TO THE EDITOR:
Targeted agent-based therapies such as covalent Bruton tyrosine kinase inhibitors (cBTKis) and venetoclax (VEN) plus anti-CD20 monoclonal antibodies have achieved superior outcomes over chemoimmunotherapy among patients with CLL with a range of fitness and comorbidity levels, in the first-line and relapsed/refractory (RR) settings.1-7 However, data to inform the optimal sequencing of targeted agents remain limited.8-10 Continuous VEN monotherapy has demonstrated efficacy in a prospective trial with patients who were heavily pretreated with either cBTKi failure or intolerance.8 However, patients with prior B-cell receptor signaling inhibitor exposure were minimally represented (n = 5) in the phase 3 MURANO study of time-limited VEN-rituximab (VEN-R). Consequently, the outcomes with time-limited VEN-R in this context are unknown.4 Given the poor survival of patients with BTKi- and VEN-resistant CLL,11,12 time-limited therapy is attractive to mitigate the emergence of resistance, potentially enabling retreatment.13-17 To address this data gap, we report outcomes of an Australian multicenter real-world cohort of patients receiving time-limited VEN-R for cBTKi-exposed CLL.
We retrospectively reviewed records of 47 consecutive patients treated at the Royal Melbourne Hospital and Peter MacCallum Cancer Centre (Melbourne), the Princess Alexandra Hospital (Brisbane), and Royal North Shore Hospital (Sydney) between November 2016 and February 2023 who received VEN-containing therapy for cBTKi-exposed CLL. Patient and disease characteristics before VEN were collected as displayed in Table 1. Genomic complexity was defined as ≥5 lesions on conventional metaphase karyotyping or single nucleotide polymorphism microarray.18,19 Best response was assessed using 2018 International Workshop on CLL criteria.20 Undetectable measurable residual disease (uMRD) was defined as <1 CLL cell per 10 000 leukocytes in the peripheral blood (PB) or bone marrow (BM) by multiparameter flow cytometry analyzing ≥200 000 leukocytes.21,22 The study was approved by the local human research ethics committee (PMC16/167). The Kaplan-Meier method was used to estimate progression-free survival (PFS) and overall survival (OS). Patients without relevant events were censored at the date of last follow-up or data cutoff (20 February 2023). Patients who underwent allogeneic stem cell transplantation (allo-SCT) were censored at that date for PFS. Associations between clinico-pathological variables and PFS were analyzed using the log-rank test with α = .05. Analyses were performed using STATA (version 17.0 for Mac) and GraphPad Prism (version 9.5.1 for Mac).
Patient characteristics before VEN-containing regimen
| . | VEN-R . | VEN monotherapy . | Whole cohort . |
|---|---|---|---|
| Clinico-pathologic characteristics before VEN | |||
| n | 32 | 15 | 47 |
| Age, y (median, range) | 70.5 (49-84) | 68 (47-86) | 70 (47-86) |
| Treatments (n) before VEN (including cBTKi)∗ | 2 (1-5) | 3 (1-7) | 2 (1-7) |
| Chemoimmunotherapy exposed | 28 (89%) | 14 (93%) | 42 (89%) |
| Fludarabine refractory (<PR or PD within 6 mos) | 3 (9%) | 1 (7%) | 4 (9%) |
| First cBTKi-containing therapy | |||
| Ibrutinib | 23 (72%) | 14 (93%) | 37 (79%) |
| Acalabrutinib | 1 (3%) | 0 (0%) | 1 (2%) |
| Zanubrutinib | 8 (25%) | 1 (7%) | 9 (19%) |
| Reason for BTKi cessation | |||
| PD | 25 (78%) | 13 (87%) | 38 (81%) |
| Toxicity | 7 (22%) | 2 (13%) | 9 (19%) |
