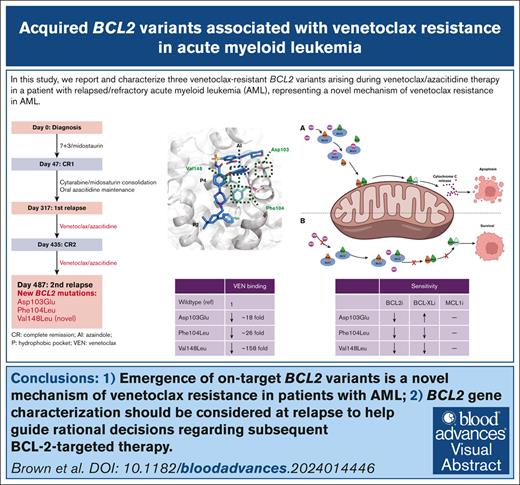
Visual Abstract
TO THE EDITOR:
The B-cell lymphoma 2 (BCL2)-selective inhibitor venetoclax is approved for therapy in combination with hypomethylating agent or low-dose cytarabine in patients with acute myeloid leukemia (AML). Despite high rates of initial hematologic response, relapsing disease is the most frequent cause of treatment failure. BCL2 variants (Gly101Val, Asp103Val/Glu/Tyr, Arg107_Arg110dup, Ala113Gly, Arg129Leu, Val156Asp, Phe104Ile)1-4 impeding capacity of venetoclax to bind the hydrophobic receptor groove on BCL2 have been reported in chronic lymphocytic leukemia and follicular lymphoma. In patients with AML, reported mechanisms of venetoclax resistance include (1) clonal selection of variants activating tyrosine kinase signaling pathways (eg, FLT3 internal tandem duplication mutation, Rat sarcoma virus [RAS]);5-7 (2) variants disrupting the function of TP536,8,9 or BCL2-associated X (BAX10); (3) upregulated expression of alternate BCL2 family proteins11; or (4) selection of monocyte differentiated blasts with overexpressed Myeloid cell leukemia-1 (MCL1).12 Venetoclax-resistant BCL2 variants analogous to those observed in chronic lymphocytic leukemia have yet to be reported in AML. Herein, we report a trio of BCL2 variants arising during venetoclax/azacitidine therapy in a patient with relapsed/refractory AML, including a novel variant (BCL2 Val148Leu) not previously reported. Venetoclax resistance for each variant was confirmed experimentally in a transgenic model system and parallel evolution in AML blasts demonstrated using multiomic single-cell technology.
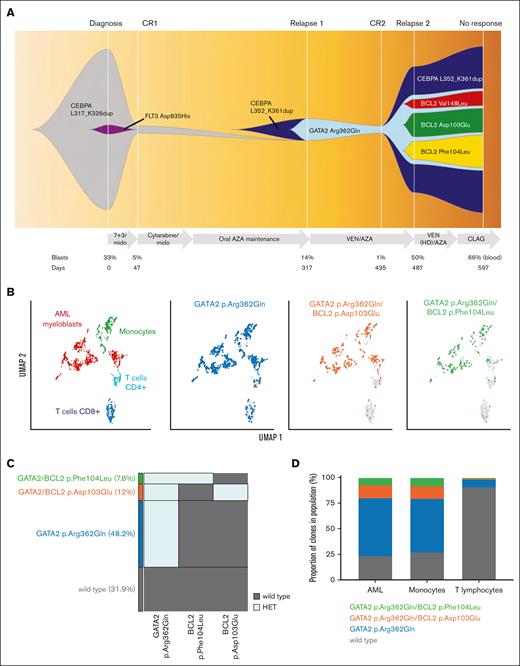
The index patient was a 67-year-old female with normal karyotype and favorable risk AML based on presence of a Basic Leucine Zipper (bZIP) domain in-frame CEBPA insertion. In addition, a subclonal FLT3 tyrosine kinase domain variant (Asp835His) at 5% variant allele frequency (VAF) was identified (Figure 1A; supplemental Table 1). Induction chemotherapy with 7 + 3 (cytarabine, daunorubicin) and midostaurin resulted in complete remission. This was consolidated with 2 cycles of intermediate dose cytarabine + midostaurin, followed by repeated cycles of maintenance oral azacitidine. Five months after initial remission, relapsed AML was diagnosed, accompanied by evolution of 2 new variants: a second bZIP in-frame CEBPA abnormality, along with a new GATA2 variant, with extinction of the previously identified FLT3 tyrosine kinase domain clone (Figure 1A; supplemental Table 1). Identification of CEBPA and GATA2 variants before relapse was limited, given that the detection limit of the bulk next generation sequencing (NGS) assay was only 4% for single nucleotide variants and 1% for insertions and deletions. AML relapse was salvaged with venetoclax/azacitidine and second morphologic remission was achieved after cycle 2. While waiting for a consolidative allogeneic stem cell transplant, the patient had poor peripheral blood count recovery and emergence of peripheral blasts after the fourth cycle of therapy. A repeat marrow examination was performed, which showed relapsed disease (60% blasts) and emergence of 3 BCL2 missense variants (c.309C>G; Asp103Glu [VAF 13%], c.312C>G; Phe104Leu [VAF 10%] and c.442G>C; Val148Leu [VAF 7%]) not previously detected in the patient after exposure to chemotherapy, midostaurin or maintenance azacitidine (Figure 1A; supplemental Table 1). A fifth cycle of azacitidine was attempted, with the venetoclax dose escalated to 400 mg daily in combination with posaconazole (a strong CYP3A4 inhibitor), with intention to increase venetoclax exposure (approximately eightfold)13; however, this failed to suppress the level of leukemic blasts. Despite further salvage with cladribine, cytarabine, and granulocyte colony-stimulating factor priming, the AML remained resistant and the patient was transitioned to hospice care. Serial molecular studies revealed further clonal expansion of the BCL2 variants despite salvage therapy (Figure 1A; supplemental Table 1).
A trio of BCL2 variants identified upon venetoclax and azacitidine progression. (A) Fish plot representation showing changes in clonal architecture over time (x-axis), with bone marrow blasts (%) and treatments administered shown. Variants were characterized using a clinical NGS panel and relapse clonal architecture from single-cell DNA sequence.14 (B-D) Multiomic single-cell characterization of bone marrow from day 487 (panel A). (B) Two-dimensional UMAP plot showing specific phenotypic populations based on antibody tags (right). Mononuclear cells clustered into 4 main blood cell compartments including AML blast cells (red). Identified DNA clones (blue, orange, and green) are overlaid onto phenotypic populations (left). (C) The proportion of cells either wild-type (WT) or heterozygote for indicated GATA2 and BCL2 variants. (D) Proportion of each mutant clone within specified blood cell lineages. AZA, azacitidine; CLAG, cladribine, cytarabine, granulocyte colony-stimulating factor; CR, complete remission; HD, high-dose; HET, heterozygous; HOM, homozygous; mido, midostaurin; UMAP, Uniform Manifold Approximation and Projection; VEN, venetoclax; 7 + 3, cytarabine/daunorubicin.
A trio of BCL2 variants identified upon venetoclax and azacitidine progression. (A) Fish plot representation showing changes in clonal architecture over time (x-axis), with bone marrow blasts (%) and treatments administered shown. Variants were characterized using a clinical NGS panel and relapse clonal architecture from single-cell DNA sequence.14 (B-D) Multiomic single-cell characterization of bone marrow from day 487 (panel A). (B) Two-dimensional UMAP plot showing specific phenotypic populations based on antibody tags (right). Mononuclear cells clustered into 4 main blood cell compartments including AML blast cells (red). Identified DNA clones (blue, orange, and green) are overlaid onto phenotypic populations (left). (C) The proportion of cells either wild-type (WT) or heterozygote for indicated GATA2 and BCL2 variants. (D) Proportion of each mutant clone within specified blood cell lineages. AZA, azacitidine; CLAG, cladribine, cytarabine, granulocyte colony-stimulating factor; CR, complete remission; HD, high-dose; HET, heterozygous; HOM, homozygous; mido, midostaurin; UMAP, Uniform Manifold Approximation and Projection; VEN, venetoclax; 7 + 3, cytarabine/daunorubicin.
