vascular occlusion, ischemia-reperfusion injury, hemolysis with resultant anemia, and chronic inflammation.1-3 In SCD, a physician diagnosis of asthma is an independent risk factor for early mortality and is associated earlier onset and increased rates of acute chest syndrome (ACS), stroke, and painful episodes.4-9 Studies have also identified individuals with SCD who demonstrate respiratory symptoms compatible with asthma (eg, cough, wheeze, and dyspnea) who never fulfill the diagnostic criteria for asthma.8,10-13 Notably, studies of children and adults with SCD have demonstrated evidence of airway hyperresponsiveness at a high rate compared with the general population.7,14-16 Although small studies have shown an association between recurrent wheezing and increased health care utilization, it remains unknown whether episodic respiratory symptoms including cough and wheeze are associated with the same patterns of health care utilization.8,13,17,18 To evaluate the association between intermittent respiratory symptoms and health care utilization among adults with SCD, we conducted a retrospective cross-sectional analysis using state-level health care utilization database. Our prespecified hypothesis was that individuals with SCD and intermittent respiratory symptoms would have higher health care utilization (overall and pain related) than individuals with SCD without a respiratory diagnosis.
Methods
Data source
This study used the State Inpatient Database and State Emergency Department Databases from the Healthcare Utilization Project and maintained by the Agency for Healthcare Research and Quality.19,20 These databases contain detailed encounter-level information on all inpatient hospitalizations and emergency department visits including discharge status, diagnosis and procedure codes, patient demographics, length of stay, and total charges across a given state. The study was reviewed by the Institutional Review Board at the University of Pennsylvania and deemed exempt from review.
Setting
The target population for this study was adults with SCD. Inclusion criteria for the study included at least 1 emergency department (ED) visit or inpatient hospitalization in Florida, Iowa, or New York between 1 January 2012 and 31 December 2014. These states were selected due to the availability of an individual visit linkage variable that allowed for the calculation of individual-level annual health care utilization (ie, annual encounter counts). The visit linkage variable is specific to a single year, which does not permit follow-up of individuals across multiple years within a given state. To identify adults with SCD, we selected encounters with an International Classification of Disease ninth revision–Clinical Modification (ICD-9-CM) diagnosis code for SCD (ICD-9-CM includes 282.6∗, 282.41, and 282.42) at any position on the discharge problem list during the study period, based on previous studies showing the validity of ICD-9-CM diagnosis codes for the detection of SCD.21,22 We excluded encounters involving children (age <18 years at the time of admission) and missing visit linkage markers.
Clinical encounter characterization and health care utilization types
Each clinical encounter was classified based on type (ED only, ED and inpatient admission, or direct inpatient admission). For each clinical encounter, diagnosis codes were screened for the presence of respiratory symptom diagnosis codes (cough, wheeze, dyspnea, and hemoptysis) as well as the presence of diagnostic codes for asthma (ICD-9-CM: 493.XX). Next, each clinical encounter’s health care utilization type was determined based on 3 potentially overlapping categories: acute SCD related, vaso-occlusive crisis related, and ACS related. Acute SCD-related pain was characterized on the basis of specific ICD-9-CM codes previously reported by Brousseau et al.23 Vaso-occlusive event (VOE)-related diagnosis codes were based on the work by Kanter et al (supplemental Table 1).24 ACS was based on the presence of a diagnostic code for ACS (517.3). A clinical encounter could be categorized in multiple health care utilization types based on diagnosis codes.
Exposure of interest: respiratory phenotype
The primary exposure of interest was the respiratory phenotype, a 3-level categorical variable. All individuals in the cohort were classified as having no asthma or respiratory symptoms, intermittent respiratory symptoms, or asthma. Asthma was defined as having ≥1 encounter with a diagnosis code for asthma (ICD-9-CM: 493.XX) at any position on the encounter record. Intermittent respiratory symptoms were defined as having ≥1 encounter with a diagnosis code for respiratory symptoms (supplemental Table 2) without a code for asthma. Individuals were classified as having no respiratory symptoms if they had no encounters with documented respiratory symptoms or asthma in the diagnosis code list.
