TO THE EDITOR:
VEXAS is a new entity encompassing a variety of autoimmune or autoinflammatory and hematological conditions, including myelodysplastic syndrome (MDS) or neoplasms.1,2
Somatic mutations of the X-linked UBA1 gene in myeloid progenitors constitute the molecular underpinnings of VEXAS, typically occurring in men during the sixth decade of life.3 Over the last 3 years since its first description, VEXAS has elicited wide medical interest resulting in the identification of pleomorphic clinical phenotypes, making this syndrome a “big masquerader” and, frequently, a clinical diagnostic challenge.
Considering the multifaceted manifestations, treatment strategies are diverse, and either aim at suppression of the UBA1-mutant clone (eg, with azacitidine or allogeneic hematopoietic cell transplant [allo-HCT]) or blockade of the downstream pleiotropic effects of hypercytokinemia.4 However, no treatment guidelines exist to date, and patients may manifest life-threatening inflammatory symptoms frequently refractory to multiple therapies. Because few literature reports have described the successful use of transplant,5-8 we hereby report the collated outcomes of patients with VEXAS undergoing allo-HCT in a multicenter European Society for Blood and Marrow Transplantation (EBMT) registry–based study.
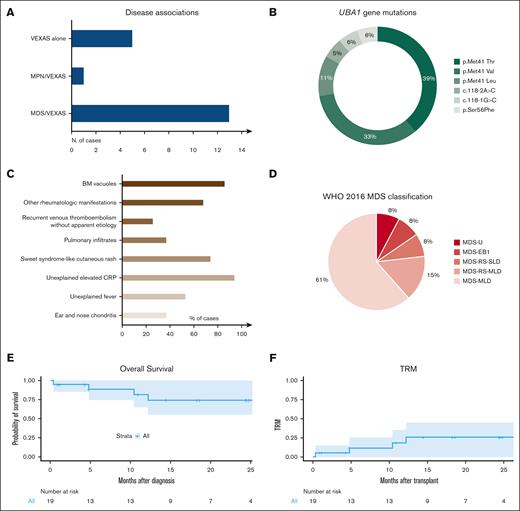
We accrued 19 patients, all males and with a median age of 59 years (interquartile range [IQR], 52-61) at the time of transplant (Table 1; supplemental Table 1). The majority had concomitant MDS (n = 13), whereas 5 presented with only autoinflammatory manifestations, and 1 had MPN (Figure 1A). UBA1 mutations consisted of missense substitutions at the Met41 hotspot in 83% of cases, followed by mutations of the splice acceptor site of exon 3 (11%), and the rare p.Ser56Phe (6%; Figure 1B). Clonal burden evaluation (available in 7 patients at diagnosis) revealed a median variant allelic frequency (VAF) of 73% (IQR, 43-88).
Patient characteristics
| Characteristic . | Total cohort (n = 19) . |
|---|---|
| Demographics | |
| Age in y∗ | 59 (52-61) |
| Male gender | 100% |
| Laboratory features | |
| UBA1 Met41 substitutions | 83% |
| Hemoglobin (g/dL)∗ | 9 (7.8-10.6) |
| MCV (fL)∗ | 100 (92-116) |
| White blood cells (×109/L)∗ | 6.9 (3.4-8.9) |
| Absolute neutrophil counts (×109/L)∗ | 4.4 (2.6-7.8) |
| Absolute monocyte counts (×109/L)∗ | 0.3 (0.1-0.4) |
| Absolute lymphocyte counts (×109/L)∗ | 0.9 (0.4-1.6) |
| Platelet counts (×109/L)∗ | 149 (61-263) |
| MN features | |
| Concomitant MDS | 68% |
| Concomitant MPN | 5% |
| BM blast in MDS (%)∗ | 2 (1-3) |
| IPSS-R MDS (n = 13) categories, (%) | |
| Very low | 15% |
| Low | 31% |
| Intermediate | 38% |
| Missing due to cytogenetic failure | 15% |
| Allo-HCT conditioning | |
| RIC | 74% |
| MAC | 26% |
| Graft source | |
| BM | 5% |
| PBSC | 95% |
| Donor choice | |
| MRD | 16% |
| MUD | 63% |
| MMUD | 5% |
| MMRD | 16% |
| GVHD prophylaxis | |
| Pt-CY | 32% |
| ATG | 58% |
| Alemtuzumab | 10% |
| Characteristic . | Total cohort (n = 19) . |
|---|---|
| Demographics | |
| Age in y∗ | 59 (52-61) |
| Male gender | 100% |
