The intrinsic coagulation pathway drives hypercoagulability in active AAV.
Drivers of hypercoagulability are most likely related disease activity, vascular inflammation, and endothelial damage.
Visual Abstract
The risk of a venous thrombotic event (VTE) is increased in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV); however, a detailed understanding of the underlying mechanisms of hypercoagulability is limited. We assessed prospectively different coagulation parameters in 71 patients with active AAV at baseline and after 6 months of follow-up. D-dimers and fibrinogen were increased in most patients at presentation and remained elevated in half of the patients. Particularly, thrombin-antithrombin (T:AT) complex and activated coagulation factors in complex with their natural inhibitors of the intrinsic coagulation pathway (ie, activated FXII:C1 esterase inhibitor [FXIIa:C1Inh], FXIa:AT, and FXIa:alpha1-antitrypsin [FXIa:α1AT]) were profoundly elevated in patients at baseline. Thrombin formation was dominantly correlated with coagulation factors of the intrinsic pathway (ie, FXIIa:AT, FXIa:AT, FXIa:α1AT, and FXIa:C1Inh) compared to the extrinsic pathway (ie, FVIIa:AT). Hypercoagulability correlated with higher disease activity, ANCA levels, C-reactive protein, serum creatinine, and proteinuria. VTEs were observed in 5 out of 71 (7%) patients within 1 month (interquartile range, 1-5) after inclusion. Baseline T:AT levels were significantly higher in patients with VTE than in those without VTE (P = .044), but other clinical or laboratory markers were comparable between both groups. Hypercoagulability is dominantly characterized by activation of the intrinsic coagulation pathway and elevated D-dimers in active AAV. The driving factors of hypercoagulability are yet to be studied but are most likely related to an interplay of increased disease activity, vascular inflammation, and endothelial damage. Future targets for intervention could include inhibitors of the intrinsic coagulation pathway and compounds specifically reducing the hyperinflammatory state.
Introduction
The risk of a venous thrombotic event (VTE) is increased in patients with antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV), with a frequency of up to 14% and an incidence rate of 2.11 per 100 patient years.1 The risk is highest among patients with active disease involving the skin, lungs, and those with impaired kidney function at presentation. Most VTEs occur during active disease, suggesting that vascular inflammation and/or endothelial damage trigger hypercoagulability.2,3 Markers of hypercoagulability and endothelial damage, however, remained elevated in patients with AAV in clinical remission, indicating low-grade inflammation that persists during quiescent disease.4 In addition, impaired fibrinolysis has been demonstrated in a subset of patients with active AAV.5,6 However, knowledge on the specific pathophysiological mechanisms that lead to hypercoagulability in patients with AAV and its translation into clinical guidance for the identification of patients at highest risk is limited.
We, therefore, investigated the coagulation cascade in a well-defined prospective cohort of patients with active AAV, using state-of-the-art assays to test extensively for activation of the intrinsic and extrinsic pathways of coagulation, at presentation and follow-up.
Methods
Patient population
The study was conducted with patients with active AAV consecutively included in a prospective observational cohort between April 2019 and April 2022 at the Maastricht University Medical Center, Maastricht, the Netherlands. Patients with AAV were defined according to the revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides.7 Patients with granulomatosis with polyangiitis or microscopic polyangiitis were included. Patients with de novo AAV or those with major relapse of the disease who needed immunosuppressive treatment for the induction of a remission according to the EULAR/ERA-EDTA recommendations were included.8 Patients with therapeutic anticoagulation at presentation were excluded. Patients provided written informed consent. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the appropriate ethics committee (reference #2020_007).
Data and sample collection
Demographic, clinical, laboratory, radiographic, and therapeutic data were prospectively assessed. Blood samples were collected by venipuncture at the time of presentation before remission-induction treatment and after 6 months when a clinical remission was achieved on 3.2% trisodium citrate and on K2-EDTA (BD Vacutainer). Blood was processed immediately, centrifuged at 2000g for 10 minutes at room temperature, and stored at −80 °C until testing.