Covariates of interest
Covariates of interest were grouped into demographics (age and sex) and social determinant of health (SDH) proxy measures (encounter insurance coverage, home county urbanization level, and home zip code median income) and year and state of care. Home county urbanization level was categorized as urban or rural according to the National Center for Health Statistics urban-rural classification schemes.25-27 Encounter insurance coverage was characterized in 4 categories: public (Medicare or Medicaid), private insurance, other/unknown insurance, and uninsured (self-pay or no charge), consistent with prior literature in this population.23
Outcomes
The primary outcome of interest was the annual encounter count (all-cause health care utilization). Secondary outcomes of interest included the various subtypes of health care utilization including SCD-related acute care, painful episode (VOE), or ACS related. Mortality rate was a binary outcome calculated as the number of individuals with a documented death divided by the total number of individuals.23 Outcomes were calculated for the overall cohort as well as stratified by respiratory phenotypes.
Statistical analysis
We used 2-tailed hypothesis tests with a prespecified alpha of 5% (α = 0.05) for all tests. All analyses were conducted using Stata version 16 (College Station, TX). Exposures and outcomes were summarized with frequency tables, with proportions and means/medians when appropriate. The association between exposures and outcomes was first evaluated using Pearson χ2 test and the Fisher exact test (inpatient mortality) performed on crosstabulated exposures and outcomes. Continuous outcomes were evaluated using the Kruskal-Wallis rank-sum test, given the nonnormality of the visit frequency counts, and post hoc pairwise comparisons were conducted using Dunn test.
For each outcome, we conducted a regression analysis to characterize its association with respiratory phenotypes. Our model-building strategy started with the respiratory phenotype as a sole independent predictor and then sequentially added the demographic covariates, SDH covariates, and state/year of care (as fixed effects). We compared the coefficient magnitudes for the respiratory phenotype with and without covariates of interest to evaluate for potential confounding. Parameters were tested for significance using the Wald test. We used logistic regression to evaluate encounter mortality (restricted to individuals who had at least 1 inpatient hospitalization), annual risk of ED visit, and annual risk of inpatient hospitalization outcomes. For annual risk, we used marginal standardization based on the final multivariable models to compute predicted probabilities of at least 1 ED visit or inpatient hospitalization. We then computed marginal predictions of risk by age group category to examine for variations by age group. We used negative binomial regression to evaluate the association between health care utilization counts (all-cause, SCD related, pain, and ACS). We chose negative binomial regression for these outcomes due to overdispersion of encounter counts, which violated the assumption of equal mean and variance required for Poisson regression. We checked model calibration using the Hosmer-Lemeshow goodness of fit statistic.
Results
Cohort description
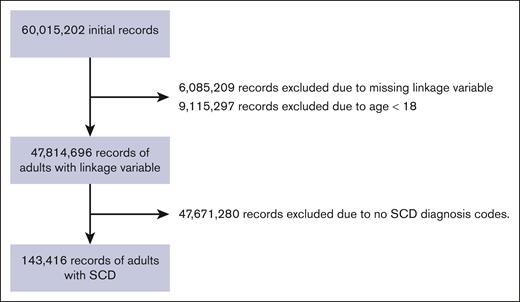
After screening 63 015 202 encounters, a total of 143 416 encounters (representing up to 29 952 individuals) were eligible for inclusion (Figure 1). The median encounter age for the cohort was 31 years (interquartile range [IQR], 24-43). Most individuals came from New York and Florida (n = 28 365 [98.5%]) and were female (n = 19 398 [61%]). Notably, 50% of individuals had home zip codes in the lowest median income quartile (Table 1). Regarding respiratory phenotype, there was a small number of individuals meeting the respiratory symptom phenotype (1030 [3.4%]), with a larger group of individuals with asthma (n = 4661 [15.6%]), although most of the cohort had no respiratory diagnoses (n = 24 261 [81%]).