We sought to clarify the cell of origin and clonal independence of evolved BCL2 variants in bone marrow mononuclear cells collected from the patient at relapse after venetoclax/azacitidine (relapse 2, Figure 1A). Targeted single-cell DNA sequencing analysis (Mission Bio Tapestri) revealed a heterozygous GATA2 Arg362Gln variant that had emerged at relapse, along with evolution of 2 independent BCL2 variant subclones marked by heterozygous BCL2 Phe104Leu (VAF 7.8%) or BCL2 Asp103Glu (VAF 12%) changes (Figure 1B-C). Neither BCL2 Val148Leu nor CEBPA was covered by the custom single-cell panel; hence, we could not detail their fate at a single-cell level. Multiomic analysis confirmed these BCL2 variants had evolved in leukemic blasts. The GATA2/BCL2 comutated clones were predominantly identified in myeloid and monocytic blast cells, rather than in T cells, enhancing the likelihood that these variants were relevant to the relapsing leukemic clone (Figure 1B,D; supplemental Figure 1). The ancestral GATA2 mutant clone was also present in both myeloid and monocytic blast cells, as well as a proportion of T cells, suggesting a preleukemic origin. No variants in TP53, BAX, FLT3, or RAS/MAPK genes were detected to infer possibility of an alternative genetic mechanism of venetoclax resistance.
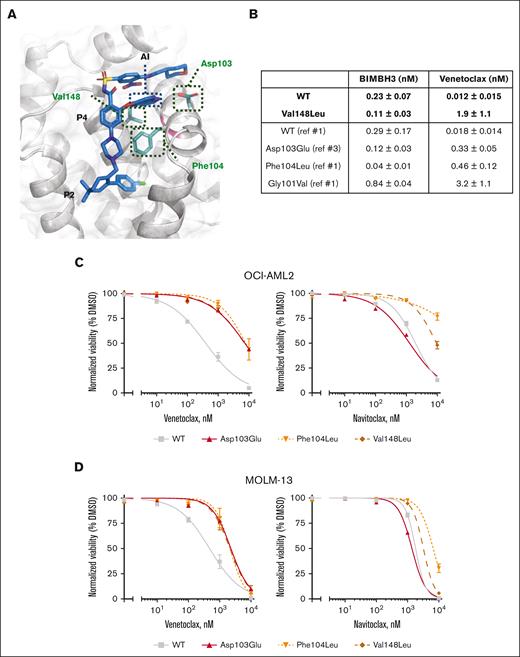
Substitution of aspartic acid for glutamic acid in BCL2 Asp103Glu may have 2 consequences for the interaction between venetoclax and BCL2, which predominantly occurs at the P2 and P4 pockets within the hydrophobic groove of BCL2. First, a hydrogen bond linking the azaindole moiety of venetoclax to amino acid 103 in the P4 pocket of BCL2 is disrupted (Figure 2A). Substitution to glutamic acid reduces the affinity of venetoclax to BCL2 by 20-fold compared with aspartic acid, while preserving the binding of BCL2 to BCL2 Interacting Mediator of cell death (BIM)-BCL2-homology domain 3 (BH3) peptide (Figure 2B). Second, BCL2 Asp103Glu alters the P4-binding pocket to more closely resemble that of B-cell lymphoma-extra large (BCL-XL).3 In vitro studies have previously shown that cell lines expressing BCL2 Asp103Glu were less sensitive to venetoclax, whereas sensitivity to navitoclax (which targets both BCL2 and BCL-XL) was enhanced.3BCL2 Phe104Leu disrupts interaction with the chlorophenyl moiety of venetoclax in the P2 pocket (Figure 2B) and has previously been reported in murine cell lines to reduce affinity to venetoclax by 25-fold, compared with wild-type BCL2.15 Amino acid 148 is situated at the base of the P4 pocket of BCL2 and lies in close proximity to the azoleindole moiety of venetoclax (Figure 2A). Although not previously reported in cancer databases, we hypothesize that substitution of valine for the larger leucine residue in the BCL2 Val148Leu variant interferes with venetoclax binding to BCL2. This was confirmed in surface plasmon resonance binding experiments, where Val148Leu was associated with almost 100-fold reduction in venetoclax binding, with preserved binding to BIM-BH3 (Figure 2B; supplemental Figures 2 and 3).