Outcome assessment
Disease activity was calculated according to the Birmingham Vasculitis Activity Score v3 (BVAS).9 Myeloperoxidase- and PR3-ANCA levels were assessed by using the Phadia ImmunoCap 250 (Thermo Fisher Scientific, Waltham, MA). Clinical remission was defined as a BVAS of 0 and a glucocorticoid dosage of ≤7.5 mg per day. Major relapse was defined as recurrent AAV (BVAS > 0) requiring the initiation of high-dose glucocorticoids with cyclophosphamide and/or rituximab.
Measurement of coagulation
The activated partial thromboplastin time was measured using Dade Actin FSL–activated PTT reagent (Siemens Healthcare Diagnostics, Marburg, Germany), prothrombin time (PT) was measured using Dade Innovin reagent (Siemens Healthcare Diagnostics), fibrinogen levels were determined with Multifibren U reagent (Siemens Healthcare Diagnostics), and D-dimer was measured using INNOVANCE D-Dimer kits (Siemens Healthcare Diagnostics). All assays were performed according to the manufacturer instruction using a BCS system (Siemens Healthineers, Erlangen, Germany). Prothrombin fragment 1+2 was measured using Enzygnost F1+2 ELISA kits (Siemens Healthineers) following the manufacturer instructions. Activated coagulation factors in complex with their natural inhibitors (ie, activated FVII:antithrombin [FVIIa:AT], FXIIa:AT, FXIIa:C1 esterase inhibitor [FXIIa:C1Inh], FXIa:AT, FXIa:alpha1-antitrypsin [α1AT], FXIa:C1Inh, FIXa:AT, FXa:AT, and thrombin:antithrombin [T:AT]) were quantified as described.10-12 In short, FVIIa:AT represents activation of the extrinsic pathway, FXIIa:AT, FXIIa:C1Inh, FXIa:AT, FXIa:α1AT, FXIa:C1Inh, and FIXa:AT represent activation of the intrinsic pathway, and FXa:AT as well as T:AT represent activation of the common pathway of coagulation.
Statistical analysis
Continuous variables were presented as mean (±standard deviation [SD]) or median (interquartile range [IQR]) and compared using the independent samples t test or Mann-Whitney U test as appropriate. Categorical variables, expressed as numbers (percentages), were compared using the χ2 test or Fisher exact test as appropriate. Paired variables were compared using the Wilcoxon signed-rank test or McNemar test as appropriate. The 95% confidence intervals (CI) of absolute differences were calculated using bootstrapping. In case of missing data, a complete-case analysis was conducted. Correlations between coagulation parameters were assessed using the Spearman rank correlation coefficient after testing for all applicable assumptions. Univariate (and multivariate) logistic regression was used to assess associations between clinical characteristics, routine laboratory markers, and the measured coagulation factors on VTE. Statistics were performed using IBM SPSS Statistics version 28 (IBM Corp, Armonk, NY) and GraphPad Prism version 9 (GraphPad Software, San Diego, CA). P values <.05 were considered significant.
Results
Patient characteristics
A total of 75 patients with active AAV were screened, and 4 patients were excluded because of the use of therapeutic anticoagulation (supplemental Figure 1). The baseline characteristics of the 71 included patients with active AAV are depicted in Table 1. Of these patients, 49 (69%) and 22 (31%) were diagnosed with granulomatosis with polyangiitis and microscopic polyangiitis, respectively. At presentation, 39 patients (55%) had de novo AAV, and 32 patients (45%) presented with a major relapse. Forty-six patients (65%) had ANCA glomerulonephritis on kidney biopsy, including 1 patient who required dialysis. Four patients (6%) presented with alveolar hemorrhage. The patient on dialysis had a central venous catheter. None of the patients were admitted to the intensive care unit or had a concurrent acute medical illness, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, at the time of inclusion. Four patients (6%) had a history of VTE, including 3 with deep venous thrombosis and 1 with pulmonary embolism. The median time of the occurrence of these VTEs before inclusion was 10 years (IQR, 2-26; supplemental Table 1). The median follow-up of the included patients was 26 months (IQR, 15-35). At 6 months, all patients achieved complete remission. During follow-up, 17 patients (25%) experienced a major relapse of AAV after a median of 17 months (IQR, 12-24). Seven patients (10%) died after a median of 7 months (IQR, 4-13); the cause of death was not related to thrombosis.
