Key Points
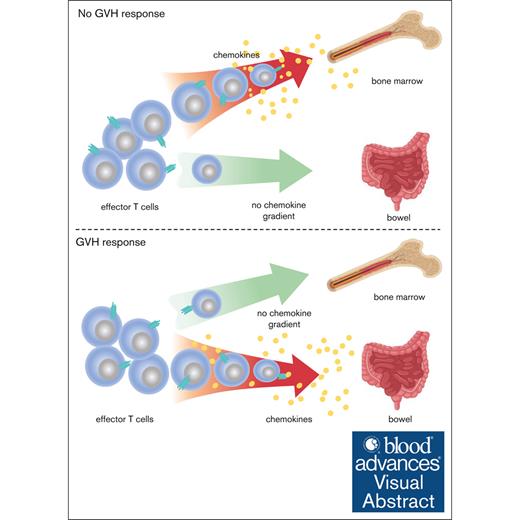
Teff trafficking to BM is chemokine dependent in unmanipulated mice or mice that received transplant without GVHD.
With GVHD, Teff trafficking to the BM loses its chemokine dependence, whereas trafficking to the intestine relies on chemokines.
Visual Abstract
In allogeneic hematopoietic stem cell transplantation (allo-SCT), alloreactive donor T cells mediate the graft-versus-leukemia effect but also attack nonhematopoietic tissues, causing graft-versus-host disease (GVHD). Reducing alloreactive T-cell trafficking to GVHD target tissues while allowing their access to bone marrow (BM) and spleen, major sites of malignant hematopoiesis, is a rational strategy for reducing the GVHD risk when using alloreactive T cells as a therapeutic. Here, we show that effector T-cell (Teff) entry into BM and spleen in unmanipulated mice and in mice that received transplantation without alloreactive T cells is augmented by pertussis toxin (PTX)-sensitive chemokine receptor signaling. However, unexpectedly, in the presence of a GVH response, chemokines no longer draw T cells into BM and spleen but remain critical for their recruitment to GVHD target tissues. Consistent with this, PTX-treated Teff cells were as efficacious as untreated T cells in killing leukemia cells in BM and spleen in mice with a concurrent GVHD response. These results suggest a strategy to improve the safety of alloreactive T-cell therapeutics in treating leukemias in the context of an allo-SCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a potentially curative therapy for patients with hematologic neoplasms, most commonly applied in the treatment of acute myeloblastic leukemia. Much of the efficacy of allo-SCT is due to alloreactive αβT cells in the allograft, which promote donor engraftment and can kill recipient leukemia cells, thereby mediating the graft-versus-leukemia (GVL) effect.1 Unfortunately, alloreactive T cells can also attack normal host tissues, causing graft-versus-host disease (GVHD).2-4 Because of the risk of GVHD, all recipients of T-cell–replete grafts are treated with immunosuppressive agents to diminish the risk and severity of GVHD. Despite alloreactive T cells expanding in all recipients of T-cell–replete grafts, relapsed malignant disease remains the greatest single cause of posttransplant mortality. A rational approach for reducing relapse would be to augment the alloreactive T-cell response. However, if broadly done, such interventions can result in severe GVHD.5
One potential method for enabling alloreactive T cells to mediate GVL with a lower risk of GVHD would be to restrict their trafficking to GVHD target tissues while allowing them to enter sites of malignant hematopoiesis, such as the bone marrow (BM) and spleen. Toward this end, studies have explored the roles of adhesion molecules and chemokine receptors in GVHD, largely by using gene-deficient T cells.6-15 For example, CCR2–/– CD8 cells and CCR6–/– T cells induced less GVHD,15 whereas CCR5–/– T cells induced more severe GVHD at least in part due to impairment of regulatory T-cell trafficking.8,9 Interferon gamma–induced C-X-C Motif Chemokine Receptor 3 (CXCR3) expression on T cells has also been identified as an important step in GVHD induction.14 In many of these studies, despite a reduction in GVHD, some GVL remained intact, supporting the idea that restricting the trafficking of newly expanding alloreactive T cells could be a strategy to widen the therapeutic window.
Here, we used 2-photon intravital microscopy (2PIM) and flow cytometry to explore the role of chemokine receptors on the trafficking of effector CD8 cells in unmanipulated mice and in mice that had undergone an allogeneic BM transplantation (BMT), with or without a concurrent GVHD response.
Materials and methods
Mice
F1 B6×BALB/c mice (CB6F1; CD45.2; H-2Kb/d), were from the Jackson Laboratories (JAX). OT-1 T-cell receptor (TCR) transgenic B6 (C57BL/6-Tg (TcraTcrb)1100Mjb/J; CD45.2; H-2Kb) were purchased from JAX and crossed with B6 Rag1–/– mice and bred at the University of Pittsburgh. All OT-1 mice and cells used were also Rag1–/– and are referred to as “OT-1.” Thy1.1 B6 (B6.PL-Thy1a/CyJ; CD45.2), B6 ubiquitin-green fluorescent protein (GFP) transgenic (B6.ubGFP) and CXCR3–/– B6 (B6.129P2-Cxcr3tm1Dgen/J; CD45.2) mice were purchased from JAX and bred at the University of Pittsburgh. OT-1 Rag1–/–CXCR3–/– mice were generated by crossing OT-1 Rag1–/– mice with CXCR3–/– B6 mice. C57BL/6J (CD45.2) were purchased from Envigo. Studies were approved by University of Pittsburgh IACUC IS00024539.
Creating OT-1 effectors
B6 mice were injected with OT-1 splenocytes (containing 1 × 105 to 5 × 105 OT-1 cells) and immunized with 50 μg of an antibody against DEC205 modified to express ovalbumin16 (laboratory-prepared) and 50 μg of anti-CD40 (clone FGK45; laboratory-prepared). Effectors were harvested from spleens 7 days later. These were purified using EasySep CD8 negative selection kits (STEMCELL Technologies) and enumerated based on staining with antibodies against Vβ5 (OT-1 TCR-β chain), sometimes with anti-Vα2 (OT-1 TCR-α chain), which identifies OT-1 TCR-α chain. Greater than 92% of purified CD8 cells were Vβ5+ (supplemental Figure 1). Nearly all Vβ5+ cells were OT-1 cells based on their staining with a SIINFEKL:Kb MHCI tetramer (supplemental Figure 1).