BCL2 variants have reduced activity to VEN in AML cells. (A) Structure of the BCL2 protein with venetoclax (blue) binding in the BCL2 groove.16 The positions of the 3 mutated residues are illustrated (green). P2 and P4 pockets and the azaindole side chain of venetoclax are indicated. (B) Table summarizing the binding affinities of BIM or venetoclax for WT or Val148Leu BCL2 as determined by direct binding assays in comparison with previously documented binding affinities for Asp103Glu, Phe104Leu, and Gly101Val BCL2 mutants. Data represent means ± 1 standard deviation (SD) of 3 independent experiments with curves and fits shown in supplemental Figures 2 and 3. (C-D) BCL2 WT, Asp103Glu, Phe104Leu, and Val148Leu were overexpressed in (C) OCI-AML2 and (D) MOLM-13 AML cell lines. In vitro viability assessed by flow cytometric enumeration of cells excluding propidium iodide upon exposure to indicated concentrations of venetoclax or navitoclax for 48 hours. Data represent means ± 1 SD of at least 3 independent experiments.
BCL2 variants have reduced activity to VEN in AML cells. (A) Structure of the BCL2 protein with venetoclax (blue) binding in the BCL2 groove.16 The positions of the 3 mutated residues are illustrated (green). P2 and P4 pockets and the azaindole side chain of venetoclax are indicated. (B) Table summarizing the binding affinities of BIM or venetoclax for WT or Val148Leu BCL2 as determined by direct binding assays in comparison with previously documented binding affinities for Asp103Glu, Phe104Leu, and Gly101Val BCL2 mutants. Data represent means ± 1 standard deviation (SD) of 3 independent experiments with curves and fits shown in supplemental Figures 2 and 3. (C-D) BCL2 WT, Asp103Glu, Phe104Leu, and Val148Leu were overexpressed in (C) OCI-AML2 and (D) MOLM-13 AML cell lines. In vitro viability assessed by flow cytometric enumeration of cells excluding propidium iodide upon exposure to indicated concentrations of venetoclax or navitoclax for 48 hours. Data represent means ± 1 SD of at least 3 independent experiments.
To study the functional effects of these BCL2 variants in AML, Asp103Glu, Phe104Leu, and Val148Leu variants were ectopically expressed in 2 human AML cell lines, OCI-AML2 and MOLM-13 (supplemental Figure 4A). As expected, AML cell lines expressing either of the 3 variants showed reduced sensitivity to venetoclax (Figure 2C-D), as well as resistance to another BCL2 antagonist: S55746 (binds P1-P3 pockets) (supplemental Figure 4B-C). Interestingly, although BCL2 variants Asp103Glu, Phe104Leu, and Val148Leu conferred comparable resistance to venetoclax, sensitivity to S55746 was differentially less impacted by Asp103Glu,3 in contrast to Phe104Leu, which was more resistant, with Val148Leu intermediate in sensitivity (supplemental Figure 4B-C). Consistent with BCL2 Asp103Glu resulting in a binding pocket conformation structurally resembling BCL-XL, AML cell lines expressing this variant had enhanced sensitivity to navitoclax (Figure 2B-C). In contrast, cell lines expressing BCL2 Phe104Leu and Val148Leu were both resistant (to navitoclax). Interestingly, BCL2 variants resistant to venetoclax had no impact on sensitivity to azacitidine (supplemental Figure 4B) or the MCL-1 inhibitor S63845 (supplemental Figure 4A-C), highlighting potential for combination therapy to be an effective treatment option for patients developing BCL2 mutated clones in AML.