Baseline characteristics of patients with active ANCA–associated vasculitis at presentation
| . | Normal range . | N = 71 . |
|---|---|---|
| Male (%) | 43 (61) | |
| Age in y (±SD) | 62 (±13) | |
| BMI (IQR) | 18.5-25 | 26.2 (24.1-29.6) |
| Diagnosis (%) | ||
| MPA | 22 (31) | |
| GPA | 49 (69) | |
| ANCA (%) | ||
| MPO | 29 (41) | |
| PR3 | 40 (56) | |
| Double positive | 2 (3) | |
| De novo presentation, (%) | 39 (55) | |
| BVAS (±SD) | 14 (±4) | |
| Organ involvement (%) | ||
| General | 42 (59) | |
| Cutaneous | 6 (9) | |
| Mucous membranes | 4 (6) | |
| ENT | 38 (54) | |
| Chest | 34 (49) | |
| Cardiovascular | 2 (3) | |
| Abdominal | 0 (0) | |
| Renal | 46 (65) | |
| Nervous system | 8 (11) | |
| Remission-induction scheme (%) | ||
| RTX | 36 (51) | |
| RTX/CYC | 16 (22) | |
| CYC | 19 (27) | |
| Dialysis at presentation (%) | 1 (1) | |
| Time to ≤5 mg prednisone in days (IQR) | 105 (77-140) | |
| Previous thrombotic events (%) | 4 (6) | |
| Laboratory parameters | ||
| Hemoglobin (±SD) | 8.2-11.0∗/7.3-9.7† mmol/L | 7.2 (±1.2) |
| Platelet (IQR) | 130-350 ×109/L | 332 (264-394) |
| Leukocytes (IQR) | 3.5-11.0 ×109/L | 8.5 (6.7-12.1) |
| Serum creatinine (IQR) | 60-115 μmol/L | 150 (95-234) |
| CRP (IQR) | <10 mg/L | 20 (8-45) |
| IgG (±SD) | 7.0-16.0 g/L | 11.7 (±3.3) |
| ANCA level (IQR) | MPO ≤ 5.0 IU/mL PR3 ≤ 3.0 IU/mL | 72 (30-120) |
| . | Normal range . | N = 71 . |
|---|---|---|
| Male (%) | 43 (61) | |
| Age in y (±SD) | 62 (±13) | |
| BMI (IQR) | 18.5-25 | 26.2 (24.1-29.6) |
| Diagnosis (%) | ||
| MPA | 22 (31) | |
| GPA | 49 (69) | |
| ANCA (%) | ||
| MPO | 29 (41) | |
| PR3 | 40 (56) | |
| Double positive | 2 (3) | |
| De novo presentation, (%) | 39 (55) | |
| BVAS (±SD) | 14 (±4) | |
| Organ involvement (%) | ||
| General | 42 (59) | |
| Cutaneous | 6 (9) | |
| Mucous membranes | 4 (6) | |
| ENT | 38 (54) | |
| Chest | 34 (49) | |
| Cardiovascular | 2 (3) | |
| Abdominal | 0 (0) | |
| Renal | 46 (65) | |
| Nervous system | 8 (11) | |
| Remission-induction scheme (%) | ||
| RTX | 36 (51) | |
| RTX/CYC | 16 (22) | |
| CYC | 19 (27) | |
| Dialysis at presentation (%) | 1 (1) | |
| Time to ≤5 mg prednisone in days (IQR) | 105 (77-140) | |
| Previous thrombotic events (%) | 4 (6) | |
| Laboratory parameters | ||
| Hemoglobin (±SD) | 8.2-11.0∗/7.3-9.7† mmol/L | 7.2 (±1.2) |
| Platelet (IQR) | 130-350 ×109/L | 332 (264-394) |
| Leukocytes (IQR) | 3.5-11.0 ×109/L | 8.5 (6.7-12.1) |
| Serum creatinine (IQR) | 60-115 μmol/L | 150 (95-234) |
| CRP (IQR) | <10 mg/L | 20 (8-45) |
| IgG (±SD) | 7.0-16.0 g/L | 11.7 (±3.3) |
| ANCA level (IQR) | MPO ≤ 5.0 IU/mL PR3 ≤ 3.0 IU/mL | 72 (30-120) |
ANCA, antineutrophil cytoplasmic antibody; BMI, body mass index; CRP, C-reactive protein; CYC, cyclophosphamide; ENT, ear, nose, and throat; GPA, granulomatosis with polyangiitis; IgG, immunoglobulin G; IQR, interquartile range; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3; RTX, rituximab; SD, standard deviation.