| Time to progression after cBTKi initiation, mos (median, range) | 32 (6.3-83.1) | 24.0 (1.1-90.7) | 31.5 (1.1-90.7) |
| Intervening therapy between cBTKi-containing regimen and VEN | |||
| None | 29 (91%) | 13 (87%) | 42 (89%) |
| Bendamustine-R | 0 (0%) | 1 (7%) | 1 (2%) |
| Methylprednisolone-R | 0 (0%) | 1 (7%) | 1 (2%) |
| Pirtobrutinib | 3 (9%) | 0 (0%) | 3 (6%) |
| Genetics before VEN-containing regimen | |||
| IGHV unmutated | 13 of 15 (87%) | 5 of 6 (83%) | 18 of 21 (86%) |
| Genomic complexity (≥5 lesions) | 8 of 16 (50%) | 3 of 8 (38%) | 11 of 24 (46%) |
| del17p and/or TP53 mutated | 17 of 24 (71%) | 9 of 14 (64%) | 26 of 38 (68%) |
| BTKi resistance mutation(s) detected | 13 of 16 (81%) | 2 of 5 (40%) | 15 of 21 (71%) |
| Outcomes after VEN-based therapy | |||
| Best iwCLL response to VEN-based regimen | |||
| CR¶ | 6 (19%) | 3 (20%) | 9 (19%) |
| PR | 20 (63%) | 4 (27%) | 24 (51%) |
| SD | 3 (9%) | 4 (27%) | 7 (15%) |
| PD | 2 (6%) | 0 (0%) | 2 (4%) |
| Not evaluated† | 1 (3%) | 4 (27%) | 5 (11%) |
| uMRD attained in PB or BM‡ | 7 of 10 (70%) | 1 of 5 (20%) | 8 of 15 (53%) |
| Reason for VEN cessation | |||
| PD | 9 (28%) | 6 (40%) | 15 (32%) |
| Remains on VEN at last follow-up | 10 (31%) | 3 (20%) | 13 (28%) |
| Completed time-limited therapy | 6 (19%) | 0 (0%) | 6 (13%) |
| Proceeded to allo-SCT | 3 (9%) | 1 (7%) | 4 (9%) |
| Toxicity§ | 1 (3%) | 3 (20%) | 4 (9%) |
| Other|| | 3 (9%) | 2 (13%) | 5 (11%) |
| . | VEN-R . | VEN monotherapy . | Whole cohort . |
|---|---|---|---|
| Clinico-pathologic characteristics before VEN | |||
| n | 32 | 15 | 47 |
| Age, y (median, range) | 70.5 (49-84) | 68 (47-86) | 70 (47-86) |
| Treatments (n) before VEN (including cBTKi)∗ | 2 (1-5) | 3 (1-7) | 2 (1-7) |
| Chemoimmunotherapy exposed | 28 (89%) | 14 (93%) | 42 (89%) |
| Fludarabine refractory (<PR or PD within 6 mos) | 3 (9%) | 1 (7%) | 4 (9%) |
| First cBTKi-containing therapy | |||
| Ibrutinib | 23 (72%) | 14 (93%) | 37 (79%) |
| Acalabrutinib | 1 (3%) | 0 (0%) | 1 (2%) |
| Zanubrutinib | 8 (25%) | 1 (7%) | 9 (19%) |
| Reason for BTKi cessation | |||
| PD | 25 (78%) | 13 (87%) | 38 (81%) |
| Toxicity | 7 (22%) | 2 (13%) | 9 (19%) |
| Time to progression after cBTKi initiation, mos (median, range) | 32 (6.3-83.1) | 24.0 (1.1-90.7) | 31.5 (1.1-90.7) |
| Intervening therapy between cBTKi-containing regimen and VEN | |||
| None | 29 (91%) | 13 (87%) | 42 (89%) |
| Bendamustine-R | 0 (0%) | 1 (7%) | 1 (2%) |
| Methylprednisolone-R | 0 (0%) | 1 (7%) | 1 (2%) |
| Pirtobrutinib | 3 (9%) | 0 (0%) | 3 (6%) |
| Genetics before VEN-containing regimen | |||
| IGHV unmutated | 13 of 15 (87%) | 5 of 6 (83%) | 18 of 21 (86%) |
| Genomic complexity (≥5 lesions) | 8 of 16 (50%) | 3 of 8 (38%) | 11 of 24 (46%) |
| del17p and/or TP53 mutated | 17 of 24 (71%) | 9 of 14 (64%) | 26 of 38 (68%) |
| BTKi resistance mutation(s) detected | 13 of 16 (81%) | 2 of 5 (40%) | 15 of 21 (71%) |
| Outcomes after VEN-based therapy | |||
| Best iwCLL response to VEN-based regimen | |||
| CR¶ | 6 (19%) | 3 (20%) | 9 (19%) |
| PR | 20 (63%) | 4 (27%) | 24 (51%) |
| SD | 3 (9%) | 4 (27%) | 7 (15%) |
| PD | 2 (6%) | 0 (0%) | 2 (4%) |
| Not evaluated† | 1 (3%) | 4 (27%) | 5 (11%) |
| uMRD attained in PB or BM‡ | 7 of 10 (70%) | 1 of 5 (20%) | 8 of 15 (53%) |
| Reason for VEN cessation | |||
| PD | 9 (28%) | 6 (40%) | 15 (32%) |
| Remains on VEN at last follow-up | 10 (31%) | 3 (20%) | 13 (28%) |
| Completed time-limited therapy | 6 (19%) | 0 (0%) | 6 (13%) |
| Proceeded to allo-SCT | 3 (9%) | 1 (7%) | 4 (9%) |
