Key Points
CD3×CD3 tetravalent antibodies cure GVHD in xenogeneic mouse models and warrant further investigation in more clinically relevant models.
Visual Abstract
Allogeneic hematopoietic stem cell transplantation is an established treatment for hematological malignancies and some genetic diseases. Acute graft-versus-host disease (GVHD) is the most common and debilitating side effect with poor survival rates of 5% to 30% for severe cases. In this manuscript, we describe a tetravalent T-cell–engaging bispecific antibody (BsAb) based on the immunoglobulin G-[L]-single-chain variable fragment (IgG-[L]-scFv) platform, with all 4 binding domains specific for CD3. In vitro, picomolar concentrations of the CD3×CD3 BsAb induced potent lysis of activated CD4 and CD8 T cells. In immunodeficient mice, in which human T cells induced xenogeneic GVHD, administration of 0.1 μg BsAb per dose depleted the majority of T cells from the peripheral blood, and 10 μg per dose completely reversed established GVHD and achieved a 100% survival rate. In mice bearing NALM6-luc xenografts, treatment with CD3×CD19 BsAb and activated human T cells induced complete remission of the leukemia, and all treated mice developed GVHD by 50 days after treatment. CD3×CD3 BsAb (3-30 μg doses) reversed clinical signs of GVHD, allowing long term follow-up beyond 250 days. T cells were undetectable by polymerase chain reaction in 4 of 5 mice in the 30 μg CD3×CD3 BsAb group 180 days after leukemia injection, and complete necropsies on day 259 revealed no evidence of human T cells or leukemia cells. Curing GVHD allows for long-term follow-up of tumor response heretofore impossible in humanized mouse models. Further studies are warranted to determine whether the CD3×CD3 BsAb has potential for treating clinical GVHD and other autoimmune diseases in humans.
Introduction
Allogeneic hematopoietic cell transplantation is a powerful treatment for diseases including leukemia, immune deficiency, metabolic defects, and hemoglobinopathies.1 One major complication of allogeneic hematopoietic cell transplantation that limits its curative potential is graft-versus-host disease (GVHD), which occurs in 35% to 50% of patients.2 Most treatment options for GVHD are based on immunosuppression. Corticosteroids, which are the mainstay for treatment of grade ≥2 acute GVHD, cause significant adverse systemic side effects, including the dampening of both innate and adaptive immunities (and increasing risk of opportunistic infections), and metabolic dysregulation.1,2 Furthermore, GVHD is frequently resistant to corticosteroid treatment. Despite these interventions, the survival of patients with grade 3 and 4 GVHD is only 25% to 30% and 1% to 2%, respectively, demanding the development of more effective and safer treatment options for these severe cases.3 To this end, several monoclonal antibodies (Abs) have been developed clinically to target T-cell antigens, including daclizumab, inolimomab, and basiliximab, which all target CD25. However, because CD25 is widely expressed on regulatory T cells, their depletion could worsen GVHD, a theory supported by evidence showing that survival was actually decreased when daclizumab was added to corticosteroids.4 In addition, although acute GVHD was blunted by daclizumab, extensive chronic GVHD can still follow.5 With the withdrawal of daclizumab from the market, the unmet need for effective anti-GVHD therapies remains unfulfilled. Here, we introduce a T-cell–engaging anti-CD3 bispecific Ab (BsAb) for treatment of GVHD. This CD3×CD3 BsAb induces T-cell fratricide and reduces clinical signs of GVHD by redirecting cytotoxic T cells to kill other T cells rather than damaging host tissues.
Methods
BsAbs
The murine OKT3 anti-CD3 Ab was humanized by grafting the heavy chain complementarity-determining region sequences onto the human framework IGHV1-2∗02-IGHJ4∗01, and the light chain complementarity-determining region sequences onto the human framework IGKV1-39∗01-IGKJ2∗02. The CD3×CD3 and CD3×CD19 BsAbs were designed by fusing the humanized OKT3 single-chain Fv fragments (scFv) to the C-terminus of the light chain of humanized immunoglobulin G1 (IgG1) Abs based on a method described previously.6,7 To remove glycosylation and complement binding, N297A and K322A mutations were included in the Fc region.
IL-15/IL-15Rα-Fc complex
Full-length ectodomain of interleukin-15 receptor α (IL-15Rα) (amino acids 1-175) was fused to the CH2-CH3 of human IgG1 Fc region. These genes were synthesized by Genscript with appropriate flanking restriction enzyme sites, subcloned into a single 2-segment mammalian expression vector, and used to transfect CHO-S cells (Invitrogen) for stable coexpression of protein complex and selection of high producers, as previously described.8
Flow cytometry analysis
For phenotyping of T cells in circulating blood, ACK lysis buffer was used to lyse red blood cells in 100 μL of blood from each mouse. Remaining white blood cells were washed and stained for flow cytometry. Anti-human antibodies against CD3 (clone UCHT1, catalog no. 300411), CD4 (clone GK1.5, catalog no. 100405), CD8 (clone SK1, catalog no. 344705), CD25 (clone BC96, catalog no. 302605), CD45 (clone HI30, catalog no. 982322), CD69 (clone FN50, catalog no. 985202), lymphocyte activation gene 3 (LAG3, clone C9B7W, catalog no. 125207), programmed cell dead protein 1 (PD-1, clone A17188A, catalog no. 379209), and T cell immunoglobulin and mucin domain 3 (TIM3, clone A18078A, catalog no. 364805; all from Biolegend) were used to stain cells. Stained cells were processed with a FACScalibur or Fortessa instrument (BD Biosciences) and analyzed with FlowJo software (FlowJo, LLC, Ashland, OR).