PTX treatment and labeling
OT-1 cells were suspended in RPMI 1640 at 2 × 107/mL with or without pertussis toxin (PTX; 200 ng/mL; Sigma) for 1 hour at 37°C. After washing with phosphate-buffered saline (ThermoFisher), PTX-treated OT-1 cells were labeled with 5 μg/mL Hoechst 33342 (ThermoFisher) or 5-μM cell trace violet (ThermoFisher), and untreated cells were labeled with 10-μM Cell Tracker Orange CMTMR Dye (ThermoFisher) for 15 minutes at 37°C. Labeled PTX-treated and untreated OT-1 cells were washed and mixed at a 1:1 ratio.
BC-CML induction
Cell purifications
CD4+ and CD8+ T cells were purified from lymph node (LN) and spleen cells using EasySep negative selection reagents according to the manufacturer’s instructions (STEMCELLTechnologies). Cell purities were >90%, with <2% of contaminating CD4 or CD8 cells. Donor BM in all experiments was depleted of T cells using anti-CD90.2 microbeads (EasySep) and is referred to as “BM.”
BMT
Irradiation was from a cesium source. CB6F1 mice received 1000 cGy and were reconstituted with 6 × 106 B6 BM cells, with or without purified B6 CD90.1+ CD4+ (0.5 × 106) and CD8+ T cells (1 × 106). A 1:1 mix of PTX-treated and untreated OT-1 cells (∼5 × 107 of each) were injected on day +14, and mice were imaged 3 hours later. For experiments analyzed by flow cytometry, F1 mice that received transplantation were injected with PTX-treated and untreated OT-1 cells on day +14. Three hours later, mice were injected with anti-CD45 3 minutes before euthanasia to identify intravascular and extravascular cells.
BM chimeras
B6 mice received 2 500 cGy fractions, followed by reconstitution with 5 × 106 B6.ubGFP BM cells. Chimeras were used 40 days later.
2PIM
2PIM is detailed in the supplemental Methods.
Flow cytometry
Flow cytometry was performed using an LSRII or Fortessa (BD). Data were analyzed using FlowJo software (FlowJo X, BD Inc; supplemental Methods).
Cell extractions
Cell extractions are given in supplemental Methods.
In vivo CTL assay
Irradiated CB6F1 mice were reconstituted with B6 BM with or without B6 T cells on day 0. On day +13, fresh BC-CML cells that were or were not pulsed with SIINFEKL were infused. These were distinguished by CMTMR labeling and mixed at a 1:1 ratio. Three hours after BC-CML injection, PTX-treated or untreated effector OT-1 cells (50 × 106 per mouse) were injected. Mice were euthanized 18 hours later for BC-CML enumeration.
Statistics
Bars on scatterplots are mean values. Significance was calculated using an unpaired Student 2-sided t test (GraphPad Prism). Paired 2-sided t tests compared absolute cell numbers between PTX-treated and untreated OT-1 cells in the same mice.
Results
We first investigated the role of chemokine receptors on CD8+ effector T-cell (Teff) entry into the BM extravascular space (site of hematopoiesis) in unmanipulated mice. Our approach was to create OT-1 Rag1–/– CD8+ TCR transgenic (“OT-1”) Teff by immunization and to use these cells, which recognize the ovalbumin-derived SIINFEKL epitope presented by Kb, as a probe for early Teff trafficking. We chose OT-1 cells because they do not recognize any antigen in B6 mice, thereby eliminating the contribution antigen might make on tissue distribution19 or Teff division, allowing for a cleaner interpretation as to the role of chemokine receptors on trafficking. Using them crossed to a Rag1–/– background assured that OT-1 Teff only expressed the OT-1 TCR, preventing unexpected reactivities from endogenous nontransgenic TCR-α or TCR-β chains. To create OT-1 effectors, we transferred CD45.2 OT-1 splenocytes (containing 1 × 105 to 5 × 105 OT-1 cells) into B6 CD45.1 mice followed by an anti-DEC205 antibody genetically modified to express ovalbumin, along with the anti-CD40 antibody FGK45 as an adjuvant.16 OT-1 effectors were harvested 7 days later, and CD8 cells were purified. Due to the expansion of OT-1 cells, >95% of harvested CD8 cells were OT-1 cells (supplemental Figure 1). To impair chemokine receptor function, effector CD8 cells were treated ex vivo with PTX, which enzymatically impairs Gαi protein–coupled chemokine receptor signaling.20,21
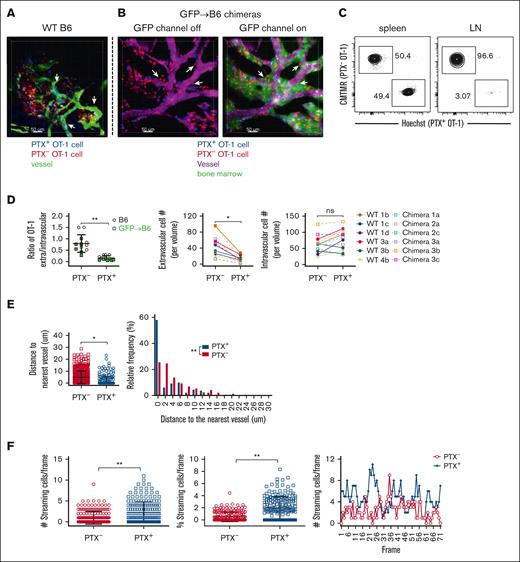
We first explored the role of chemokine receptors in the trafficking of Teff to the BM of unmanipulated mice. OT-1 effectors were PTX treated or untreated, differentially labeled with fluorescent dyes, and coinfused IV into unmanipulated B6 mice (5 × 107 of each). Three hours later, mice were injected with dextran-Fluorescein isothiocyanate (FITC) or Q-dot655 to visualize blood vessels and then subjected to imaging (Figure 1A; supplemental Video 1). The effectiveness of PTX treatment was confirmed by the near complete exclusion of PTX-treated OT-1 effectors from LN but not from spleen (Figure 1C). We enumerated OT-1 cells in imaged volumes in which at least some OT-1 cells were extravascular and had migrated to regions not contiguous with blood vessels. OT-1 cells were classified as being intravascular or extravascular using Imaris software, and the ratios of extravascular to intravascular (E/I) OT-1 cells were calculated. E/I ratios were higher for untreated (PTX–) than for PTX-treated (PTX+) OT-1 cells, and this difference was driven by fewer PTX+ cells being extravascular (Figure 1D), demonstrating a role for PTX-sensitive chemokine receptors in OT-1 effector egress into the marrow space.