| Toxicity§ | 1 (3%) | 3 (20%) | 4 (9%) |
| Other|| | 3 (9%) | 2 (13%) | 5 (11%) |
IGHV, immunoglobulin heavy-chain variable region gene; iwCLL, International Working Group on CLL; PR, partial response; SCC, squamous cell carcinoma; SD, stable disease; t-MN, therapy-related myeloid neoplasm.
One patient previously had undergone allo-SCT; 5 patients received a BTKi-containing regimen as first-line therapy;
Not evaluated because of early death (infection, n =3; t-MN, n =2);
uMRD, defined as <1 CLL cell per 10 000 leukocytes by flow cytometry;
Fatal infection (n = 2), fatal intracranial hemorrhage (n = 1), and recurrent neutropenia (n = 1);
t-MN (n = 2), radiotherapy for anal SCC (n =1), metastatic SCC (n =1), metastatic melanoma (n =1);
BM and CT evaluation was performed in 11 of 23 (48%) patients with clinical parameters consistent with CR, patients not assessed by both methods were classified as achieving PR.
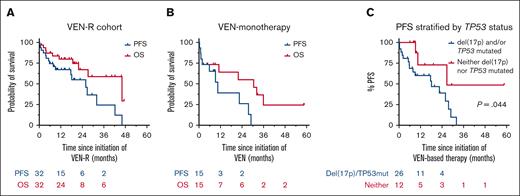
In total, 32 (68%) patients received VEN-R intended as time-limited therapy, 15 (32%) patients received continuous VEN monotherapy (Table 1). Among the VEN-R cohort (summarized in supplemental Figure 1), 25 (78%) patients discontinued cBTKi because of progressive disease (PD) and the median time to progression from cBTKi initiation was 32 months (range, 6.3-83.1 months). cBTKi-resistance mutations were detected in 13 of 16 patients tested (81%; supplemental Table 1). VEN-R therapy was the immediate next treatment in 91% of cases; 3 patients received intervening pirtobrutinib. The median time from cessation of BTKi to initiation of VEN-R was 26 days (range, 0-37 months). Before VEN-R commencement, most patients had high-risk disease genetics: unmutated immunoglobulin heavy-chain variable region gene (13 of 15; 87%), genomic complexity (8 of 16; 50%), and del(17p) and/or TP53 mutation (17 of 24; 71% [both lesions in 7 of 20 (35%) patients]). The objective response rate (ORR) to VEN-R was 81% (26 of 32). Of the 21 patients with clinical parameters consistent with complete response (CR), computed tomography and BM evaluation was performed in 8 (38%) patients. The formal CR rate was 19% (6 of 32 patients). Among the 10 patients assessed (6 in partial response and 4 in CR), PB/BM uMRD was attained in 7 (70%) patients. At a median follow-up of 20.6 months (range, <1-58.6 months), 10 (31%) patients remain on VEN and 22 (69%) have ceased therapy because of: PD (n = 9; 28%), completion of time-limited therapy (n = 6; 19%), planned allo-SCT (n = 3, 9%), toxicity (n = 1, 3%), or intercurrent malignancy (n = 3, 9%). The median duration of VEN exposure was 10.0 months (range, <1-27 months). Of 6 patients who completed time-limited therapy, 3 developed progressive CLL at 3, 5, and 17 months off treatment, respectively; 2 remain in remission at <1 and 3 months off treatment; and 1 died of infection in remission, 22 months after VEN cessation. The median PFS after VEN-R initiation was 25.9 months (95% confidence interval [CI], 9.2-42.2) and the median OS was 46.1 months (95% CI, 21.9-not estimable [NE]; Figure 1A). Among 3 patients previously treated with pirtobrutinib, 2 proceeded to allo-SCT in CR and remain alive in remission at 3 and 8 months after transplant, respectively; the third died of therapy-related myeloid neoplasm, 2 months after initiating VEN-R. The median PFS and OS for patients receiving continuous VEN monotherapy was 10.5 months (95% CI, 1.1-28.9) and 30.5 months (95% CI, 1.1-NE), respectively (Figure 1B).