T-cell isolation and activation
T cells were purified from human peripheral blood mononuclear cells (PBMCs) using a Pan T Cell Isolation Kit (catalog no. 130-096-535, Miltenyi Biotech) and were activated and expanded with CD3/CD28 Dynabeads (catalog no. 11131D, Gibco) according to the manufacturer’s protocol. For in vitro and in vivo experiments, T cells were activated in culture for 7 to 10 days before being used.
In vitro T-cell fratricide assay
Activated T cells were expanded for 9 days and cultured with 350 pM Ab in the presence of IL-2 (30 IU/mL). The number of live CD4 and CD8 cells was calculated using CountBright Absolute Counting Beads (catalog no. C36950, Invitrogen). Dead cells were excluded using annexin-V staining. Experiments were repeated 3 times. Representative results are shown from 1 experiment with 3 technical replicates.
In vivo studies
All mouse experiments were performed in compliance with the institutional animal care and use committee guidelines. The immunodeficient NOD.Cg-Prkdcscid IL-2R-γtm1Wjl/SzJ (NSG) mice were purchased from The Jackson Laboratory (Maine), bred in house, and provided with Sulfatrim food.
For GVHD experiments, 6- to 10-week-old mice were inoculated with human PBMCs or activated T cells. GVHD scoring was based on skin integrity, fur texture, activity, and posture, as described previously (see Table 1).9 Dead mice received a score of 5. For in vivo fratricide studies, mice were treated with 0.1 or 1 μg CD3×CD3 BsAb or negative control CD3×CD19 BsAb. For the experiment comparing CD3×CD3 BsAb with standard anti-GVHD therapy, treatment with dexamethasone (0.8 mg/mouse intraperitoneal [IP] twice per week), cyclophosphamide (1.25 mg/mouse IP twice per week), or CD3×CD3 BsAb (3, 10, or 30 μg per mouse IP or IV twice per week) commenced when GVHD was clinically evident and mice had a score of ∼2.
GVHD scoring system
| . | Skin . | Fur . | Posture . | Mobility . | Weight . |
|---|---|---|---|---|---|
| 0.5 | Flaking | Ventral ruffling | Slight kyphosis | Decreased activity | - |
| 1 | Erythema | Ventral lines, slight back ruffling | Obvious kyphosis | Stationary >50% of the time | 10%-25% weight loss |
| 1.5 | Open lesion | Ruffling on >50% of body | Kyphosis | Moves only when stimulated | - |
| 2 | Multiple open lesions | Ruffling on entire body, denuded skin | Severe kyphosis | No movement | >25% weight loss |
| . | Skin . | Fur . | Posture . | Mobility . | Weight . |
|---|---|---|---|---|---|
| 0.5 | Flaking | Ventral ruffling | Slight kyphosis | Decreased activity | - |
| 1 | Erythema | Ventral lines, slight back ruffling | Obvious kyphosis | Stationary >50% of the time | 10%-25% weight loss |
| 1.5 | Open lesion | Ruffling on >50% of body | Kyphosis | Moves only when stimulated | - |
| 2 | Multiple open lesions | Ruffling on entire body, denuded skin | Severe kyphosis | No movement | >25% weight loss |
For the leukemia study, mice were injected IV (via tail vein) with 1 × 106 NALM6-luc human leukemia cells transduced with luciferase; 4 days later, in vivo bioluminescent imaging was performed to confirm engraftment of leukemia cells. Treatment with activated human T cells (ATCs; 10 million per dose), CD3×CD19 BsAb (1 μg per dose, to treat the CD19+ leukemia), and recombinant human IL-15/IL-15Rα-Fc complex (to support T-cell survival in vivo) began on day 5. An untreated group and a group treated with only ATC + IL-15/IL-15Rα-Fc complex were included as negative controls. Tumor growth was monitored using bioluminescent imaging. Mice in the negative control groups were euthanized when they developed hind limb paralysis, a known complication of NALM6 leukemia xenografts caused by infiltration into the bone marrow and central nervous system.10 This experiment was repeated twice with near identical results, however, for the sake of conciseness, only 1 experiment is shown in these figures.
Pathology
Complete necropsies of 6 mice were performed by the ’s Laboratory of Comparative Pathology. Mice were euthanized with CO2 overdose. Following gross examination, all organs were fixed in 10% neutral-buffered formalin for 72 hours, followed by decalcification of bone in a formic acid solution (Surgipath Decalcifier I, Leica Biosystems, Buffalo Grove, IL). Formalin-fixed tissues were then routinely processed in ethanol and xylene and embedded in paraffin in a Leica ASP6025 tissue processor. Paraffin blocks were sectioned at 5 microns and stained with hematoxylin and eosin. The following tissues were processed and examined: heart, lungs, liver, gallbladder, kidneys, pancreas, stomach, duodenum, jejunum, ileum, cecum, colon, salivary glands, skin (perigenital, trunk, and head), urinary bladder, uterus, cervix, vagina, ovaries, oviducts, adrenal glands, spleen, thyroid gland, esophagus, trachea, spinal cord, bone marrow, vertebrae, sternum, femur, tibia, stifle joint, skeletal muscle, nerves, skull, nasal cavity, oral cavity, teeth, ears, eyes, pituitary gland, and brain. Immunohistochemistry (IHC) was performed on the lungs of 1 mouse to confirm that infiltrating lymphocytes were indeed CD3+ human T cells. The IHC assay was performed on a Leica Bond RX automated stainer (Leica Biosystems). After deparaffinization and heat-induced epitope retrieval in a citrate buffer at pH 6.0, the primary Ab against human CD3 was applied (LifeSpan Biosciences, catalog LS-C202826, rabbit monoclonal, clone MRQ-39, 1:500 dilution), followed by application of a polymer detection system (DS9800, Novocastra Bond Polymer Refine Detection, Leica Biosystems). The chromogen was 3,3-diaminobenzidine-tetrachloride, and sections were counterstained with hematoxylin. Interpretation of gross findings, hematoxylin and eosin–stained slides, and IHC was conducted by a board-certified comparative pathologist.