Teff cell entry into BM of unmanipulated mice is chemokine receptor-dependent. B6 mice (A; supplemental Video 1) or B6.ubGFP→ B6 BM chimeric mice (B; supplemental Video 2) were IV injected with untreated (red) or PTX-treated (blue) OT-1 effectors. Three hours later, mice were injected with dextran-FITC (A) or Qdot655 (B) to visualize blood vessels and then underwent 2PIM imaging of the calvarium. Representative images (A-B). Arrows highlight extravascular OT-1 cells adjacent to blood vessels. Scale bar represents 50 μm. PTX-treated OT-1 cells were excluded from LN but not spleen (C). PTX treatment reduced the E/I ratio of OT-1 effectors, the absolute number of extravascular OT-1 effectors (D), and the distance extravascular OT-1 cells traveled from the nearest vessel (E). (F) Rapidly moving (streaming) intravascular OT-1 cells (supplemental Video 3) were more numerous and more frequent among PTX-treated cells. Number of streaming cells per frame (left panel); the percentages of PTX– and PTX+ cells that were streaming (middle panel); the numbers of streaming PTX+ and PTX– cells per image frame (right panel). (D-E) Data from 3 nonchimeric and 3 chimeric mice with a total of 12 imaged volumes. (F) Data from 2 mice with 2 imaged volumes. Significance was determined by a 2-tailed Student t test. ∗P < .001; ∗∗P < .0001. ns, not significant.
Teff cell entry into BM of unmanipulated mice is chemokine receptor-dependent. B6 mice (A; supplemental Video 1) or B6.ubGFP→ B6 BM chimeric mice (B; supplemental Video 2) were IV injected with untreated (red) or PTX-treated (blue) OT-1 effectors. Three hours later, mice were injected with dextran-FITC (A) or Qdot655 (B) to visualize blood vessels and then underwent 2PIM imaging of the calvarium. Representative images (A-B). Arrows highlight extravascular OT-1 cells adjacent to blood vessels. Scale bar represents 50 μm. PTX-treated OT-1 cells were excluded from LN but not spleen (C). PTX treatment reduced the E/I ratio of OT-1 effectors, the absolute number of extravascular OT-1 effectors (D), and the distance extravascular OT-1 cells traveled from the nearest vessel (E). (F) Rapidly moving (streaming) intravascular OT-1 cells (supplemental Video 3) were more numerous and more frequent among PTX-treated cells. Number of streaming cells per frame (left panel); the percentages of PTX– and PTX+ cells that were streaming (middle panel); the numbers of streaming PTX+ and PTX– cells per image frame (right panel). (D-E) Data from 3 nonchimeric and 3 chimeric mice with a total of 12 imaged volumes. (F) Data from 2 mice with 2 imaged volumes. Significance was determined by a 2-tailed Student t test. ∗P < .001; ∗∗P < .0001. ns, not significant.
In some volumes, OT-1 cells were immediately outside and abutting the vessel (see arrows in Figure 1A). We were unsure as to whether these were areas without marrow (ie, bone or cartilage) or with only a small, nearly 1-cell diameter thick marrow volume. To better define these areas, we repeated OT-1 migration experiments with B6.ubGFP→B6 BM chimeras in which only hematopoietic cells were GFP+. In these chimeras, extravascular OT-1 cells were only present in volumes with surrounding GFP+ cells, although the volumes could be small (Figure 1B; supplemental Video 2). The locations (extravascular or intravascular) of PTX-treated and untreated cells in these chimeras were similar as those in nonchimeric B6 mice (Figure 1D).
Although PTX treatment retarded OT-1 entry into extravascular marrow, some PTX-treated cells were nonetheless extravascular. These were closer to the nearest blood vessel than untreated OT-1 cells, suggesting that, after egress, chemokine gradients continued to affect OT-1 cell positioning (Figure 1E). We also used 2PIM to analyze OT-1 cell motility. Some intravascular OT-1 cells streamed relatively rapidly such that within a given frame they occupied more than 1 location and traveled long distances between scans (“streaming” cells; supplemental Video 3). These streaming cells were more common among PTX+ OT-1 cells (Figure 1F), suggesting that PTX treatment reduced the likelihood that they would stably adhere to vascular endothelial cells.20,22 Other intravascular OT-1 cells moved more slowly (PTX treated or untreated), presumably in contact with the endothelium (supplemental Video 4), with some cells crawling against the direction of blood flow as has been observed for monocytes23 (supplemental Video 5).
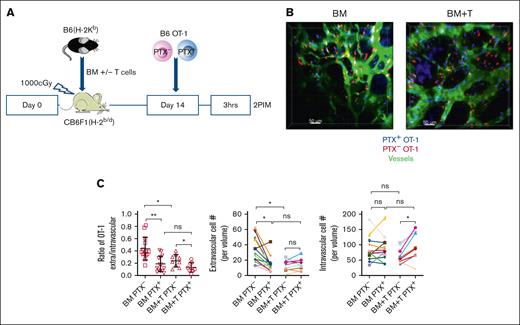
We next examined the impact of an allogeneic BMT, with or without a GVH response, on effector OT-1 trafficking. To do so, irradiated F1(B6×BALB/c) recipient (CBF1) mice were reconstituted with B6 BM, with or without unmanipulated B6 T cells (0.5 × 106 CD4+ and 1 × 106 CD8+ T cells). On day +14, PTX-treated and untreated OT-1 effectors were coinfused, and the calvarium of mice were imaged 3 hours later (Figure 2A, experimental design). In the absence of a GVH response, PTX− OT-1 cells had similar E/I ratios and numbers of extravascular cells similar to that observed in unmanipulated mice (Figure 2B-C, left panel and quantitation, respectively; supplemental Video 6). Similarly, PTX treatment reduced the E/I ratio, driven by a decrease in extravascular OT-1 cells. In contrast, relative to mice that received transplant with no T cells, the GVH response reduced the E/I ratio of PTX- OT-1 due to fewer extravascular OT-1 cells (Figure 2B-C, right panel and quantitation, respectively; supplemental Video 7). PTX treatment of OT-1 cells modestly further reduced their E/I ratios (Figure 2C); however, in this case, the additional reduction in the E/I ratio was driven by increases in the intravascular OT-1 numbers, whereas PTX treatment surprisingly did not affect the number of extravascular OT-1 cells, suggesting that, with a GVH response, T-cell entry into the extravascular marrow was less strongly driven by chemokine gradients.