Normal hemoglobin ranges for males.
Normal hemoglobin ranges for females.
Coagulation markers at baseline and after 6 months
At presentation, all patients were sampled before the initiation of remission-induction treatment; none of the patients who relapsed were administered prednisolone >5 mg per day, whereas 4 of these patients were on maintenance treatment using azathioprine. At 6 months, samples from 52 out of 71 (73%) patients were available to test for coagulation markers; all patients had achieved a remission and were on maintenance treatment, that is, low-dose prednisolone combined with rituximab (n = 38) or azathioprine (n = 14). No effects of immunosuppressive treatment on coagulation markers should be expected.13
Table 2 shows coagulation markers at presentation and remission in patients with AAV. D-dimer levels above the upper limit of normal were found in 50 out of 69 (73%) patients and 23 out of 52 (44%) patients at presentation and remission (P = .035), respectively. Fibrinogen was elevated at presentation in most patients (n = 63/69 [91%]), which decreased after 6 months (n = 36/52 [69%]; P = .012). T:AT, an activation marker of the common pathway, and activation markers of the intrinsic pathway (ie, FXIIa:C1Inh, FXIa:AT, and FXIa:α1AT) were increased in the majority of patients at presentation. In contrast, baseline FVIIa:AT, a marker of the extrinsic pathway, was normal in 62 out of 69 (90%) patients. These observations point toward the intrinsic pathway as the main route of thrombin generation. Coagulation markers remained stable or decreased over time.
Coagulation parameters at presentation with active disease and at follow-up after 6 months of treatment in patients with active ANCA–associated vasculitis
| . | Normal range . | N . | Baseline (IQR) . | N . | Follow-up (IQR) . | Absolute difference (95% CI) . | Overall P value . |
|---|---|---|---|---|---|---|---|
| D-dimers, μg/mL | <0.5∗ | 69 | 1.3 (0.6-2.6) | 52 | 0.6 (0.3-1.0) | 0.7 (0.3-1.1) | .017† |
| High D-dimers (%) | 50 (73) | 23 (44) | - | .035† | |||
| Fibrinogen, g/L | 1.7-4.0 | 69 | 6.3 (5.2-8.6) | 52 | 4.9 (3.7-5.7) | 1.4 (0.7-2.7) | <.001† |
| High fibrinogen (%) | 63 (91) | 36 (69) | - | .012† | |||
| PT, s | 10-12 | 69 | 8.7 (8.3-9.3) | 52 | 8.3 (8.2-8.9) | 0.4 (0.0-0.6) | .006† |
| Prolonged PT (%) | 4 (6) | 1 (2) | - | .625 | |||