Statistics
Statistical analysis was performed using Graphpad Prism software. All data were assumed to be normally distributed and parametric analyses were used. Comparison between 2 groups was performed using the Student t test, and comparisons between multiple groups were determined by analysis of variance and Tukey test for multiple comparisons analysis. A P value of <.05 was considered statistically significant. Error bars denote the standard error of the mean. Survival analysis was performed using a log-rank test.
Memorial Sloan-Kettering Cancer Center animal care and use committee approval 09-05-010.
Results
Generation of a T-cell–engaging anti-CD3 BsAb with an IgG-[L]-scFv format and silenced Fc functions (CD3×CD3 BsAb)
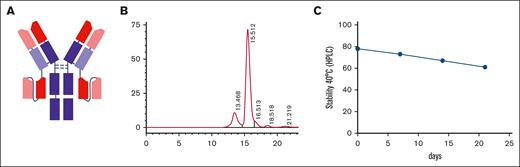
One of the applications for depleting T cells is to treat diseases caused primarily by T cells. Such diseases include GVHD, autoimmune diseases (directly induced by T cells or in which T cells play a role in activating B cells to generate autoantibodies), and T-cell malignancies. We have systematically investigated the relationship between BsAb format and potency both in vitro and in murine models of cancer and showed that the IgG-[L]-scFv format, in which the scFv of a T-cell–engaging Ab was fused to the C-terminus of the light chain of a tumor-specific monoclonal IgG Ab, is more potent than other BsAb platforms,11-13 including Bispecific T cell Engager (BiTE) and heterodimer, which are the only 2 US Food and Drug Administration–approved BsAb formats in the clinic.13 Here, we embedded the humanized anti-CD3 domains both in the scFv and the IgG-Fab (Figure 1A). The Fc was silenced to eliminate any Fc-mediated functions.14 The purity, low endotoxin level, and stability under heat stress were acceptable (Figure 1B-C).
The physicochemical structure of the CD3×CD3 BsAb. (A) schematic diagram of the IgG-scFv BsAb. (B) The purity of the CD3×CD3 BsAb was measured using high-performance liquid chromatography (HPLC). (C) The temperature stability of the CD3×CD3 BsAb was tested at 40°C over time using HPLC (percentage of the area under the curve for the main peak is shown in the diagram). n = 1 sample per time point.
The physicochemical structure of the CD3×CD3 BsAb. (A) schematic diagram of the IgG-scFv BsAb. (B) The purity of the CD3×CD3 BsAb was measured using high-performance liquid chromatography (HPLC). (C) The temperature stability of the CD3×CD3 BsAb was tested at 40°C over time using HPLC (percentage of the area under the curve for the main peak is shown in the diagram). n = 1 sample per time point.
The CD3×CD3 BsAb induces potent T-cell fratricide in vitro
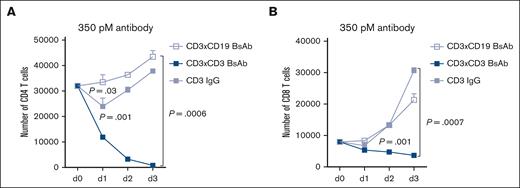
To test the T-cell killing effect of the CD3 BsAb, T cells were cultured with 350 pM of the Ab in the presence of IL-2 to support T-cell proliferation. As shown in Figure 2, as low as 350 pM CD3×CD3 BsAb induces strong T-cell fratricide among both CD4 (>97% depletion) and CD8 (54% depletion) T-cell populations, although T-cell death was more prominent among CD4 T cells. Two different antibodies were used as controls. The first was an IgG-[L]-scFv BsAb specific for CD19 (CD3×CD19, anti-CD19 on both Fab arms) and CD3 (CD3×CD3, both scFvs connected to the C-terminal of the IgG common light chain). The second control Ab was the humanized anti-CD3 IgG. Interestingly, none of these control antibodies induced significant T-cell depletion and, in fact, T cells ultimately proliferated in their presence (Figure 2).
The T-cell–engaging anti-CD3 BsAb induces potent T-cell fratricide in vitro. Activated T cells were expanded for 9 days and cultured with 350 pM of the Ab in the presence of IL-2 (30 IU/mL). The number of live CD4 (A) and CD8 cells (B) was calculated using CountBright Absolute Counting Beads (Invitrogen). Dead cells were excluded using annexin-V staining. Area under the curve was compared between groups using analysis of variance (ANOVA) and multiple comparison analysis. n = 3 technical replicates per time point per condition. Experiment was repeated 3 times.