GVHD reduces chemokine-driven OT-1 effector entry into extravascular BM. (A) Experimental design. (B) Representative images from mice that received transplant with only donor BM (left; supplemental Video 6) and with BM and T cells (BM + T; right; supplemental Video 7). Scale bar represents 50 μm. (C) Quantitation of the E/I ratio and absolute numbers of extravascular and intravascular OT-1 cells. BM alone data are from 14 fields from 6 mice. GVH data are from 9 fields from 6 mice. Significance was determined by a 2-tailed Student t test. ∗P < .001; ∗∗P < .0001.
GVHD reduces chemokine-driven OT-1 effector entry into extravascular BM. (A) Experimental design. (B) Representative images from mice that received transplant with only donor BM (left; supplemental Video 6) and with BM and T cells (BM + T; right; supplemental Video 7). Scale bar represents 50 μm. (C) Quantitation of the E/I ratio and absolute numbers of extravascular and intravascular OT-1 cells. BM alone data are from 14 fields from 6 mice. GVH data are from 9 fields from 6 mice. Significance was determined by a 2-tailed Student t test. ∗P < .001; ∗∗P < .0001.
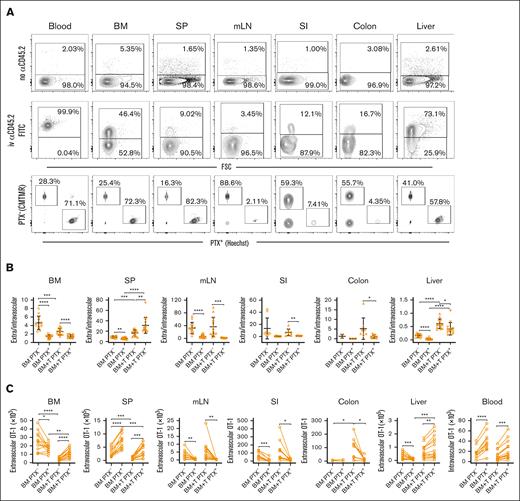
We also used flow cytometry to study the role of PTX-sensitive pathways in Teff trafficking. Flow cytometry allowed the study of OT-1 effectors in long bones (femurs and tibias), which is difficult with 2PIM due to the cortex thickness, and the assessment of changes on a scale beyond what can be captured by 2PIM. To do so, irradiated CBF1 mice were reconstituted with B6 BM with or without unmanipulated B6 T cells to induce GVHD. On day +14, differentially labeled PTX-treated or untreated OT-1 effectors (∼5 × 105 of each) were coinfused. Three hours later, mice were euthanized, and OT-1 cells were enumerated by flow cytometry. To determine whether OT-1 cells were intravascular or extravascular, mice were injected with a labeled antibody against CD45.2 3 minutes before euthanasia (Figure 3A, representative flow cytometry). Cells with bright anti-CD45 staining were classified as being intravascular.
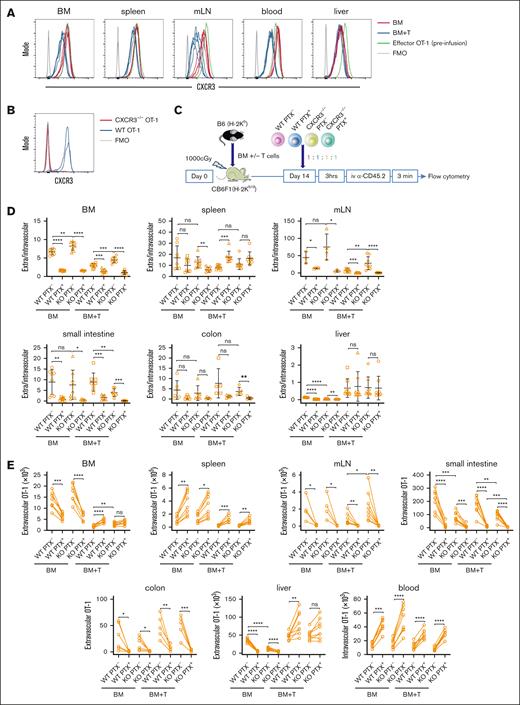
GVHD reduces chemokine-driven OT-1 effector entry into extravascular BM but promotes PTX-sensitive OT-1 effector entry into GVHD target tissues. Irradiated CB6F1 mice were reconstituted with B6 BM with T cells (BM + T) or without B6 T cells (BM). On day +14 after transplantation, PTX-treated OT-1 effectors (Hoechst labeled) or untreated OT-1 cells (CMTMR labeled) were infused. Mice were euthanized 3 hours later, 3 minutes after being injected with an α-CD45.2 FITC antibody to identify intravascular cells. (A) Representative flow cytometry showing OT-1 cells from mice not injected with (top row) and injected with α-CD45.2 (second row). Representative CMTMR and Hoechst staining of CD8+vβ5+ cells is in the third row. Ratios of extravascular (CD45.2–) to intravascular (CD45.2+) OT-1 cells (B). Enumeration of PTX+ and PTX– OT-1 cells, paired from individual mice (C). Data are combined from 4 independent experiments with a total of 13 BM alone and 14 BM + T recipients. Too few OT-1 cells were recovered from some mice, especially in the colon, accounting for some groups having fewer than 13 or 14 data points. Significance was determined by a 2-tailed Student t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. SP, spleen.
GVHD reduces chemokine-driven OT-1 effector entry into extravascular BM but promotes PTX-sensitive OT-1 effector entry into GVHD target tissues. Irradiated CB6F1 mice were reconstituted with B6 BM with T cells (BM + T) or without B6 T cells (BM). On day +14 after transplantation, PTX-treated OT-1 effectors (Hoechst labeled) or untreated OT-1 cells (CMTMR labeled) were infused. Mice were euthanized 3 hours later, 3 minutes after being injected with an α-CD45.2 FITC antibody to identify intravascular cells. (A) Representative flow cytometry showing OT-1 cells from mice not injected with (top row) and injected with α-CD45.2 (second row). Representative CMTMR and Hoechst staining of CD8+vβ5+ cells is in the third row. Ratios of extravascular (CD45.2–) to intravascular (CD45.2+) OT-1 cells (B). Enumeration of PTX+ and PTX– OT-1 cells, paired from individual mice (C). Data are combined from 4 independent experiments with a total of 13 BM alone and 14 BM + T recipients. Too few OT-1 cells were recovered from some mice, especially in the colon, accounting for some groups having fewer than 13 or 14 data points. Significance was determined by a 2-tailed Student t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. SP, spleen.