Cohort description
| . | . | Total . |
|---|---|---|
| N = 29 952 . | ||
| Respiratory phenotype? | No asthma or intermittent respiratory symptoms | 24 261 (81.0%) |
| Intermittent respiratory symptoms | 1 030 (3.4%) | |
| Asthma | 4 661 (15.6%) | |
| Year | 2012 | 9 669 (32.3%) |
| 2013 | 9 885 (33.0%) | |
| 2014 | 10 398 (34.7%) | |
| State | FL | 14 546 (48.6%) |
| IA | 438 (1.5%) | |
| NY | 14 968 (50.0%) | |
| Encounter age, y | 31 (24-43) | |
| Age group | 18-30 y | 14 238 (47.5%) |
| 31-45 y | 9 172 (30.6%) | |
| 46-64 y | 5 282 (17.6%) | |
| 65+ y | 1 260 (4.2%) | |
| Race | White | 1 068 (3.6%) |
| Black | 25 304 (84.5%) | |
| Hispanic | 2 261 (7.5%) | |
| Asian/PI | 128 (0.4%) | |
| American Indian or Alaskan native | 10 (0.0%) | |
| Other | 1 126 (3.8%) | |
| 55 (0.2%) | ||
| Sex | Male | 11 554 (38.6%) |
| Female | 18 398 (61.4%) | |
| Home zip median income | Quartile 1 | 14 977 (50.0%) |
| Quartile 2 | 6 556 (21.9%) | |
| Quartile 3 | 4 375 (14.6%) | |
| Quartile 4 | 3 085 (10.3%) | |
| 959 (3.2%) | ||
| Home urbanization | Urban | 29 088 (97.1%) |
| Rural | 788 (2.6%) | |
| 76 (0.3%) | ||
| Insurance coverage | Medicaid | 12 608 (42.1%) |
| Private insurance | 6 324 (21.1%) | |
| Self-pay | 3 213 (10.7%) | |
| Other | 633 (2.1%) | |
| No charge | 190 (0.6%) | |
| Medicare | 6 984 (23.3%) |
| . | . | Total . |
|---|---|---|
| N = 29 952 . | ||
| Respiratory phenotype? | No asthma or intermittent respiratory symptoms | 24 261 (81.0%) |
| Intermittent respiratory symptoms | 1 030 (3.4%) | |
| Asthma | 4 661 (15.6%) | |
| Year | 2012 | 9 669 (32.3%) |
| 2013 | 9 885 (33.0%) | |
| 2014 | 10 398 (34.7%) | |
| State | FL | 14 546 (48.6%) |
| IA | 438 (1.5%) | |
| NY | 14 968 (50.0%) | |
| Encounter age, y | 31 (24-43) | |
| Age group | 18-30 y | 14 238 (47.5%) |
| 31-45 y | 9 172 (30.6%) | |
| 46-64 y | 5 282 (17.6%) | |
| 65+ y | 1 260 (4.2%) | |
| Race | White | 1 068 (3.6%) |
| Black | 25 304 (84.5%) | |
| Hispanic | 2 261 (7.5%) | |
| Asian/PI | 128 (0.4%) | |
| American Indian or Alaskan native | 10 (0.0%) | |
| Other | 1 126 (3.8%) | |
| 55 (0.2%) | ||
| Sex | Male | 11 554 (38.6%) |
| Female | 18 398 (61.4%) | |
| Home zip median income | Quartile 1 | 14 977 (50.0%) |
| Quartile 2 | 6 556 (21.9%) | |
| Quartile 3 | 4 375 (14.6%) | |
| Quartile 4 | 3 085 (10.3%) | |
| 959 (3.2%) | ||
| Home urbanization | Urban | 29 088 (97.1%) |
| Rural | 788 (2.6%) | |
| 76 (0.3%) | ||
| Insurance coverage | Medicaid | 12 608 (42.1%) |
| Private insurance | 6 324 (21.1%) | |
| Self-pay | 3 213 (10.7%) | |
| Other | 633 (2.1%) | |
| No charge | 190 (0.6%) | |
| Medicare | 6 984 (23.3%) |
Data are presented as median (IQR) for continuous measures and n (%) for categorical measures.
Mortality, health care utilization, and hospitalization outcomes
The overall mortality rate was 1.7% (n = 524 [of 29 952]) and not significantly different across the respiratory phenotype groups (P = .32; Table 2). Overall, the median annual encounter count was 2 clinical encounters (IQR, 1-4; Table 2). The median annual encounter count was highest in the respiratory symptom group (median, 4 [IQR, 2-10]) and lower in the asthma (median, 3 [IQR, 1-7]) and no respiratory symptoms (median, 2 [IQR, 1-3]) groups. The difference in medians was significant overall in the Kruskal-Wallis test (P < .003) and in the post hoc Dunn test (P < .003 in each pairwise comparison). The median length of stay for inpatient hospitalizations was 1 day (IQR, 0-4; Table 2). A similar pattern of health care utilization was seen across other clinical encounter types including SCD-related acute care encounters and painful episode encounters. The median and IQR for ACS encounters was low across all groups.