The T-cell–engaging anti-CD3 BsAb induces potent T-cell fratricide in vitro. Activated T cells were expanded for 9 days and cultured with 350 pM of the Ab in the presence of IL-2 (30 IU/mL). The number of live CD4 (A) and CD8 cells (B) was calculated using CountBright Absolute Counting Beads (Invitrogen). Dead cells were excluded using annexin-V staining. Area under the curve was compared between groups using analysis of variance (ANOVA) and multiple comparison analysis. n = 3 technical replicates per time point per condition. Experiment was repeated 3 times.
The CD3×CD3 BsAb induces profound T-cell depletion in mice engrafted with human T cells
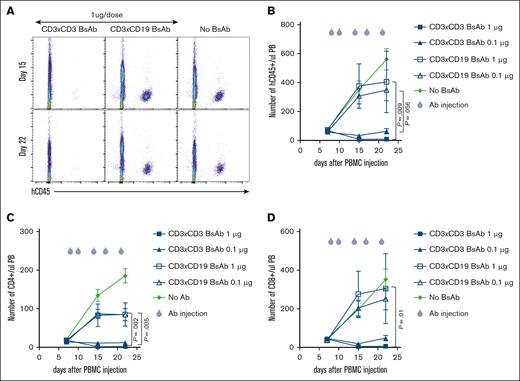
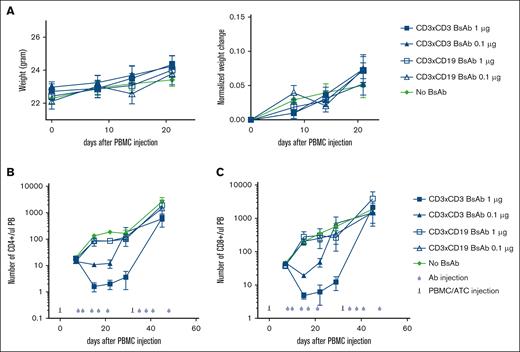
To test whether the CD3×CD3 BsAb can induce T-cell depletion in vivo, NSG mice were injected with 30 million PBMCs (10 million each from 3 separate donors) IP to induce GVHD. Peripheral blood was stained for the presence of T cells on day 7, and treatment with 0.1 μg or 1 μg CD3×CD3 BsAb or negative control CD3×CD19 BsAb was started on day 8. Although the CD3×CD3 BsAb depleted almost all T cells from the peripheral blood (Figure 3A-B), the negative control CD3×CD19 BsAb had no effect. Both 1 μg and 0.1 μg CD3×CD3 BsAb induced potent T-cell depletion, although at 1 μg the depletion of T cells was more profound (Figure 3B). Analysis of T-cell subpopulations revealed that both CD4 and CD8 T cells were depleted in vivo (Figure 3C-D). One concern about using this strategy in vivo is that treatment with a CD3×CD3 BsAb may result in massive T-cell activation leading to cytokine release syndrome. Previous studies have shown that mice experiencing cytokine release syndrome after treatment with chimeric antigen receptor (CAR) T cells exhibit symptoms such as hunching, piloerection, lethargy, and weight loss.15,16 In our experiments, mice that received CD3×CD3 BsAb did not show any signs of distress (Figure 4A). In a separate experiment, NSG mice were injected with 20 million T cells and 3 days later treated with either a single dose of 3 μg CD3×CD3 BsAb or no Ab. Blood was drawn 24 hours after treatment with CD3×CD3 BsAb and flow cytometry was performed to determine the frequency and phenotype of circulating T cells. Supplemental Figure 1 shows that the frequency of human CD45+ cells was significantly decreased in mice treated with CD3×CD3 BsAb, particularly of CD4+ cells. Expression of the activation markers CD25 and CD69 were significantly upregulated on T cells in CD3×CD3 BsAb–treated mice, as were PD-1, LAG3, and TIM3 (supplemental Figure 2).
The CD3×CD3 BsAb depletes human T cells in vivo. NSG mice were injected with 30 million PBMCs IP. Peripheral blood was stained for the presence of T cells on day 7 and treatment was started the day after. Treatment was composed of IV injection of 1 μg or 0.1 μg CD3×CD3 or a CD19×CD3 BsAb. (A) At different time points, blood was taken and stained for the presence of human cells. The number of human CD45 cells (B), CD4 (C), and CD8 (D) T cells was determined using flow cytometry. Area under the curve was compared between groups using ANOVA and multiple comparison analysis. n = 5 mice per group.
The CD3×CD3 BsAb depletes human T cells in vivo. NSG mice were injected with 30 million PBMCs IP. Peripheral blood was stained for the presence of T cells on day 7 and treatment was started the day after. Treatment was composed of IV injection of 1 μg or 0.1 μg CD3×CD3 or a CD19×CD3 BsAb. (A) At different time points, blood was taken and stained for the presence of human cells. The number of human CD45 cells (B), CD4 (C), and CD8 (D) T cells was determined using flow cytometry. Area under the curve was compared between groups using ANOVA and multiple comparison analysis. n = 5 mice per group.
Depletion of T cells via the CD3×CD3 BsAb is not associated with clinical side effects. Mice were treated as in Figure 3 and were weighed weekly (A). At different time points, blood was taken and stained for the presence of human cells. The number of human CD4 (B) and CD8 (C) T cells was determined using flow cytometry. n = 5 mice per group. Experiment was repeated 2 times.