| APTT, s | 28-32 | 69 | 27 (25-29) | 52 | 26 (24-29) | 0.7 (−0.8 to 2.3) | .040† |
| Prolonged APTT (%) | 5 (7) | 3 (6) | - | .687 | |||
| FVIIa:AT, pM | ≤2641 | 69 | 770 (480-906) | 52 | 664 (399-863) | 106 (−125 to 302) | .174 |
| High FVIIa:AT (%) | 7 (10) | 0 (0) | - | .125 | |||
| FXIIa:AT, pM | ≤118 | 70 | 59 (36-80) | 51 | 47 (32-67) | 11 (−7 to 21) | .401 |
| High FXIIa:AT (%) | 11 (16) | 4 (8) | - | 1.000 | |||
| FXIIa:C1Inh, pM | ≤2166 | 70 | 3889 (3595-4286) | 51 | 3894 (3502-4163) | 6 (−202 to 298) | .162 |
| High FXIIa:C1Inh (%) | 70 (100) | 49 (96) | - | .500 | |||
| FXIa:AT, pM | ≤43 | 69 | 47 (25-94) | 52 | 51 (23-82) | 4 (−31 to 29) | .629 |
| High FXIa:AT (%) | 38 (55) | 29 (56) | - | .687 | |||
| FXIa:α1AT, pM | ≤29 | 69 | 26 (16-39) | 52 | 14 (0.4-22) | 11 (5-19) | <.001† |
| High FXIa:α1AT (%) | 29 (42) | 4 (8) | - | .004† | |||
| FXIa:C1Inh, pM | ≤514 | 69 | 71 (71-235) | 52 | 71 (71-71) | 0 (0-0) | .048† |
| High FXIa:C1Inh (%) | 12 (17) | 2 (4) | - | .125 | |||
| FIXa:AT, pM | ≤472 | 69 | 259 (197-363) | 52 | 246 (187-337) | 13 (−22 to 79) | .376 |
| High FIXa:AT (%) | 11 (16) | 6 (12) | - | 1.000 | |||
| FXa:AT, pM | ≤279 | 69 | 177 (158-206) | 52 | 190 (162-211) | 14 (−826 to 26) | .685 |
| High FXa:AT (%) | 8 (12) | 1 (2) | - | .125 | |||
| T:AT, μg/L | ≤4.7 | 69 | 2.9 (1.8-6.0) | 52 | 2.0 (1.5-2.8) | 0.9 (0.0-1.7) | .009† |
| High T:AT (%) | 20 (29) | 4 (8) | - | .065 |
| . | Normal range . | N . | Baseline (IQR) . | N . | Follow-up (IQR) . | Absolute difference (95% CI) . | Overall P value . |
|---|---|---|---|---|---|---|---|
| D-dimers, μg/mL | <0.5∗ | 69 | 1.3 (0.6-2.6) | 52 | 0.6 (0.3-1.0) | 0.7 (0.3-1.1) | .017† |
| High D-dimers (%) | 50 (73) | 23 (44) | - | .035† | |||
| Fibrinogen, g/L | 1.7-4.0 | 69 | 6.3 (5.2-8.6) | 52 | 4.9 (3.7-5.7) | 1.4 (0.7-2.7) | <.001† |
| High fibrinogen (%) | 63 (91) | 36 (69) | - | .012† | |||
| PT, s | 10-12 | 69 | 8.7 (8.3-9.3) | 52 | 8.3 (8.2-8.9) | 0.4 (0.0-0.6) | .006† |
| Prolonged PT (%) | 4 (6) | 1 (2) | - | .625 | |||
| APTT, s | 28-32 | 69 | 27 (25-29) | 52 | 26 (24-29) | 0.7 (−0.8 to 2.3) | .040† |
| Prolonged APTT (%) | 5 (7) | 3 (6) | - | .687 | |||
| FVIIa:AT, pM | ≤2641 | 69 | 770 (480-906) | 52 | 664 (399-863) | 106 (−125 to 302) | .174 |
| High FVIIa:AT (%) | 7 (10) | 0 (0) | - | .125 | |||
| FXIIa:AT, pM | ≤118 | 70 | 59 (36-80) | 51 | 47 (32-67) | 11 (−7 to 21) | .401 |