Annual health care utilization patterns/counts
| . | Total . | No asthma or intermittent respiratory symptoms . | Intermittent respiratory symptoms . | Asthma . | P value . |
|---|---|---|---|---|---|
| N = 29 952 . | n = 24 261 . | n = 1030 . | n = 4661 . | ||
| Died during encounter | 524 (1.7%) | 416 (1.7%) | 15 (1.5%) | 93 (2.0%) | .32 |
| Total encounter count | 2 (1-4) | 2 (1-3) | 4 (2-10) | 3 (1-7) | <.001 |
| Hospitalization count | 0 (0-1) | 0 (0-1) | 0 (0-1) | 1 (0-2) | <.001 |
| Length of stay, d | 1 (0-4) | 1 (0-4) | 0 (0-2.5) | 1.5 (0-4) | <.001 |
| ED visit count | 1 (0-3) | 1 (0-2) | 4 (1-8) | 1 (0-4) | <.001 |
| Acute care utilization | 1 (1-3) | 1 (1-3) | 3 (1-10) | 2 (1-5) | <.001 |
| Painful episode encounter count | 1 (0-3) | 1 (0-2) | 3 (0-8) | 1 (0-4) | <.001 |
| ACS encounter count | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <.001 |
| . | Total . | No asthma or intermittent respiratory symptoms . | Intermittent respiratory symptoms . | Asthma . | P value . |
|---|---|---|---|---|---|
| N = 29 952 . | n = 24 261 . | n = 1030 . | n = 4661 . | ||
| Died during encounter | 524 (1.7%) | 416 (1.7%) | 15 (1.5%) | 93 (2.0%) | .32 |
| Total encounter count | 2 (1-4) | 2 (1-3) | 4 (2-10) | 3 (1-7) | <.001 |
| Hospitalization count | 0 (0-1) | 0 (0-1) | 0 (0-1) | 1 (0-2) | <.001 |
| Length of stay, d | 1 (0-4) | 1 (0-4) | 0 (0-2.5) | 1.5 (0-4) | <.001 |
| ED visit count | 1 (0-3) | 1 (0-2) | 4 (1-8) | 1 (0-4) | <.001 |
| Acute care utilization | 1 (1-3) | 1 (1-3) | 3 (1-10) | 2 (1-5) | <.001 |
| Painful episode encounter count | 1 (0-3) | 1 (0-2) | 3 (0-8) | 1 (0-4) | <.001 |
| ACS encounter count | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | <.001 |
Data are presented as median (IQR) for continuous measures and n (%) for categorical measures.
Health care utilization and age group
The overall annual risk of inpatient hospitalization was 42% (95% confidence interval [CI], 41.5-42.5; Table 3), based on the logistic regression model. The annual risk of inpatient hospitalization was highest in the asthma phenotype (47.6%; 95% CI, 46.4-48.9) and lowest in the intermittent respiratory symptom phenotype (37.2%; 95% CI, 34.6-39.8). Based on age group, the annual risk of inpatient hospitalization was highest in the 65+ years age group (50.2%; 95% CI, 47.7-52.6; Table 3) and lowest in the 31 to 45 years age group (40.4%; 95% CI, 39.5-41.2).
Annual chance (%) of inpatient hospitalization based on respiratory phenotype and age
| Age group . | Overall . | No asthma or intermittent respiratory symptoms . | Intermittent respiratory symptoms . | Asthma . |
|---|---|---|---|---|
| 18-30 | 41.7 (41.0-42.4) | 40.4 (39.7-41.1) | 33.7 (31.2-36.2) | 50.3 (49.0-51.7) |
| 31-45 | 40.4 (39.5-41.2) | 39.1 (38.1-40.0) | 32.4 (29.9-35.0) | 48.9 (47.5-40.4) |
| 46-64 | 43.6 (42.5-44.8) | 42.3 (41.1-43.5) | 35.5 (32.8-38.2) | 52.3 (50.7-53.9) |
| 65+ | 50.2 (47.7-52.6) | 48.9 (46.4-51.3) | 41.8 (38.3-45.4) | 58.9 (56.2-61.6) |
| Overall | 42.0 (41.5-42.5) | 41.1 (40.5-41.6) | 37.2 (34.6-39.8) | 47.6 (46.4-48.9) |
| Age group . | Overall . | No asthma or intermittent respiratory symptoms . | Intermittent respiratory symptoms . | Asthma . |
|---|---|---|---|---|
| 18-30 | 41.7 (41.0-42.4) | 40.4 (39.7-41.1) | 33.7 (31.2-36.2) | 50.3 (49.0-51.7) |
| 31-45 | 40.4 (39.5-41.2) | 39.1 (38.1-40.0) | 32.4 (29.9-35.0) | 48.9 (47.5-40.4) |
| 46-64 | 43.6 (42.5-44.8) | 42.3 (41.1-43.5) | 35.5 (32.8-38.2) | 52.3 (50.7-53.9) |
| 65+ | 50.2 (47.7-52.6) | 48.9 (46.4-51.3) | 41.8 (38.3-45.4) | 58.9 (56.2-61.6) |
| Overall | 42.0 (41.5-42.5) | 41.1 (40.5-41.6) | 37.2 (34.6-39.8) | 47.6 (46.4-48.9) |
Estimates are presented as % point estimate (95% CI).