Depletion of T cells via the CD3×CD3 BsAb is not associated with clinical side effects. Mice were treated as in Figure 3 and were weighed weekly (A). At different time points, blood was taken and stained for the presence of human cells. The number of human CD4 (B) and CD8 (C) T cells was determined using flow cytometry. n = 5 mice per group. Experiment was repeated 2 times.
The CD3×CD3 BsAb reverses xenogeneic GVHD in mice and extends survival
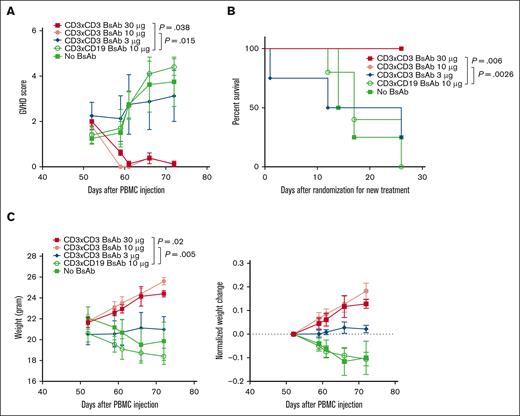
To accelerate the development of GVHD in the mice treated in the previous section, a second dose of effector cells (22 million activated T cells per mouse) was injected on day 32, followed by anti–T-cell BsAb treatment. As shown in Figure 4B-C, 1 μg CD3×CD3 BsAb was insufficient in the presence of ex vivo activated T cells for T-cell depletion. All the mice were then randomized into 5 groups: 3 groups received increasing doses of the CD3×CD3 BsAb (3 μg, 10 μg, and 30 μg), 1 group received 10 μg CD3×CD19 BsAb, and the fifth group did not receive any Ab.
GVHD assessment showed that 30 μg and 10 μg CD3×CD3 BsAb reduced the GVHD score from 2 to 0.12 and from 1.8 to 0.12, respectively. In contrast, the GVHD score continued to increase despite treatment in the mice treated with only 3 μg of the CD3×CD3 BsAb, or those treated with 10 μg of the control BsAb, or those left untreated (Figure 5A). In addition, the mice in the CD3×CD3 BsAb group (30 μg and 10 μg) gained weight whereas the mice in the other groups lost weight, providing further evidence for the therapeutic effect of higher dose of the CD3×CD3 BsAb against GVHD (Figure 5B). Finally, all mice that received 30 μg and 10 μg CD3×CD3 BsAb survived whereas those in the other groups succumbed to GVHD (Figure 5C).
The CD3×CD3 BsAb treats mice with established xenogeneic GVHD. Mice were treated as in Figure 3. (A,C) Mice were frequently weighed, observed, and scored for GVHD. Area under the curve was compared between groups using ANOVA and multiple comparison analysis. (B) Survival analysis was done by log-rank test. n = 5 mice per group. Experiment was repeated 2 times.
The CD3×CD3 BsAb treats mice with established xenogeneic GVHD. Mice were treated as in Figure 3. (A,C) Mice were frequently weighed, observed, and scored for GVHD. Area under the curve was compared between groups using ANOVA and multiple comparison analysis. (B) Survival analysis was done by log-rank test. n = 5 mice per group. Experiment was repeated 2 times.
The CD3×CD3 BsAb reverses xenogeneic GVHD in mice cured of human leukemia without allowing leukemia relapse
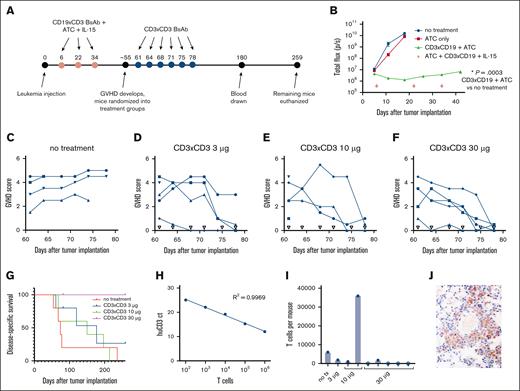
Because 1 of the concerns about T-cell–depleting therapy for the treatment of GVHD is the loss of the graft-versus-leukemia effect and subsequent relapse of leukemia, we wanted to test our CD3×CD3 BsAb in this context using a xenograft mouse model of CD19+ leukemia. NALM6-luc leukemia-bearing mice were treated with 3 doses of ATC, CD3×CD19 BsAb, and IL-15/IL-15Rα-Fc complex to cure the leukemia (Figure 6A). One week after the first treatment, luciferase signal from the leukemia cells in the treated group was no longer visible by bioluminescent imaging (Figure 6B). Mice in the no-treatment and ATC-only groups developed hind-limb paralysis at approximately days 20 to 30 (a known consequence of NALM6 xenografts) and were euthanized. None of the mice lost weight during this initial phase of treatment.