In conclusion, this report highlights several novel findings of relevance to patients receiving venetoclax for AML. These include clinical emergence of on-target BCL2 variants as a relevant mechanism of venetoclax resistance; experimental confirmation that BCL2 Asp103Glu, Phe104Leu, and Val148Leu are mediators of venetoclax resistance; and rapid emergence of polyclonal variants in AML blasts resistant to venetoclax dose escalation. Studies of resistance mechanisms to venetoclax combined with low-intensity chemotherapy for AML are ongoing from large-scale trials and will be important to estimate the frequency with which BCL2 variants emerge. With potential for BCL2 variants to be underreported as a mechanism of treatment failure after venetoclax therapy, our findings encourage consideration of BCL2 gene characterization at relapse to guide rational decisions regarding BCL2-targeted therapy in AML.
Acknowledgments: This work was supported by grants from the Australian National Health and Medical Research Council (A.H.W., F.C.B., R.B., and P.C.) and Veski (R.B.).
Contribution: F.C.B., X.W., M.C., A.H.W., and A.E.P. conceived and designed the study; X.W., M.C., and A.E.P. were responsible for patient care; F.C.B, X.W., R.B., C.C.C., T.M., S.K., G.P., P.B., D.C.S.H., and P.C. collected, analyzed, and interpreted the data; S.F.P. and G.Y. provided methodological support and interpretation of the bulk sequencing data; F.C.B, X.W., M.C., A.H.W., and A.E.P. wrote the manuscript; and all authors reviewed the data and contributed to critical revision of the manuscript.
Conflict-of-interest disclosure: A.H.W. has served on advisory boards for Novartis, AstraZeneca, Astellas, Janssen, Jazz, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, Bristol Myers Squibb, and BeiGene; has consulted for AbbVie, Servier, Novartis, Shoreline, and Aculeus; receives research funding to the institution from Novartis, AbbVie, Servier, Bristol Myers Squibb, Syndax, Astex, AstraZeneca, and Amgen; and serves on speaker’s bureau for AbbVie, Novartis, Bristol Myers Squibb, Servier, and Astellas. A.H.W., F.C.B., T.M., R.B., G.P., D.C.S.H., and P.C. are employees of the Walter and Eliza Hall Institute (WEHI). WEHI receives milestone and royalty payments related to the development of venetoclax. Current and past employees of WEHI may be eligible for financial benefits related to these payments. A.H.W. and P.C. receive such a financial benefit. C.C.C. has served on advisory boards for AbbVie, Pfizer, and Sumitomo Pharma Oncology and on speaker’s bureau for Bristol Myers Squibb, AstraZeneca, and AbbVie. A.E.P. has served on advisory boards or consulted for AbbVie, Genentech, Bristol Myers Squibb, Astellas, Daiichi Sankyo, Immunogen, Rigel, Syndax, Schrödinger, Aptose, Curis, and Foghorn; and receives research funding to the institution from AbbVie, Astellas, Daiichi Sankyo, FujiFilm, and Syndax. The remaining authors declare no competing financial interests.
Correspondence: Alexander E. Perl, Division of Hematology-Oncology, Abramson Cancer Center, University of Pennsylvania, 3400 Civic Blvd, PCAM, 12-154 South Tower, Philadelphia, PA 19104; email: alexander.perl@pennmedicine.upenn.edu; and Andrew H. Wei. Division of Blood Cells and Blood Cancer, Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia; email: wei.a@wehi.edu.au.
References
Author notes
F.C.B. and X.W. are joint first authors and contributed equally to this study.
A.H.W. and A.E.P. are joint senior authors.
Data are available on request from the corresponding author, Alexander E. Perl (alexander.perl@pennmedicine.upenn.edu).
The full-text version of this article contains a data supplement.