| Laboratory features | |
| UBA1 Met41 substitutions | 83% |
| Hemoglobin (g/dL)∗ | 9 (7.8-10.6) |
| MCV (fL)∗ | 100 (92-116) |
| White blood cells (×109/L)∗ | 6.9 (3.4-8.9) |
| Absolute neutrophil counts (×109/L)∗ | 4.4 (2.6-7.8) |
| Absolute monocyte counts (×109/L)∗ | 0.3 (0.1-0.4) |
| Absolute lymphocyte counts (×109/L)∗ | 0.9 (0.4-1.6) |
| Platelet counts (×109/L)∗ | 149 (61-263) |
| MN features | |
| Concomitant MDS | 68% |
| Concomitant MPN | 5% |
| BM blast in MDS (%)∗ | 2 (1-3) |
| IPSS-R MDS (n = 13) categories, (%) | |
| Very low | 15% |
| Low | 31% |
| Intermediate | 38% |
| Missing due to cytogenetic failure | 15% |
| Allo-HCT conditioning | |
| RIC | 74% |
| MAC | 26% |
| Graft source | |
| BM | 5% |
| PBSC | 95% |
| Donor choice | |
| MRD | 16% |
| MUD | 63% |
| MMUD | 5% |
| MMRD | 16% |
| GVHD prophylaxis | |
| Pt-CY | 32% |
| ATG | 58% |
| Alemtuzumab | 10% |
BM, bone marrow; IPSS-R, Revised International Prognostic Scoring System; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MMRD, mismatched related donor, MMUD, mismatched unrelated donor; MN, myeloid neoplasia; MPN, myeloproliferative neoplasm; MRD, matched related donor; MUD, matched unrelated donor; RIC, reduced-intensity conditioning; PBSC, peripheral blood stem cells.
Data are presented as median (IQR).
VEXAS features and post-transplant outcomes. (A) Bar graph shows the distribution of patients according to disease phenotypes and association with myeloid neoplasia (myelodysplastic syndrome, MDS or myeloproliferative neoplasia, MPN). (B) Pie chart detailing the frequency of the UBA1 mutations identified in our cohort. (C) Bar graph shows VEXAS clinical manifestations. (D) Pie chart showcases the MDS diagnosis according to the WHO 2016 MDS classification. (E) Kaplan-Meier curve shows the probability of OS in 19 patients with VEXAS undergoing allo-HCT. Numbers at risk are indicated below the curve. (F) Kaplan-Meier curve shows TRM in 19 patients with VEXAS undergoing allo-HCT. Numbers at risk are indicated below the curve.
VEXAS features and post-transplant outcomes. (A) Bar graph shows the distribution of patients according to disease phenotypes and association with myeloid neoplasia (myelodysplastic syndrome, MDS or myeloproliferative neoplasia, MPN). (B) Pie chart detailing the frequency of the UBA1 mutations identified in our cohort. (C) Bar graph shows VEXAS clinical manifestations. (D) Pie chart showcases the MDS diagnosis according to the WHO 2016 MDS classification. (E) Kaplan-Meier curve shows the probability of OS in 19 patients with VEXAS undergoing allo-HCT. Numbers at risk are indicated below the curve. (F) Kaplan-Meier curve shows TRM in 19 patients with VEXAS undergoing allo-HCT. Numbers at risk are indicated below the curve.
All patients presented with macrocytic anemia with a median hemoglobin of 9 g/dL (IQR, 7.8-10.6) and a median mean corpuscular volume (MCV) of 100 fL (IQR, 92-116). Rheumatologic manifestations (eg, Sweet syndrome–like cutaneous rash9) were present at diagnosis in all patients (Figure 1C), and apart from steroids, which were universally used, a specific treatment was required in 74% of patients.