In the BM, consistent with the imaging results, the GVH response reduced the E/I ratios (ratio of cells that did not stain with the infused anti-CD45.2 antibody [extravascular] to cells that did stain with it [intravascular]) of PTX- OT-1 (Figure 3B), again driven by a reduction of extravascular OT-1 cells (Figure 3C; number of cells not staining with anti-CD45). In recipients of only donor BM, PTX treatment reduced the E/I ratio, also due to a decrease in extracellular OT-1 cells. In the presence of a GVH response, however, although PTX reduced the E/I ratio, it unexpectedly increased the number of extravascular OT-1 cells (Figure 3C); whereas by imaging, the numbers were equivalent. The discrepancy between the flow and imaging results could have been due to a true difference between long bones (studied by the flow cytometry approach) and the calvarium or the overcalling of extravascular cells based on antibody exclusion. But even with this modest difference, as measured by both techniques, PTX treatment in the context of GVHD did not reduce effector OT-1 cell trafficking to extravascular BM, consistent with GVH diminishing chemokine-driven OT-1 cell entry.
Because we used flow cytometry instead of imaging, we were able to analyze OT-1 effector entry into other tissues (Figure 3). GVH reduced the E/I ratios of PTX– and PTX+ OT-1 cells in spleen, driven in both cases by a reduction in extravascular OT-1 cells. With or without GVHD, PTX treatment further reduced the E/I ratios in the spleen (Figure 3B). However, in both situations, this was despite PTX unexpectedly increasing the number of extravascular OT-1 cells (Figure 3C), which was offset by a greater number of PTX+ OT-1 cells present in blood (Figure 3C, right most panel). As anticipated, and confirming the efficacy of PTX treatment, PTX+ OT-1 cells were nearly completely excluded from mesenteric LN (Figure 3C). Few OT-1 cells, PTX treated or not, were detected in small intestine (SI) and colon. Nonetheless, the SI E/I ratio was reduced by PTX (with or without GVHD), driven by a reduction in extravascular OT-1 cells (Figure 3B-C). In the colon, too few OT-1 cells were detected in the absence of GVHD to assess the effect of PTX treatment. However, GVHD increased the recruitment of PTX– OT-1 cells to the colon, and that recruitment was reduced by PTX treatment. In the liver, GVHD increased the E/I ratios of both PTX+ and PTX– OT-1 cells. PTX treatment reduced E/I ratios, with or without GVHD. In the absence of GVHD, the reduced E/I ratios of PTX-treated cells were driven by fewer extravascular hepatic OT-1 cells. In contrast, in the presence of GVHD, the number of extravascular hepatic OT-1 cells was modestly increased by PTX treatment, but the E/I ratio was still decreased because this increase was offset by an increase in blood OT-1 cells (Figure 3B-C).
In summary, the GVH response reduced but did not eliminate OT-1 effector entry into BM and spleen, sites of malignant hematopoiesis, while increasing OT-1 effector recruitment to key GVHD target tissues. However, although PTX treatment of OT-1 cells greatly diminished their recruitment to SI and colon in mice with GVHD, it increased the number of extravascular OT-1 cells in the BM, spleen, and liver, sites of malignant hematopoiesis. Of note, PTX treatment, with or without GVH, increased blood OT-1 cells (Figure 3C). This increase, perhaps due to less egress into tissues that require chemokine receptor engagement for T-cell entry, may have made more OT-1 cells available to enter tissues that were permissive for chemokine receptor–independent T-cell entry, such as the BM, spleen, and liver.
CXCR3 has been reported to have an important role in alloreactive T-cell migration to GVHD target tissues.14 We, therefore, explored the role of CXCR3 in OT-1 effector recruitment to BM and other tissues, with and without a GVH response. We first confirmed that the OT-1 effectors we generated expressed CXCR3 (Figure 4A). We then crossed OT-1 mice with CXCR3–/– mice (knockout [KO]; Figure 4B) and compared the trafficking of wild-type (wt) and KO OT-1 effectors, with or without PTX treatment. Irradiated CB6F1 mice were reconstituted with B6 BM, with or without B6 T cells. Approximately 2 weeks later, a mix of wt and KO OT-1 effectors that were or were not PTX treated were coinjected into mice. Three hours later, mice were euthanized for analysis of OT-1 cell migration by flow cytometry (experimental design, Figure 4C). CXCR3 did not contribute to OT-1 entry into the extravascular BM, with or without GVHD (E/I ratios, Figure 4D; number of extravascular OT-1 cells, Figure 4E). If anything, there was a trend for KO OT-1 cells to enter the BM more readily as indicated by KO PTX– OT-1 cells having higher E/I ratios and modestly but significantly more extravascular OT-1 cells in the presence of GVHD. This difference was unlikely to be attributable to differences in wt and KO blood OT-1 numbers, which were similar (Figure 4E). Without a GVH response, PTX treatment of wt and KO OT-1 cells reduced BM E/I ratios, driven by fewer extravascular OT-1 cells. In mice with GVHD, however, PTX treatment increased BM extravascular wt but not KO OT-1 cells, suggesting that the absence of CXCR3 alone was sufficient to modestly increase OT-1 cell entry. The absence of CXCR3 only affected PTX– OT-1 trafficking to Mesenteric lymph node (mLN) in the presence of GVHD, and unexpectedly, this was manifest by a higher E/I ratio and an increased number of extravascular KO OT-1 cells. Nonetheless, consistent with the importance of chemokine receptors in enabling T-cell entry to mLN, PTX treatment of KO and wt OT-1 cells reduced E/I ratios and the number of extravascular OT-1 cells.
CXCR3 contributes to but is not required for OT-1 effector recruitment. (A) OT-1 effectors generate by vaccination before (green line in all plots as a reference) and recovered after transfer expressed CXCR3. Each line is data from an individual mouse. (B) CXCR3–/– OT-1 effectors do not stain with the anti-CXCR3 antibody. (C) Experimental design. Irradiated CB6F1 mice received equal numbers of wt or CXCR3–/– OT-1 effectors that were or were not PTX treated. Three hours later, mice were injected with anti-CD45.2 to identify intravascular cells and were euthanized immediately thereafter. (D) Ratios of extravascular (CD45.2–) to intravascular (CD45.2+) OT-1 cells. (E) Numbers of extravascular (CD45.2) cells recovered from each tissue. Paired values are from the same mice. Data are from 2 experiments with 4 mice per group in each. Significance was determined by a 2-tailed Student t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. FMO, Fluorescent minus one.