The CD3×CD3 BsAb treats GVHD in mice previously cured of xenograft leukemia without allowing leukemia relapse. (A) Timeline of experiment and treatments. (B) CD19×CD3 BsAb cures mice of CD19+ NALM6-luc leukemia. Luciferase (luc)–transduced human leukemia NALM6-luc was monitored using in vivo bioluminescent imaging. n = 5 mice per group. (C-F) GHVD scores for each individual mouse, grouped by treatment. Open triangles represent treatment days. (G) Survival of mice treated with CD3×CD3 BsAb. (H) Standard curve of cycle threshold (ct) values for human CD3 in complementary DNA generated from RNA purified from mouse blood spiked with known numbers of T cells. (I) Calculated number of T cells per mouse. Open circles indicate that the number was incalculably low because the ct value of for human CD3 was >40. (J) Immunohistochemical staining (600× original magnification) showing human CD3+ T cells in the lung tissue of the mouse euthanized on day 197, the mouse from the 10-μg group with the highest number of T cells in its peripheral blood. Experiment was repeated 2 times.
The CD3×CD3 BsAb treats GVHD in mice previously cured of xenograft leukemia without allowing leukemia relapse. (A) Timeline of experiment and treatments. (B) CD19×CD3 BsAb cures mice of CD19+ NALM6-luc leukemia. Luciferase (luc)–transduced human leukemia NALM6-luc was monitored using in vivo bioluminescent imaging. n = 5 mice per group. (C-F) GHVD scores for each individual mouse, grouped by treatment. Open triangles represent treatment days. (G) Survival of mice treated with CD3×CD3 BsAb. (H) Standard curve of cycle threshold (ct) values for human CD3 in complementary DNA generated from RNA purified from mouse blood spiked with known numbers of T cells. (I) Calculated number of T cells per mouse. Open circles indicate that the number was incalculably low because the ct value of for human CD3 was >40. (J) Immunohistochemical staining (600× original magnification) showing human CD3+ T cells in the lung tissue of the mouse euthanized on day 197, the mouse from the 10-μg group with the highest number of T cells in its peripheral blood. Experiment was repeated 2 times.
The mice that had been cured of leukemia then went on to develop clinical signs of GVHD (weight loss, hunched posture, skin flaking and reddening, fur loss, and lethargy; see Table 1) ∼55 days after the first administration of T cells. Mice were scored for GVHD and randomized into 4 treatment groups: no treatment and CD3×CD3 BsAb at 3, 10, and 30 μg CD3×CD3 BsAb per dose. Figure 6C shows that mice that were not treated for GVHD had persistent GVHD symptoms for a∼20 days and eventually died or were euthanized when their GVHD scores reached 5. Mice treated with the CD3×CD3 BsAb had a significant reduction in GVHD symptoms that began almost immediately after the first dose of CD3×CD3 BsAb (Figure 6D-F). They eventually recovered completely, with the GVHD scores for all the mice in the 10-μg and 30-μg groups regressing to ≤1. Persistent scores for GVHD were mainly because of ventral ruffling of fur. Figure 6G shows the survival of the mice treated with CD3×CD3 BsAb. Although 3 and 10 μg CD3×CD3 BsAb extended survival somewhat compared with the untreated group, survival for the group treated with 30 μg was significantly extended compared with the untreated mice, with this group surviving >250 days after leukemia injection.
To determine whether human T cells persisted in the mice several months after administration of the CD3×CD3 BsAb, peripheral blood was collected from the surviving mice on day 180 and reverse transcription polymerase chain reaction was performed to identify human T cells. Reverse transcription polymerase chain reaction was chosen over flow cytometry for its increased sensitivity. A standard curve was generated using blood samples spiked with known numbers of cultured T cells (Figure 6H). Using this standard curve and the assumption that an average mouse has a 2.0 mL total blood volume,17 we calculated the number of T cells per mouse (Figure 6I). Overall, 4 of 5 mice in the 30 μg group had no detectable T cells, whereas the mice in the remaining groups all had detectable T cells, albeit very low numbers. The 1 mouse in the 10 μg group with >30 000 T cells was found to be severely lethargic on day 197 and was euthanized. IHC revealed the presence of small numbers of human T cells in the lungs (Figure 6J), and occasional basal epidermal keratinocyte degeneration and apoptosis were found in the skin, thus indicating mild vestiges of GVHD. The other mouse in the 10 μg group was found similarly lethargic with labored breathing and euthanized on day 214, and the mouse in the no treatment group was found lethargic with an ocular mass on day 236 and euthanized. The remaining 5 mice in the 30 μg group were euthanized on day 259 and complete necropsies with thorough histologic examination of all major organs revealed no evidence of residual T cells or GVHD in these animals.
The CD3×CD3 BsAb is superior to standard therapy at reversing xenogeneic GVHD
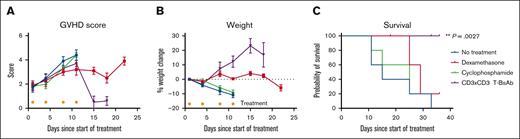
Although no standard-of-care treatment for GVHD exists, corticosteroids and are commonly used to suppress lymphocyte activation in both acute and chronic GVHD.1 Cyclophosphamide is used in multiple settings: as pretransplant conditioning; as primary posttransplant prophylaxis; and, less commonly, to treat steroid-resistant GVHD.18-20 In order to compare the efficacy of our novel CD3×CD3 BsAb to standard treatment options, we induced GVHD in NSG mice by injecting 25 × 106 ATCs per mouse IV and waiting 42 days until GVHD symptoms became clinically evident in the majority of the mice per our scoring system (Table 1). GVHD was scored and mice were randomized into 4 groups so that each group had an average starting GVHD score of ∼2. Mice were given no treatment, dexamethasone (corticosteroid, 0.8 mg), cyclophosphamide (1.25 mg), or CD3×CD3 BsAb (10 μg) IP twice per week for 2 weeks. The mice were weighed and scored twice weekly. Figure 7A shows that although the clinical GVHD score continued to increase in all 4 groups during the 2 weeks of treatment, it fell precipitously in the CD3×CD3 BsAb–treated group and remained relatively stable in the dexamethasone-treated group during the week after treatment. Mice in the CD3×CD3 BsAb–treated group started to gain weight after 1 week of treatment, whereas the weight of the dexamethasone-treated mice remained stable and the weight of the mice in the cyclophosphamide-treated group and untreated group continued to decline (Figure 7B). The CD3×CD3 BsAb significantly extended survival compared with the untreated group (Figure 7C), whereas no other treatment had a statistically significant effect on survival outcomes. These data suggest that in our model, the CD3×CD3 BsAb is a more powerful treatment against acute xenogeneic GVHD compared with standard therapeutic strategies.