Outcomes after VEN-based regimens for patients with cBTKi-exposed CLL. (A) PFS and OS for patients who received VEN-R; (B) PFS and OS for patients who received VEN monotherapy; and (C) PFS, stratified by TP53 status for patients receiving VEN-based therapy.
Outcomes after VEN-based regimens for patients with cBTKi-exposed CLL. (A) PFS and OS for patients who received VEN-R; (B) PFS and OS for patients who received VEN monotherapy; and (C) PFS, stratified by TP53 status for patients receiving VEN-based therapy.
Among the 12 patients who developed PD after VEN-R, disease histology was CLL in 4 (33%), diffuse large B-cell lymphoma–type Richter transformation (RT) in 6 (50%), with 1 case each of Hodgkin-type RT (8%) and interdigitating dendritic cell neoplasm (8%). From the time of PD after VEN, the median OS was 11.4 months (95% CI, 3.3-NE). Histology at PD and causes of death are shown in supplemental Table 2.
Univariate analyses were performed to assess correlation between pre-VEN clinico-pathologic variables with PFS after VEN-based therapy (supplemental Table 3). The presence of del(17p) and/or TP53 mutations was significantly associated with inferior PFS (hazard ratio, 3.55; 95% CI, 1.03-12.17; P = .044; Figure 1C). Among 5 patients without prior chemoimmunotherapy exposure, 2 developed PD at 6 and 9 months after VEN initiation, respectively, and 3 remain on VEN at last follow-up (range, 8-17 months). Among 8 patients who attained uMRD on VEN-based therapy, 2 proceeded to allo-SCT in ongoing remission; 2 remain on VEN at last follow-up at 14 and 19 months, respectively; 2 developed PD at 8 and 27 months after VEN-initiation, respectively; 1 died of heart failure in remission 46 months after VEN-initiation; and 1 remains in remission at 59 months despite ceasing VEN because of recurrent neutropenia after 2.5 months of therapy.