CXCR3 contributes to but is not required for OT-1 effector recruitment. (A) OT-1 effectors generate by vaccination before (green line in all plots as a reference) and recovered after transfer expressed CXCR3. Each line is data from an individual mouse. (B) CXCR3–/– OT-1 effectors do not stain with the anti-CXCR3 antibody. (C) Experimental design. Irradiated CB6F1 mice received equal numbers of wt or CXCR3–/– OT-1 effectors that were or were not PTX treated. Three hours later, mice were injected with anti-CD45.2 to identify intravascular cells and were euthanized immediately thereafter. (D) Ratios of extravascular (CD45.2–) to intravascular (CD45.2+) OT-1 cells. (E) Numbers of extravascular (CD45.2) cells recovered from each tissue. Paired values are from the same mice. Data are from 2 experiments with 4 mice per group in each. Significance was determined by a 2-tailed Student t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. FMO, Fluorescent minus one.
In contrast to what was observed in BM and spleen, with or without GVHD, KO PTX– OT-1 cells were less efficiently recruited to the SI, although CXCR3 expression was not absolutely required. PTX treatment further reduced KO OT-1 recruitment to the SI, demonstrating that other chemokine receptors also contribute to Teff entry (Figure 4D-E). Similar to prior experiments (Figure 2), few OT-1 cells were recovered from the colon. With that limitation, there were no differences in the numbers of untreated wt and KO OT-1 cells in colon with or without of GVHD, whereas PTX treatment further reduced the numbers of both (Figure 4D-E). In the liver, without GVHD, KO OT-1 cells were reduced relative to wt OT-1 cells, and recruitment of wt and KO OT-1 effectors were similarly reduced by PTX treatment (Figure 4D-E). However, with GVHD, the absence of CXCR3 had no effect on OT-1 recruitment; PTX treatment increased extravascular hepatic wt but not KO OT-1 cells.
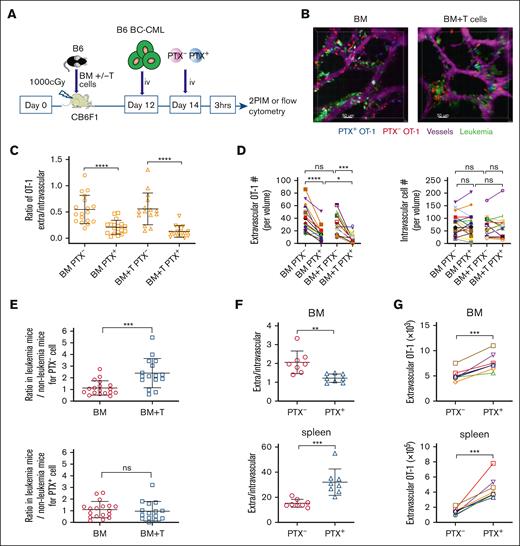
We next explored how leukemia cells affect OT-1 Teff entry into the BM. To do so, irradiated CB6F1 mice were reconstituted with B6 BM, with or without B6 T cells. B6 BC-CML cells18,24 and OT-1 effectors were infused on days +12 and +14, respectively. Calvaria were imaged by 2PIM 3 hours later (Figure 5A-B, experimental design and representative images, respectively; supplemental Videos 8 and 9 [BM alone and BM + T, respectively]). We used B6 and not CB6F1 BC-CML cells so as alloreactive donor B6 T-cell killing of host background BC-CML cells would not confound comparisons between mice with and without a GVH response. In contrast to experiments without leukemia cells, with BC-CML cells, the GVH response neither decreased the E/I ratio nor reduced the number of extravascular OT-1 cells (Figure 5C-D). PTX treatment similarly reduced the OT-1 E/I ratio, with or without GVHD (Figure 5C). The decline in the E/I ratio was driven by a decrease in extravascular OT-1 cells; nonetheless, PTX+ OT-1 cells were still able to enter the extravascular marrow. To better understand the differences between OT-1 trafficking in GVH mice that did or did not have leukemia, we compared results from experiments with BC-CML with experiments without BC-CML (Figure 2). To do so, we normalized E/I ratios in mice with BC-CML to the average E/I ratio of mice from experiments without BC-CML cells (Figure 5E). Without a GVH response, the mean ratio was close to 1, suggesting similar behavior with and without leukemia. However, with GVH, the mean ratio was >2, indicating that leukemia cells diminished the GVH-induced disruption of the chemokine drive for effector OT-1 entry.
BC-CML cells promote OT-1 recruitment in the presence of a GVH response. (A) Experimental design. (B) Representative images from the calvarium of mice transplanted as described in panel A; Scale bar represents 50 μm. See also supplemental Videos 8 and 9. (C) Ratios of E/I OT-1 cells. (D) Numbers of extravascular and intravascular OT-1 cells. Symbols connected by lines represent data from the same mice. BM alone data are from 18 volumes from 7 mice. BM + T data are from 15 volumes in 9 mice. We compared the E/I ratios of OT-1 cells in mice transplanted with leukemia cells to those measured in mice transplanted without leukemia cells by normalizing the ratios with leukemia cells to the mean of the ratios observed in mice transplanted without leukemia cells (E). (F-G) The experimental design was similar as in panel A, except intravascular and extravascular OT-1 cells were enumerated by flow cytometry based on extravascular cells staining with αCD45.2 FITC, which was administered 3 minutes before euthanizing. BM cells from femurs and tibias were analyzed. (F) Ratios of extravascular (CD45.2–) to intravascular (CD45.2+) OT-1 cells as determined by flow cytometry. (G) Extravascular OT-1 cell number in BM and spleen. Data are from 2 experiments with 4 mice per group in each. Significance was determined by a 2-tailed Student t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
BC-CML cells promote OT-1 recruitment in the presence of a GVH response. (A) Experimental design. (B) Representative images from the calvarium of mice transplanted as described in panel A; Scale bar represents 50 μm. See also supplemental Videos 8 and 9. (C) Ratios of E/I OT-1 cells. (D) Numbers of extravascular and intravascular OT-1 cells. Symbols connected by lines represent data from the same mice. BM alone data are from 18 volumes from 7 mice. BM + T data are from 15 volumes in 9 mice. We compared the E/I ratios of OT-1 cells in mice transplanted with leukemia cells to those measured in mice transplanted without leukemia cells by normalizing the ratios with leukemia cells to the mean of the ratios observed in mice transplanted without leukemia cells (E). (F-G) The experimental design was similar as in panel A, except intravascular and extravascular OT-1 cells were enumerated by flow cytometry based on extravascular cells staining with αCD45.2 FITC, which was administered 3 minutes before euthanizing. BM cells from femurs and tibias were analyzed. (F) Ratios of extravascular (CD45.2–) to intravascular (CD45.2+) OT-1 cells as determined by flow cytometry. (G) Extravascular OT-1 cell number in BM and spleen. Data are from 2 experiments with 4 mice per group in each. Significance was determined by a 2-tailed Student t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. ns, not significant.