The CD3×CD3 BsAb is superior to steroids or cyclophosphamide in a xenogeneic mouse model of GVHD. (A) GVHD score of mice treated with dexamethasone (0.8 mg/mouse per dose), cyclophosphamide (1.25 mg/mouse per dose), or CD3×CD3 BsAb (10 μg/mouse per dose) IP twice per week for 2 weeks. (B) Weight change of the same animals plotted as percent change from starting day. (C) Survival of the same animals. Note that the 2 mice that were censored from the survival graph were euthanized because of neurological impairment (head tilt) attributed on necropsy to eye and ear infections. n = 5 mice per group. Gold asterisks indicate treatment days. Experiment was conducted once.
The CD3×CD3 BsAb is superior to steroids or cyclophosphamide in a xenogeneic mouse model of GVHD. (A) GVHD score of mice treated with dexamethasone (0.8 mg/mouse per dose), cyclophosphamide (1.25 mg/mouse per dose), or CD3×CD3 BsAb (10 μg/mouse per dose) IP twice per week for 2 weeks. (B) Weight change of the same animals plotted as percent change from starting day. (C) Survival of the same animals. Note that the 2 mice that were censored from the survival graph were euthanized because of neurological impairment (head tilt) attributed on necropsy to eye and ear infections. n = 5 mice per group. Gold asterisks indicate treatment days. Experiment was conducted once.
Discussion
T cells are the main effectors in the pathogenesis of acute GVHD.21 In this manuscript we introduced a T-cell–engaging BsAb against CD3 to deplete T cells in vitro and in a xenogeneic mouse model of GVHD. The IgG-[L]-scFv format in which both the Fab fragments and the scFvs bind to CD3 (tetravalency for CD3) was essential for depletion of T cells whereas neither a similar BsAb with only 2 scFvs directed at CD3 nor 2 anti-CD3 Fab in the IgG format could deplete T cells in vitro. The tetravalent BsAb was also highly effective in mice with established GVHD. In this manuscript we describe experiments using mice xenografted with human leukemia, but we have performed other experiments with various solid tumor models with the same effect.
A major limitation of therapeutic studies where human T cells are adoptively transferred to immunodeficient mice is xenogeneic GVHD, which confounds immunologic end points and causes lethal complications 30 to 60 days after the first T-cell infusion. GVHD in this context prevents researchers from assessing the durability of the antitumor response beyond 2 months and also prevents investigation of clonal evolution since this process does not proceed normally under selective immune pressure. For example, the emergence of tumor clones with antigen loss or spliced variants (eg, CD19 and CD22) are difficult to measure or to study, unless they appear rapidly within weeks and in sufficient clonal frequencies. Hence, studies of clonal escape and drug resistance cannot be easily implemented in such models. The ability of CD3×CD3 BsAb to effectively remove T cells that cause both clinical and histopathologic features of GVHD should allow much longer term studies in these mouse models. It should be possible to study the effect of sequential cycles of human T cells, each time terminated with a short course of CD3×CD3 BsAb, whether the T cells are driven by a CAR or tumor-targeted BsAbs.
Recently, a phase 3 trial of GVHD prophylaxis in patients with HLA-matched donors proved the superiority of combination posttransplant cyclophosphamide, tacrolimus, and mycophenolate mofetil compared with the standard combination of tacrolimus and methotrexate.19 However, only 52.7% of patients treated with the experimental regimen remained free of relapse or GVHD 1 year after transplant and 6.3% of the patients in this group developed grade 3/4 acute GVHD within a year of transplant.19 Breakthrough GVHD is commonly treated with corticosteroids to suppress immune function and the majority of these patients experience metabolic side effects and infections, likely contributing to morbidity and mortality.22 Steroid resistance develops in up to 50% of the patients, significantly affecting quality of life and decreasing overall survival to 5% to 30%.23 Patients with steroid-resistant GVHD are treated with the Janus kinase (JAK) 1/JAK2 inhibitor ruxolitinib, which blocks the action of cytokines through the JAK-STAT pathway. Ruxolitinib induced a 57% overall response rate in steroid refractory GVHD patients by day 28 after treatment, however, half of the patients still died and the median overall survival was 0.9 months.24 Several other therapies for steroid refractory GVHD are under investigation in clinical trials including extracorporeal photopheresis, α1-antitrypsin infusion, fecal microbiota transplantation, anti-CD3/CD7 immunotoxin, anti-α4β7 Ab vedolizumab, antithymocyte globulin, anti-CD25 antibodies (daclizumab, basiliximab, and inolimomab), anti–tumor necrosis factor α Abs (infliximab and etanercept), and mesenchymal stem cells.23 CD3/CD7-immunotoxin was given fast-track designation for the treatment of steroid refractory acute GVHD by the US Food and Drug Administration in 2019, and a phase 3 trial is ongoing (ClinicalTrials.gov identifier: NCT04128319).