The annual risk of an ED visit was 61.5% (95% CI, 60.9-62.0; Table 4) based on the logistic regression model. In contrast to inpatient hospitalizations, the annual risk of an ED visit was highest in the intermittent respiratory symptom phenotype (86.3%; 95% CI, 84.1-88.4; Table 4) and similar between the asthma phenotype (62.3%; 95% CI, 60.9-63.7) and the no respiratory symptom phenotype (60.3%; 95% CI, 59.7-60.9). There was a declining annual risk of ED visit based on age group, with the highest annual risk in the 18- to 30-year-old age group (68.0%; 95% CI, 67.2-68.8) and lowest among the 65+ years age group (31.0%; 95% CI, 28.2-33.7).
Annual chance (%) of ED encounter based on respiratory phenotype and age
| Age group . | Overall . | No asthma or intermittent respiratory symptoms . | Intermittent respiratory symptoms . | Asthma . |
|---|---|---|---|---|
| 18-30 | 68.0 (67.2 – 68.8) | 66.8 (66.0-67.7) | 90.2 (88.5-91.8) | 69.1 (67.7-70.5) |
| 31-45 | 60.9 (59.9 - 61.9) | 59.6 (58.5-60.6) | 87.0 (84.8-89.1) | 62.0 (60.3-63.7) |
| 46-64 | 51.9 (50.5-53.2) | 50.4 (49.0-51.8) | 82.1 (79.2-84.9) | 52.9 (51.0-54.9) |
| 65+ | 31.0 (28.2-33.7) | 29.4 (26.7-32.2) | 65.1 (60.0-70.2) | 31.5 (28.4-34.6) |
| Overall | 61.5 (60.9-62.0) | 60.3 (59.7-60.9) | 86.3 (84.1-88.4) | 62.3 (60.9-63.7) |
| Age group . | Overall . | No asthma or intermittent respiratory symptoms . | Intermittent respiratory symptoms . | Asthma . |
|---|---|---|---|---|
| 18-30 | 68.0 (67.2 – 68.8) | 66.8 (66.0-67.7) | 90.2 (88.5-91.8) | 69.1 (67.7-70.5) |
| 31-45 | 60.9 (59.9 - 61.9) | 59.6 (58.5-60.6) | 87.0 (84.8-89.1) | 62.0 (60.3-63.7) |
| 46-64 | 51.9 (50.5-53.2) | 50.4 (49.0-51.8) | 82.1 (79.2-84.9) | 52.9 (51.0-54.9) |
| 65+ | 31.0 (28.2-33.7) | 29.4 (26.7-32.2) | 65.1 (60.0-70.2) | 31.5 (28.4-34.6) |
| Overall | 61.5 (60.9-62.0) | 60.3 (59.7-60.9) | 86.3 (84.1-88.4) | 62.3 (60.9-63.7) |
Estimates are presented as % point estimate (95% CI).
When stratified based on respiratory phenotype and age group, there were 2 notable patterns of health care utilization. First, for inpatient hospitalizations, the annual risk of hospitalizations increased with increasing age but was consistently highest in the asthma phenotype (Table 3). Secondly, with respect to ED visits, the annual risk was similar between the no asthma/intermittent respiratory symptom group and asthma group, with a significantly higher risk in the intermittent respiratory symptom phenotype (70%-90%) and declining risk with increasing age group (Table 4).