Herein, we report, to our knowledge, the largest series of patients receiving VEN-R intended as time-limited therapy for cBTKi-exposed CLL, an increasingly commonly encountered clinical scenario. In the phase 3 MURANO trial of VEN-R in RR CLL, the end of combination treatment rate of PB uMRD was 62%, the median PFS was 53.6 months, and the majority of patients experienced durable treatment-free remissions.4,23 Among this cBTKi-exposed cohort, despite frequent responses, the median PFS was 25.9 months and durable treatment-free remissions were infrequently observed. RT at disease progression was common (8 of 12; 67%). These outcomes are consistent with other reports of VEN monotherapy among patients with cBTKi-exposed CLL, and comparable with alternative therapeutic options for such patients. Although more heavily pretreated (median, 4 prior lines of therapy), continuous VEN monotherapy achieved a similar ORR (65%) and PFS (median 24.7 months) among 91 patients with cBTKi-exposed CLL in a prospective trial.8 In a subanalysis of the phase 1/2 BRUIN trial, the noncovalent BTKi pirtobrutinib achieved an ORR of 73% and a median PFS of 19.6 months among patients with cBTKi-exposed disease (n = 247; median, 3 prior lines of therapy; TP53 aberrant in 90 of 193 [47%] patients).24 These analyses, and our data set, are notable for the prominent chemoimmunotherapy pretreatment, enriched adverse biology, and shorter duration of BTKi benefit (median time to progression was 32 months in this cohort) than observed for the majority of unselected patients starting BTKi.25 Therefore, the generalizability of these data to patients with lower-risk, chemoimmunotherapy-naïve CLL progressing after first-line BTKi is uncertain. In the CLL13 trial, obinutuzumab achieved superior remission depth and duration compared with R in combination with VEN for patients with treatment-naïve CLL and may be the superior anti-CD20 partner in RR, BTKi-exposed disease, however further study is required.1
In conclusion, VEN-R is an effective treatment for cBTKi- and chemoimmunotherapy-exposed RR CLL, although PFS appears shorter than described in patients who are cBTKi naïve. Clinicians and patients should be cognizant that prolonged treatment-free remissions are uncommon after prior BTKi exposure, particularly among patients with TP53-aberrant disease, limiting the potential for future VEN retreatment. Given the high rate of RT and poor salvageability anticipated at PD, consolidative strategies such as allo-SCT or other immune-based therapies should be considered in appropriate patients with responsive disease.
Contribution: T.E.L., R.B., P.A.T., and M.A.A. conceived the project and designed the study; T.E.L., R.B., A.W., S.M.H., P.M., Y.S., S.P.M., J.C., P.B., C.S.T., J.F.S., A.W.R., P.A.T., and M.A.A. were responsible for patient care; P.B. contributed molecular data, and performed and analyzed genomic data; T.E.L., R.B., V.S.L., S.M.H., P.M., Y.S., and S.P.M. collected the data; T.E.L. analyzed the data; T.E.L. and R.B. wrote the first version of the manuscript; and all authors reviewed the data and contributed to critical revision of the manuscript.
Conflict-of-interest disclosure: A.W.R., M.A.A., T.E.L., and V.S.L. are employees of the Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to VEN. A.W.R., M.A.A., and T.E.L. are recipients of a share in royalty payments paid to the Walter and Eliza Hall Institute of Medical Research. T.E.L. has received honoraria from AbbVie. R.B. has received sponsorship from AbbVie. S.M.H. has received honoraria from AstraZeneca, Janssen, and Gilead, and nonfinancial assistance from AbbVie. P.M. has served on advisory boards for, and received honoraria or speaker fees from, AbbVie, BeiGene, Janssen, AstraZeneca, Astellas, Otsuka, Menarini, and Jazz. S.P.M. has acted as a consultant/advisor for AbbVie, AstraZeneca, BeiGene, Janssen, Gilead, and Roche. A.W. is an advisory board member for AbbVie and AstraZeneca, and has received honoraria from AbbVie, Roche, AstraZeneca, Novartis, and BeiGene. C.S.T. has received honoraria and research funding from AbbVie and Janssen, and received honoraria from BeiGene. A.W.R. received research funding from AbbVie, Genentech, Servier, Janssen, and BeiGene. J.F.S. receives research funding from AbbVie, Genentech, Celgene, and Janssen, and is an advisory board member for, and has received honoraria from, AbbVie, Acerta, Celgene, Genentech, Janssen, Roche, Sunesis, and Takeda. M.A.A. has received honoraria from AbbVie, Janssen, AstraZeneca, Novartis, Takeda, Kite, and CSL Behring. P.A.T. has received honoraria from AbbVie, Adaptive Biotechnologies, AstraZeneca, BeiGene, Genentech, Janssen, Lilly, Merck, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Mary Ann Anderson, Walter and Eliza Hall Institute, Department of Clinical Haematology, Royal Melbourne Hospital and Peter MacCallum Cancer Centre, 1G Royal Parade, Parkville, Melbourne, VIC 3141, Australia; email: maryann.anderson2@mh.org.au.
References
Author notes
T.E.L. and R.B. contributed equally to this study.
P.A.T. and M.A.A. contributed equally to this study.
Data are available on request from the corresponding author, Mary Ann Anderson (maryann.anderson2@mh.org.au).
The full-text version of this article contains a data supplement.