Generation of CAR T cells against T cell antigens such as CD5 and CD7 is another option for treatment of T cell diseases, however, poor in vitro expansion of the cells because of fratricide necessitates the use of gene editing technologies or inducible gene expression systems that could further complicate the manufacturing process or clinical implementation.25,26 Additionally, the incorporation of suicide genes into the CAR construct is necessary to prevent long-term T-cell depletion in vivo. In contrast, CD3×CD3 BsAbs could be used as an off-the-shelf drug and side effects could be controlled simply by titrating the dose. Although leukopenia is typical among patients after chemotherapy or radiation and immunosuppressants used to treat GVHD may impair T-cell activity, patients with GVHD generally have enough functioning, circulating T cells for this T-cell–engaging BsAb to function.
Although this xenogeneic mouse model is useful for testing the efficacy of our BsAbs to induce T-cell fratricide in vivo for the purpose of translational drug development, we recognize that its ability to recapitulate the biology of GVHD in immunocompromised mice is limited. Improved models will be needed to evaluate T-cell reconstitution, toxicity due to cytokine release, and the ability of the undepleted T cells to effectively respond to various pathogens. Using tools such as in vitro or in vivo challenge models and hematopoietic stem cells as the graft in lieu of purified T cells or PBMCs would allow for the testing of BsAbs at doses that are insufficient to deplete all target cells but are sufficient to reverse or control GVHD, a strategy that could be useful in human translation in order to preserve antipathogen immune function in patients. Determining how many residual T cells are needed to fight infections or leukemia will need further investigation, but the first priority is developing a treatment that will allow patients survive acute GVHD before these other considerations are clinically relevant.
An important aspect of this T-cell depletion strategy is its dose dependency, in that the clinical dose could be precisely titrated based on T-cell number, cytokine release, and other toxicities. If given continuously, the CD3×CD3 BsAb could cause deep T-cell aplasia, given its potency at depleting T cells through fratricide. However, its relatively short half-life in vivo (days) mitigates this risk. Additionally, cytokine release syndrome may occur in humans, although this syndrome has by now been well-studied and can typically be well managed through supportive care and drugs such as tocilizumab. To ameliorate these risks, scFvs targeting other T-cell antigens (CD1, CD4, CD8, CD25, etc) could be used in in lieu of the CD3 scFv moiety on our CD3×CD3 BsAb to more specifically target certain subsets of T cells, whereas the BsAb described in these experiments is not selective for alloreactive T cells and depends on reducing the overall number of cytotoxic T cells for its mechanism of action. The work described above using the CD3×CD3 BsAb provides proof of concept for this principle that could be expanded on in the future. Although these results are preliminary and the model is inherently limited in its utility, a tetravalent CD3×CD3 BsAb merits further investigation as to its suitability for translation into the clinic for patients with steroid-refractory GVHD.
Acknowledgments
The authors thank Hong Xu for performing the Biacore experiments and Mahiuddin Ahmed for help with designing humanized sequences. The authors also thank Hongfen Guo for her technical support. The authors thank the Laboratory of Comparative Pathology (Cancer Center Support Grant P30 CA008748) for conducting the pathological analysis and interpretation.
This work was supported, in part, by funds from Enid A. Haupt Endowed Chair, Kids Walk for Kids with Cancer NYC, Isabella Santos Foundation, Katie Find a Cure Foundation, the Robert Steel Foundation, National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support Grant P30 CA008748, and NIH/NCI Predoctoral to Postdoctoral Fellow Transition Award K00 CA223062. Funding support for purchase of the IVIS Spectrum CT was provided by NIH Shared Instrumentation Grant 1 S10 OD01627-01.
Authorship
Contribution: S.S.H. conceived the study, performed the initial mouse experiments, analyzed the data, designed the figures, and drafted the manuscript; N.-K.V.C. conceived the study, supervised the project, helped analyze the data, and wrote and edited the manuscript; M.E.-C. performed the tumor xenograft experiments, analyzed the data, designed the figures, and wrote and edited the manuscript; I.C.M. performed the pathological analysis and interpretation and contributed to the manuscript; and J.H. performed the polymerase chain reaction experiments, analyzed the data, designed the figures, and contributed to the manuscript.
Conflict-of-interest disclosure: S.S.H. and N.-K.V.C. were named as coinventors in a patent on CD3 BsAb filed by the Memorial Sloan Kettering Cancer Center (MSK). Both MSK and N.-K.V.C. have financial interest in Y-mAbs, Abpro Laboratories, and Eureka Therapeutics. N.-K.V.C. reports receiving commercial research grants from Y-mabs Therapeutics; was named as inventor on multiple patents filed by MSK, including those licensed to Ymabs Therapeutics, Biotec Pharmacon, and Abpro Laboratories; and is a scientific advisory board member for Eureka Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Nai-Kong V. Cheung, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 170, New York, NY 10065; email: cheungn@mskcc.org.
References
Author notes
Publication-related are available on request from the corresponding author, Nai-Kong V. Cheung (cheungn@mskcc.org).
The full-text version of this article contains a data supplement.