VEXAS symptoms preceded the diagnosis of MDS/MPN in the majority of cases (median time, 27 months [IQR, 10-39]), whereas in 3 patients the presentation was concomitant, and in 1 patient the MDS was diagnosed almost 2 years (22 months) before VEXAS symptoms onset. According to the 2016 World Health Organization criteria,10 MDS was classified as detailed in Figure 1D. Karyotype was normal in 11 VEXAS/MDS cases (n = 2 culture failure), and allocation according to Revised International Prognostic Scoring System was as follows: intermediate n = 5, low n = 4, and very low n = 2 patients. Consistently, median bone marrow blast count was 2% (IQR, 1-3).
Overall, mutational screening was available for 17 cases (n = 12 cases with MDS, n = 1 with MPN, and n = 4 with VEXAS alone). In line with previous reports,11,12DNMT3A was the most frequently mutated gene (n = 4), followed by TET2 (n = 3), KRAS (n = 2), and IDH2, NRAS, SRSF2, ASXL1, and RUNX1 (n = 1 each).
Allo-HCT was performed at a median of 41 months (IQR, 22-58) from VEXAS onset. Before transplant, patients received a variety of both rheumatological and hematological treatments, with a high degree of refractoriness (median number of lines 5; IQR, 1-6). Hematological therapy consisted of azacitidine in 8 cases (complete response [CR; n = 3], stable disease [SD; n = 2], refractory [n = 3]), and erythropoietin-stimulating agents in 5 cases to lower transfusion burden. Rheumatological therapy was extremely varied and mostly dependent on disease-specific manifestations. Indeed, patients were administered sequential combinations of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate, hydroxychloroquine, and azathioprine; biologics (bDMARDs), such as anti-IL1, anti-IL6, and anti-TNF; and targeted synthetic DMARDs (tsDMARDs). Given previous reports regarding the efficacy of tsDMARDs,13 we specifically inquired on the response to this category in our cohort. Patients who were administered ruxolitinib achieved CR (n = 2) and partial response (n = 2), whereas the 2 cases treated with baricitinib achieved a partial response.
In all cases, stability of the clinical phenotype with no flares or uncontrolled manifestations was attained at the time of allo-HCT. Of particular note is that in 4 cases (n = 3 MDS and 1 MPN), clonal progression with acquisition of additional chromosomal alterations (+8, +21, t[12;16], del[13][q14]) was evident. Overall, 56% of patients had a Karnofsky score >80%, and 5 were classified as high-risk (≥ 3) as per the hematopoietic cell transplantation (HCT)–specific comorbidity index score. Donor choice consisted of matched related in 16%, matched unrelated in 63%, mismatched related in 16%, and mismatched unrelated in 5% of the cohort. Peripheral blood was the preferred stem cell source in all but 1 case, and a reduced-intensity regimen was used in 14 patients (74%).14 Overall, conditioning was based on a backbone of fludarabine plus busulfan (n = 7), treosulfan (n = 4), busulfan and thiotepa (n = 3), melphalan (n = 2, of whom 1 with total body irradiation), melphalan and thiotepa (n = 1), and the FLAMSA/busulfan-cyclophosphamide sequential combination (n = 2). Graft-versus-host disease (GVHD) prophylaxis was based on posttransplant cyclophosphamide (n = 6), or serotherapy (n = 11 ATG, n = 2 alemtuzumab), in combination with cyclosporine and short-course methotrexate (n = 7) or mycophenolate (n = 5). Only 1 patient received an ex vivo manipulated graft after TCRαβ/CD19 depletion.
All but 1 patient, experiencing primary graft failure, reached neutrophils and platelets engraftment at a median time of 16 days (range, 8-32) and 15 days (range, 4-47) from allo-HCT, respectively. Overall, 94% of patients achieved full-donor chimerism at last follow-up and in 2 cases donor-lymphocyte infusion were administered because of an initial (<6 months) mixed chimeric state. Acute GVHD occurred in 58% of cases, with grade 2 to 4 noted in 26% of patients at a median of 1.9 months (range, 0.3-4) from graft infusion. Chronic GVHD presented in 4 patients at a median of 4.6 months (range, 3.3-5) from transplant, which in 2 cases resolved by the first year after the procedure. Conversely, 1 patient died in CR because of bacterial infection and severe, multiple-refractory chronic GVHD, which in an additional case was still unresolved at the last follow-up.