Regression analysis
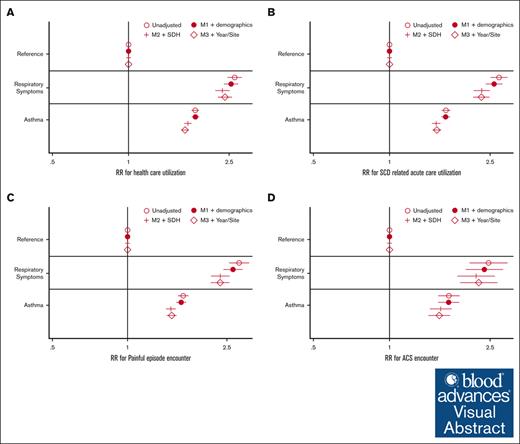
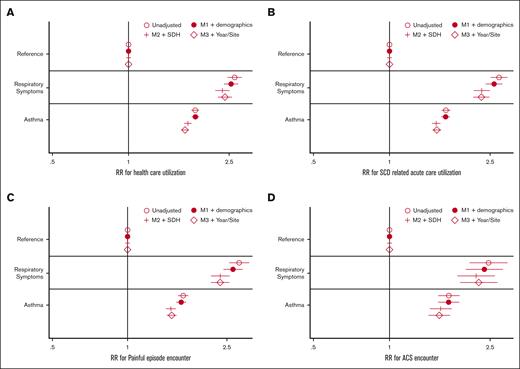
In regression analysis, there was a significant association between the respiratory phenotype and each of the health care utilization outcomes (total encounters, SCD-related acute care, painful episode encounters, and ACS encounters) but not for inpatient mortality. The annual relative risk for each outcome was more than double the risk of the no respiratory diagnosis group (Figure 2). The association between respiratory phenotype remained significant after adjusting for demographic covariates (age and sex), proxies for SDH, year of care, and state.
Regression analysis of health care utilization by respiratory symptoms. (A) Model outcome: all cause health care utilization. (B) SCD-related acute health care utilization. (C) VOE health care utilization. (D) ACS acute care utilization. Estimates are exponentiated coefficients (eg, RR, due to negative binomial regression). Final model includes M3 + fixed effects for year and site (state) of care. M1, unadjusted model (respiratory phenotypes as only covariate); M2, M1 + demographic variables (age, sex, and race); M3, M2 + SDH proxy variables (home median income quartile and home urbanization). RR, relative risk; VOE, vaso-occlusive event.
Regression analysis of health care utilization by respiratory symptoms. (A) Model outcome: all cause health care utilization. (B) SCD-related acute health care utilization. (C) VOE health care utilization. (D) ACS acute care utilization. Estimates are exponentiated coefficients (eg, RR, due to negative binomial regression). Final model includes M3 + fixed effects for year and site (state) of care. M1, unadjusted model (respiratory phenotypes as only covariate); M2, M1 + demographic variables (age, sex, and race); M3, M2 + SDH proxy variables (home median income quartile and home urbanization). RR, relative risk; VOE, vaso-occlusive event.
Discussion
In this large, cross-sectional analysis of adults with SCD presenting for acute care in 3 geographically diverse states, we found a small but identifiable group of individuals with intermittent respiratory symptoms without asthma (3%). There was a significant association between respiratory phenotype and health care utilization, with the intermittent respiratory symptoms phenotype uniformly associated with a higher rate of health care utilization than the other 2 phenotypes. The differences in health care utilization spanned all-cause utilization, SCD-related acute care, painful episode encounters, and ACS clinical encounters. Finally, there was no significant difference in mortality based on respiratory phenotype.
The pulmonary system is particularly at risk due to the underlying pathophysiology of SCD. Studies have shown that children and adults with SCD have abnormalities on pulmonary function testing, including signs of both restrictive and obstructive respiratory disease, as well as airway hyper reactiveness and pulmonary hypertension.1,3,12,14,16,28 Because the pulmonary vasculature carries deoxygenated blood, erythrocytes in this vasculature system are likely to undergo increased sickling due to the lower oxygen tension in pulmonary arterial blood than other arterial systems.