Using the same transplant approach, we used flow cytometry instead of 2PIM to analyze OT-1 migration in the presence of a GVH response and leukemia cells. Similar to the case without leukemia cells, PTX treatment reduced the E/I ratio in BM, although the number of extravascular OT-1 cells were increased (Figure 5F-G). This increase differed from the PTX-induced reduction in extravascular cells in mice with GVHD when measured by 2PIM, which parallels what was observed when comparing 2PIM and flow-based methods of quantitating extravascular OT-1 cells in mice that received transplant without BC-CML (Figure 3C). This discrepancy could reflect the overcalling of extravascular cells by flow cytometry or a difference in OT-1 cell behavior in calvarium vs the femur. In spleen, PTX increased the E/I ratio, again with an increase in extravascular OT-1 cells.
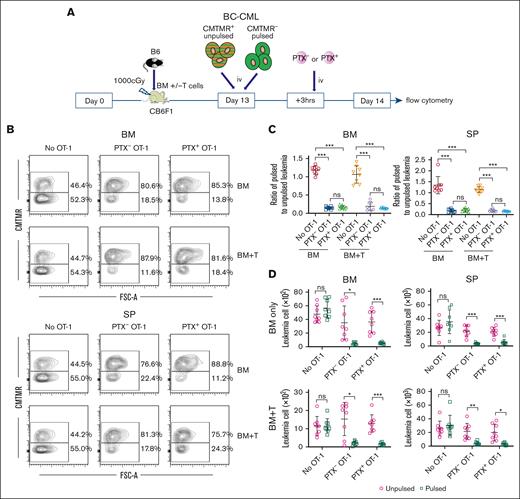
Taken together, these data indicate that in the presence of a GVH response, blockade of chemokine receptor signaling reduces Teff access to key GVHD target tissues while being permissive for Teff entry into extravascular BM and spleen, primary sites of malignant hematopoiesis. We, therefore, tested how PTX treatment of OT-1 effectors affects the killing of BC-CML cells pulsed with the peptide SIINFEKL, the Kb-restricted ovalbumin (OVA)–derived epitope recognized by OT-1 cells. Irradiated CB6F1 mice were reconstituted with B6 BM with or without B6 T cells (1 × 106 CD8 and 0.5 × 106 CD4 cells). On day +13, mice were injected with BC-CML cells that were unpulsed (CMTMR+) and SIINFKEL pulsed (CMTMR–), mixed at a 1:1 ratio (107 of each). Three hours later, OT-1 effectors (∼5 × 107) that were or were not PTX treated were injected (Figure 6A, experimental design). An additional control group did not receive OT-1 cells. On day +14, mice were euthanized, and BC-CML cells were enumerated by flow cytometry (Figure 6B, representative flow cytometry). In mice with or without GVHD and in both BM and spleen, PTX-treated and untreated OT-1 effectors killed SIINFEKL-pulsed BC-CML cells, with similar ratios of recovered unpulsed/pulsed BC-CML cells (Figure 6C) and absolute numbers of BC-CML cells recovered (Figure 6D).
PTX+ and PTX- OT-1 cells kill SIINFEKL-pulsed BC-CML cells in mice that received transplant. (A) Experimental design. Irradiated CB6F1 mice were reconstituted with B6 BM, without or with B6 T cells. On day +13, mice received BC-CML cells that were SIINFEKL pulsed (unlabeled) or unpulsed (CMTMR labeled). Three hours later, mice were injected with PTX+ or PTX– OT-1 effectors. Another group received BC-CML cells but not OT-1 cells. Mice were euthanized the next day, and pulsed and unpulsed BC-CML cells in spleen and BM were enumerated. (B) Representative flow cytometry (gated on BC-CML cells based on expression of a nonsignaling form of the human NGFR and EGFP). (C) Ratios of SIINFEKL pulsed to unpulsed cells in BM and spleen. (D) Numbers of BC-CML cells enumerated in each experimental group. Data are combined from 2 experiments, with 4 mice per group in each. Significance was determined by a 2-tailed Student t test. ∗P < .01; ∗∗P < .001; ∗∗∗P < .0001. EGFP, enhanced green fluorescent protein; NGFR, nerve growth factor receptor; ns, not significant; SP, spleen.
PTX+ and PTX- OT-1 cells kill SIINFEKL-pulsed BC-CML cells in mice that received transplant. (A) Experimental design. Irradiated CB6F1 mice were reconstituted with B6 BM, without or with B6 T cells. On day +13, mice received BC-CML cells that were SIINFEKL pulsed (unlabeled) or unpulsed (CMTMR labeled). Three hours later, mice were injected with PTX+ or PTX– OT-1 effectors. Another group received BC-CML cells but not OT-1 cells. Mice were euthanized the next day, and pulsed and unpulsed BC-CML cells in spleen and BM were enumerated. (B) Representative flow cytometry (gated on BC-CML cells based on expression of a nonsignaling form of the human NGFR and EGFP). (C) Ratios of SIINFEKL pulsed to unpulsed cells in BM and spleen. (D) Numbers of BC-CML cells enumerated in each experimental group. Data are combined from 2 experiments, with 4 mice per group in each. Significance was determined by a 2-tailed Student t test. ∗P < .01; ∗∗P < .001; ∗∗∗P < .0001. EGFP, enhanced green fluorescent protein; NGFR, nerve growth factor receptor; ns, not significant; SP, spleen.
Discussion
Adoptive immunotherapy with CD8+ T cells that target minor histocompatibility antigens (miHAs) presented by MHCI molecules is an attractive approach for treating hematopoietic neoplasms in the context of an allo-SCT.25-28 Ideally such therapies would target miHAs with expression limited to hematopoietic cells, thereby reducing the risk of GVHD.29 However, the levels of miHA expression in GVHD target tissues are incompletely understood, and moreover, the transplant environment could induce genes (eg, those induced by interferon gamma) that have lower expression under homeostatic conditions typical for reference specimens. Altering T cells in ways that restrict their entry to GVHD target tissues, but which are permissive for migration to locations where malignant cells reside, is a rational approach for improving the safety of anti-miHA–targeted T-cell therapies.