| High FXIIa:AT (%) | 11 (16) | 4 (8) | - | 1.000 | |||
| FXIIa:C1Inh, pM | ≤2166 | 70 | 3889 (3595-4286) | 51 | 3894 (3502-4163) | 6 (−202 to 298) | .162 |
| High FXIIa:C1Inh (%) | 70 (100) | 49 (96) | - | .500 | |||
| FXIa:AT, pM | ≤43 | 69 | 47 (25-94) | 52 | 51 (23-82) | 4 (−31 to 29) | .629 |
| High FXIa:AT (%) | 38 (55) | 29 (56) | - | .687 | |||
| FXIa:α1AT, pM | ≤29 | 69 | 26 (16-39) | 52 | 14 (0.4-22) | 11 (5-19) | <.001† |
| High FXIa:α1AT (%) | 29 (42) | 4 (8) | - | .004† | |||
| FXIa:C1Inh, pM | ≤514 | 69 | 71 (71-235) | 52 | 71 (71-71) | 0 (0-0) | .048† |
| High FXIa:C1Inh (%) | 12 (17) | 2 (4) | - | .125 | |||
| FIXa:AT, pM | ≤472 | 69 | 259 (197-363) | 52 | 246 (187-337) | 13 (−22 to 79) | .376 |
| High FIXa:AT (%) | 11 (16) | 6 (12) | - | 1.000 | |||
| FXa:AT, pM | ≤279 | 69 | 177 (158-206) | 52 | 190 (162-211) | 14 (−826 to 26) | .685 |
| High FXa:AT (%) | 8 (12) | 1 (2) | - | .125 | |||
| T:AT, μg/L | ≤4.7 | 69 | 2.9 (1.8-6.0) | 52 | 2.0 (1.5-2.8) | 0.9 (0.0-1.7) | .009† |
| High T:AT (%) | 20 (29) | 4 (8) | - | .065 |
Wilcoxon signed-ranks test was used for continuous variables and McNemar test was used for categorical variables. The 95% CIs of absolute differences were calculated using bootstrapping. High is defined as above normal range.
α1AT, α1-antitrypsin; aPTT, activated partial thromboplastin time; AT, antithrombin; C1Inh, C1 esterase inhibitor; FIXa, activated factor IX; FVIIa, activated FVII; FXa, activated factor X; FXIa, activated factor XI; FXIIa, activated factor XII; PT, prothrombin time; T:AT, thrombin in complex with antithrombin.
D-dimers normal range for age 50 years or younger, with an incremental increase of 0.1 for each year >50 years.
Statistically significant.
Baseline coagulation markers and their associations
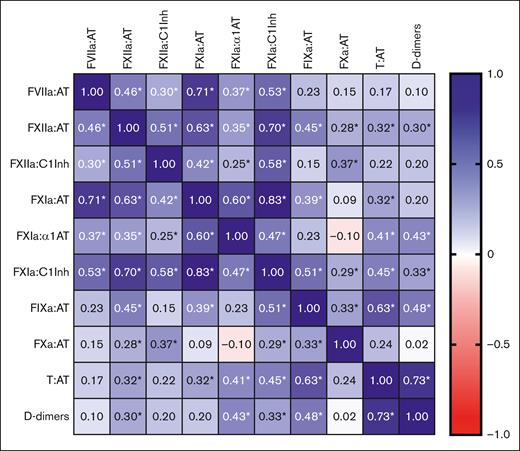
The interaction between coagulation markers was explored by calculating the Spearman rank correlation coefficients (r) (Figure 1). There was a strong positive correlation between T:AT and D-dimers (r = 0.73; P < .001), T:AT and FIXa:AT (r = 0.63; P < .001), and a moderate positive correlation between T:AT and FXIIa:AT (r = 0.32; P = .007), FXIa:AT (r = 0.32; P = .007), FXIa:α1AT (r = 0.41, P < .001), and FXIa:C1Inh (r = 0.45; P < .001), pointing to activation of the intrinsic pathway. No correlation was found between T:AT and FVIIa:AT (r = 0.17; P = .167).