With a median follow-up of 14 months (range, 0.4-86) from allo-HCT, 2-year overall survival (OS) and transplant-related mortality (TRM) were 74.2% (95% confidence interval [CI], 55.1-100) and 25.8% (95% CI, 7.3-49.6), respectively (Figure 1E-F). Of note, 4 patients died of bacterial infection (n = 3) and CNS toxicity (n = 1). Remarkably, no patient experienced relapse of VEXAS symptoms or MDS/MPN, and all cases (n = 11) with >1 year follow-up displayed resolution of the VEXAS-specific phenotypes and were able to discontinue any immunosuppression between 2 and 10 months after transplant. Finally, molecular studies (n = 6) revealed disappearance (by Sanger) or eradication (digital-droplet polymerase chain reaction) of UBA1 mutations by 3 to 6 months after allo-HCT, paralleling the reversion of the disease clinical phenotype.
VEXAS is a complex syndrome characterized by inflammatory and hematological features, including propensity to MDS development.15 Currently, allo-HCT remains the only curative option for MDS and is also used to treat rheumatologic conditions refractory to conventional treatments.16 Although indication for transplant is controversial in lower-risk MDS, a previous EBMT study on 246 such patients reported OS and TRM rates of 58% and 30%, respectively at 3 years after allo-HCT.17 Analogous results were reported allografting rheumatological or autoimmune disorders, with OS and TRM rates of 70% and 20.5%, respectively at 5 years.18 However, allo-HCT is burdened by a nonnegligible risk of morbidity and mortality, which needs to be judiciously considered when identifying patients suitable for transplant. Recently, the autoimmune diseases and chronic malignancies working parties of the EBMT offered guidance with regard to transplant strategies in VEXAS.1,4 Although waiting for the results of the prospective phase 2 clinical trial ongoing (NCT05027945), we here present the largest series of VEXAS patients who received allograft so far, highlighting the curative potential of allo-HCT. Although acknowledging the limitations of a small, retrospective study with a short follow-up, fludarabine-based conditioning regimens appeared able to facilitate good transplant-related outcomes with limited GVHD and TRM in line with previous experiences in VEXAS,19 MDS17 and rheumatological disorders,18 also reversing the clinical phenotype of the disease. Notably, in 6 patients we registered the abatement of UBA1 VAF after transplant, adding to the very limited evidence on the UBA1 clonal kinetics after this procedure.20 Given the hemato-inflammatory nature of the disease, allo-HCT may be a viable option in selected patients with VEXAS. As experience accumulates, the optimal transplant conditioning platform, GVHD prophylaxis, and timing of the procedure will hopefully become more apparent.
Acknowledgments: The authors thank the European Society for Blood and Marrow Transplantation centers participating in the study. The authors thank AIRC 5 × 1000 call “Metastatic disease: the key unmet need in oncology” to MYNERVA project, #21267 to M.T.V. (Myeloid Neoplasms Research Venture AIRC. A detailed description of the MYNERVA project is available at http://www.progettoagimm.it).
C.G. was supported by a grant from the Edward P. Evans Foundation.
Contribution: C.G. and D.M.L. supervised the project, collected, analyzed, interpreted clinical data, and wrote the manuscript; and all authors participated in patient recruitment, collected clinical and molecular data, and edited the manuscript, read, and approved the final version of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carmelo Gurnari, Department of Biomedicine and Prevention, Tor Vergata University, Viale Oxford 81, 00133, Rome, Italy;.
References
Author notes
Informed consent was obtained according to the protocols approved by the institutional review board of the participating institutions, European Society for Blood and Marrow Transplantation regulations, and in accordance with the ethical principles set forth by the Declaration of Helsinki.
All data are presented in the manuscript. Requests for additional information should be sent to the corresponding author, Carmelo Gurnari (gurnarc@ccf.org).
The full-text version of this article contains a data supplement.