This study confirms and extends prior literature about respiratory symptoms in individuals with SCD.4,5 Our findings are consistent with other small studies in which coughing and wheezing symptoms were found to be independent predictors of ED care in adults with SCD.8,18 Notably, our study shows increased health care utilization across multiple domains of SCD, both pulmonary (eg, ACS encounters) and other (SCD-related acute care and painful episodes) for the intermittent respiratory symptom phenotype, for which a previous study had only demonstrated increased painful episodes and ACS with respiratory symptoms.18
There are 2 main limitations to our findings: risk of misclassification bias and limited individual-level clinical data. Because the data source is an administrative health database being used secondarily, it is possible that diagnoses on the health record do not correlate with individual clinical symptoms. Additionally, it is possible that individuals had chronic health diagnoses such as asthma on outpatient visits, which were not available in this data set, such that individuals we classified as intermittent respiratory symptoms had diagnoses of asthma that we were unable to ascertain. Another diagnostic possibility is that, with the newly implemented race-neutral spirometry classifications, the individuals with intermittent respiratory symptoms will meet the diagnostic criteria for asthma or other chronic pulmonary disorder.29 However, if the intermittent respiratory symptom cohort was primarily composed of individuals with asthma, we would have expected their health care utilization patterns to be more similar. As a cross-sectional analysis, we also have limited ability for causal inference from study data. Because we could not clearly separate exposure and outcome temporally, it remains unclear whether intermittent respiratory symptoms are present before the increased health care utilization. A final risk of our study is that because the data end in 2014, patterns of health care utilization and respiratory symptoms in this population have changed, especially after the advent of the coronavirus pandemic in 2020.
The first major implication of our study is the need for further characterization of respiratory phenotype. In particular, understanding whether individuals with the intermittent respiratory symptom phenotype have underlying pulmonary disease driving their symptoms is an important next step, because it could lead to appropriate therapy when indicated. Respiratory symptoms in SCD could represent a variety of underlying pathology including but not limited to pulmonary inflammation associated with SCD, acute respiratory infections, chronic lung disease, or even local air quality. Secondly, the long-term significance of intermittent respiratory symptoms is important to help guide clinicians as to its significance. For example, the implications of intermittent respiratory symptom phenotype being a clinical prodrome to an eventual diagnosis of asthma are different from the implications of intermittent respiratory symptoms as a sign of viral-induced broncho-constriction or restrictive lung disease. This study suggests that individuals with SCD who present to the ED with respiratory symptoms may benefit from further pulmonary evaluation, although the timing of the evaluation remains unclear.
In summary, there is a small group of adults with SCD who have intermittent respiratory symptoms without an accompanying diagnosis of asthma. These adults have significantly increased annual health care utilization (but not mortality), driven primarily by high emergency department utilization. Future studies should focus on longitudinal assessment of respiratory symptoms in adults with SCD to help provide individual-level data useful for clinicians and patients, consistent with the clinical and research priorities for sickle cell lung disease.
Acknowledgments
The authors thank Meena Bewtra for her assistance and helpful comments and David Buckler and Carmen Vargas-Torres for their help with data cleaning and management.
This work was supported in part through the computational and data resources and staff expertise provided by Scientific Computing and Data at the Icahn School of Medicine at Mount Sinai and supported by the Clinical and Translational Science Awards (grant UL1TR004419) from the National Center for Advancing Translational Sciences. Research reported in this publication was also supported by the Office of Research Infrastructure of the National Institutes of Health (award numbers S10OD026880 and S10OD030463). This work was supported by a grant from the National Heart, Lung, and Blood Institute (NHLBI; 5R01-HL142671). A.A. is supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (3T32DK007740-24S1). M.P.L. receives funding from the NHLBI (K23-HL143042).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors of this study are public or nonprofit organizations that support science in general. They had no role in gathering, analyzing, or interpreting data. The content of this publication does not necessarily reflect the views or polices of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: J.G. and N.N. conceptualized the study; M.P.L. provided access to data; A.A. performed study design and analysis, with support from J.G., C.J., M.P.L., and N.N.; A.A. drafted the initial manuscript; and all authors revised the manuscript for key intellectual content and read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.P.L. is Department of Emergency Medicine, Stanford University, Palo Alto, CA.
The current affiliation for A.A. is Department of Pediatrics, Keck School of Medicine at the University of Southern California, Los Angeles, CA.
Correspondence: Atu Agawu, Department of Pediatrics, University of Southern California, Keck School of Medicine, 4650 Sunset Blvd, Mailstop #78, Los Angeles, CA 90007; email: agawu@usc.edu.
References
Author notes
Presented in abstract form at the 2022 ATS International Conference, San Francisco, CA, 16 May 2022.
The data used for this study are publicly available for purchase from the Agency for Healthcare Research and Quality with use governed by a data use agreement. The code used to create analytic datasets and produce results/figures is available by request.
The full-text version of this article contains a data supplement.