Correlation matrix between the different coagulation factors and D-dimers in patients with active AAV. Spearman rank correlation coefficients were used. Blue indicates a positive correlation, and red indicates a negative correlation. ∗Indicates a P value <.05. α1AT, α1-antitrypsin; AT, antithrombin; C1Inh, C1 esterase inhibitor; FVIIa, activated FVII; FXa, activated factor X; FXIa, activated factor XI; FXIIa, activated factor XII; FIXa, activated factor IX; T:AT, thrombin in complex with antithrombin.
Correlation matrix between the different coagulation factors and D-dimers in patients with active AAV. Spearman rank correlation coefficients were used. Blue indicates a positive correlation, and red indicates a negative correlation. ∗Indicates a P value <.05. α1AT, α1-antitrypsin; AT, antithrombin; C1Inh, C1 esterase inhibitor; FVIIa, activated FVII; FXa, activated factor X; FXIa, activated factor XI; FXIIa, activated factor XII; FIXa, activated factor IX; T:AT, thrombin in complex with antithrombin.
Next, we focused on patients with elevated T:AT and D-dimers to better understand hypercoagulability among these patients in particular (Table 3). T:AT was associated with elevated D-dimers (absolute difference, 1.8 μg/L [95% CI, 1.3-3.8]; P < .001). Patients with increased T:AT showed higher levels of FXIIa:AT (absolute difference, 26 pM [95% CI, 3-126]; P = .005), FXIa:α1AT (absolute difference, 15 pM [95% CI, 2-107]; P = .007), FXIa:C1Inh (absolute difference, 198 pM [95% CI, 0-1662]; P < .001), and FIXa:AT (absolute difference, 215 pM [95% CI, 91-562]; P < .001) as compared with those with normal T:AT. Notably, elevated D-dimers were also associated with activation of the intrinsic pathway as reflected by higher levels of FXIIa:AT (absolute difference, 27 pM [95% CI, 8-40]; P = .002), FXIa:α1AT (absolute difference, 10 pM [95% CI, 3-25]; P = .002), FXIa:C1Inh (absolute difference, 0 pM [95% CI, 0-97]; P = .016), and FIXa:AT (absolute difference, 97 pM [95% CI, 32-152]; P < .001). Of note, T:AT but not D-dimers was also associated with higher levels of FVIIa:AT (absolute difference, 192 pM [95% CI, 24-1014]; P = .023), although most of these patients presented with FVIIa:AT levels within the normal range. Thus, hypercoagulability appears to be associated with the intrinsic rather than extrinsic pathway.
Coagulation parameters of patients with elevated vs normal T:AT and D-dimers at presentation
| . | Elevated T:AT . | Normal T:AT . | Absolute difference (95% CI) . | Overall P value . |
|---|---|---|---|---|
| n = 20 | n = 49 | |||
| FVIIa:AT, pM | 859 (658-2627) | 667 (414-876) | 192 (24-1014) | .023∗ |
| FXIIa:AT, pM | 72 (57-423) | 46 (35-69) | 26 (3-126) | .005∗ |
| FXIIa:C1Inh, pM | 3870 (3492-6105) | 3889 (3616-4142) | 19 (−312 to 1176) | .336 |
| FXIa:AT, pM | 83 (27-186) | 43 (24-79) | 40 (−14 to 86) | .085 |