Previous studies exploring the roles of chemokine receptors expressed by T cells in GVHD6-15 mostly used chemokine receptor gene–deficient T cells infused at the time of transplant. Our use of OT-1 effectors to interrogate chemokine receptor requirements for effector migration into tissues adds to this prior work in several ways: (1) studying effectors that were or were not PTX treated excluded the impact that a given gene deficiency would have on the migration of naïve T cells to sites of priming,6,30-33 which could contribute to a given phenotype; (2) PTX treatment provides a broader impairment of chemokine receptors than is the case with chemokine receptor gene–deficient T cells; (3) OT-1 effectors did not recognize any antigen in our recipients, which eliminated contributions that variations in tissue antigen expression might have on T-cell trafficking19,34; (4) the use of OT-1 cells combined with the analysis of mice only a few hours after transfer minimized the contributions that antigen-driven cell division after transfer might make on T-cell quantitation; (5) Teff are more representative of antigen receptor–modified adoptive immunotherapy T-cell products than are naïve T cells; and (6) our approaches were quantitative, relying on complementary 2PIM and flow cytometry enumerations.
OT-1 effector entry into the extravascular marrow space of unmanipulated mice and irradiated mice that received transplant with donor BM but without GVH-inducing donor T cells was dependent, although incompletely, on T-cell chemokine receptor signaling. Surprisingly, however, in the presence of GVHD, chemokine receptors were no longer major drivers of OT-1 effector entry into the extravascular marrow and spleen. Rather, more PTX+ than PTX– OT-1 cells were recruited into both BM and spleen in mice with GVHD. In contrast, PTX treatment reduced the migration of OT-1 effectors into GVHD target tissues, even as GVHD itself promoted the recruitment of PTX– OT-1 cells. This difference suggested that in the context of a T-cell–replete allo-SCT, inhibition of chemokine receptors on Teff could preserve GVL while diminishing the risk of GVHD. Consistent with this idea, with or without a GVHD response, PTX+ and PTX– OT-1 both killed BC-CML cells in both the spleen and BM. However, we did not stress the system by testing killing with lower numbers of transferred PTX– and PTX+ OT-1 cells, and it is possible such studies would reveal that 1 or the other is more effective.
In contrast to the broad impact of impairing of all PTX-sensitive chemokine receptors, we found only a modest role for CXCR3 in OT-1 effector trafficking, despite all OT-1 effectors expressing it. With or without GVHD, CXCR3–/– and wt OT-1 effectors were similarly capable of entering extravascular marrow and spleen, although the absence of CXCR3 modestly reduced OT-1 effector recruitment to the SI and liver. Nevertheless, PTX treatment was more potent than the lone absence of CXCR3 in reducing OT-1 effector entry into SI and colon, major sites of clinically significant GVHD, indicating that other PTX-sensitive chemokine receptors participate.
The use of 2PIM enabled observations beyond what could have been made by only flow cytometry. With microscopy, we could definitively determine whether T cells were intravascular or extravascular and assure that they were in areas of hematopoiesis. That our 2PIM and flow cytometry results were mostly concordant increases the confidence that the flow cytometric approach for excluding intravascular OT-1 cells by their availability to IV antibodies was accurate. With 2PIM, we also were able to better understand T-cell movements within the vessels that supply the marrow and in the extravascular space. In this regard, we observed cells streaming rapidly with blood flow, whereas others were adherent to the vasculature and even moving upstream. Quantitating these movements was not the focus of this work, but in future studies, we could apply 2PIM more systematically to better understand how GVHD and the presence of leukemia cells alter T-cell trafficking and T-cell positioning in the extravascular marrow. Integrins and other adhesion molecules also play a role in T-cell egress from vasculature and positioning in tissues.35-40 We did not evaluate their roles in Teff trafficking with and without GVHD; this would be worthy of future investigations.
An important unanswered question is how the GVH response diminishes chemokine-dependent T-cell recruitment into the extravascular marrow space. That overall numbers of extravascular OT-1 cells were reduced with GVHD relative to mice that received transplant without donor T cells suggests that the chemokine independence was real and not that a chemokine-dependent component was masked by a larger magnitude chemokine-independent migration. One potential mechanism is that the chemokine gradient was disrupted by alloreactive T cells that directly or indirectly killed or suppressed radio-resistant host cells that produce and release chemokines. Leptin receptor–positive cells in the marrow microenvironment are major producers of chemokines, and such cells would be candidate targets of direct or indirect action.41 The GVH response might also have disrupted the presentation of chemokines on the lumen of marrow blood vessels. Hematopoietic cells can also produce chemokines, and it is possible that the alloresponse more completely eliminated key host hematopoietic elements than radiation alone. That GVHD did not reduce chemokine-dependent OT-1 recruitment when donor B6 background BC-CML cells, which were resistant to alloreactive T-cell killing, were infused is consistent with a role for marrow hematopoietic cells in some way contributing to the maintenance of a marrow chemokine gradient. This effect could be dependent on the type of leukemia. The investigation of these potential mechanisms will be the subject of future studies.
Taken together, our results suggest that impairment of chemokine receptor function could improve the safety of adoptive T-cell products targeting hematopoietic malignancies. We used PTX treatment to impair chemokine receptor function. This enzymatic approach is transient and may not be ideal for clinical application. An alternative would be genome editing of genes encoding GαI subunits, which would lead to a permanent reduction in chemokine receptor signaling. The increased therapeutic window that chemokine receptor interference affords may only be realized in the presence of a sufficient GVH response, which could be difficult to reliably engineer in the clinic. A better understanding of how GVH reduces chemokine receptor–dependent recruitment of Teff to extravascular BM would be an important first step in discovering a more reliable approach.
Acknowledgments
The authors thank the animal technicians at the University of Pittsburgh for their diligent care.
This work was supported by NIH R01 HL117855.
Authorship
Contribution: W.D.S. conceived experiments, analyzed data, and wrote the manuscript; K.Z. designed and executed experiments, analyzed data, and wrote the manuscript; J.Z. designed experiments and provided expertise on the 2-photon intravital microscopy imaging; M.Z. provided technical advice and assisted in designing and executing experiments; and S.R. assisted in the execution of experiments.
Conflict-of-interest disclosure: W.D.S. is a founder, stock option holder, and compensated consultant for BlueSphere Bio; and is also an option holder in Orca Bio in exchange for consulting services. The remaining authors declare no competing financial interests.
Correspondence: Warren D. Shlomchik, University of Pittsburgh School of Medicine, Assembly Building Room 3055 5051 Centre Ave, Pittsburgh, PA 15261; email: warrens@pitt.edu.
References
Author notes
Data are available on request from the corresponding author, Warren D. Shlomchik (warrens@pitt.edu).
The full-text version of this article contains a data supplement.