| FXIa:α1AT, pM | 39 (23-172) | 24 (14-33) | 15 (2-107) | .007∗ |
| FXIa:C1Inh, pM | 269 (71-2842) | 71 (71-71) | 198 (0-1662) | <.001∗ |
| FIXa:AT, pM | 446 (297-1529) | 231 (191-294) | 215 (91-562) | <.001∗ |
| FXa:AT, pM | 185 (165-330) | 177 (152-198) | 9 (−16 to 84) | .247 |
| . | Elevated T:AT . | Normal T:AT . | Absolute difference (95% CI) . | Overall P value . |
|---|---|---|---|---|
| n = 20 | n = 49 | |||
| FVIIa:AT, pM | 859 (658-2627) | 667 (414-876) | 192 (24-1014) | .023∗ |
| FXIIa:AT, pM | 72 (57-423) | 46 (35-69) | 26 (3-126) | .005∗ |
| FXIIa:C1Inh, pM | 3870 (3492-6105) | 3889 (3616-4142) | 19 (−312 to 1176) | .336 |
| FXIa:AT, pM | 83 (27-186) | 43 (24-79) | 40 (−14 to 86) | .085 |
| FXIa:α1AT, pM | 39 (23-172) | 24 (14-33) | 15 (2-107) | .007∗ |
| FXIa:C1Inh, pM | 269 (71-2842) | 71 (71-71) | 198 (0-1662) | <.001∗ |
| FIXa:AT, pM | 446 (297-1529) | 231 (191-294) | 215 (91-562) | <.001∗ |
| FXa:AT, pM | 185 (165-330) | 177 (152-198) | 9 (−16 to 84) | .247 |
| . | Elevated D-dimers . | Normal D-dimers . | Absolute difference (95% CI) . | Overall P value . |
|---|---|---|---|---|
| n = 50 | n = 19 | |||
| FVIIa:AT, pM | 728 (494-917) | 841 (416-898) | 113 (−251 to 317) | .883 |
| FXIIa:AT, pM | 63 (44-113) | 36 (29-55) | 27 (8-40) | .002∗ |
| FXIIa:C1Inh, pM | 3951 (3635-4418) | 3824 (3542-4131) | 127 (−161 to 397) | .172 |
| FXIa:AT, pM | 51 (24-94) | 46 (28-95) | 5 (−41 to 32) | .846 |
| FXIa:α1AT, pM | 31 (17-63) | 21 (2-26) | 10 (3-25) | .002∗ |
| FXIa:C1Inh, pM | 71 (71-471) | 71 (71-71) | 0 (0-97) | .016∗ |
| FIXa:AT, pM | 295 (231-422) | 197 (145-270) | 97 (32-152) | <.001∗ |
| FXa:AT, pM | 176 (161-218) | 177 (153-197) | 0 (−18 to 26) | .600 |
| T:AT, μg/L | 3.3 (2.3-9.0) | 1.5 (1.3-2.2) | 1.8 (1.3-3.8) | <.001∗ |
| . | Elevated D-dimers . | Normal D-dimers . | Absolute difference (95% CI) . | Overall P value . |
|---|---|---|---|---|
| n = 50 | n = 19 | |||
| FVIIa:AT, pM | 728 (494-917) | 841 (416-898) | 113 (−251 to 317) | .883 |
| FXIIa:AT, pM | 63 (44-113) | 36 (29-55) | 27 (8-40) | .002∗ |
| FXIIa:C1Inh, pM | 3951 (3635-4418) | 3824 (3542-4131) | 127 (−161 to 397) | .172 |
| FXIa:AT, pM | 51 (24-94) | 46 (28-95) | 5 (−41 to 32) | .846 |
| FXIa:α1AT, pM | 31 (17-63) | 21 (2-26) | 10 (3-25) | .002∗ |
| FXIa:C1Inh, pM | 71 (71-471) | 71 (71-71) | 0 (0-97) | .016∗ |
| FIXa:AT, pM | 295 (231-422) | 197 (145-270) | 97 (32-152) | <.001∗ |
| FXa:AT, pM | 176 (161-218) | 177 (153-197) | 0 (−18 to 26) | .600 |
| T:AT, μg/L | 3.3 (2.3-9.0) | 1.5 (1.3-2.2) | 1.8 (1.3-3.8) | <.001∗ |
The Mann-Whitney U test was used for continuous variables. The 95% CIs of absolute differences were calculated using bootstrapping.
α1AT, α1-antitrypsin; AT, antithrombin; C1Inh, C1 esterase inhibitor; FIXa, activated factor IX; FVIIa, activated FVII; FXa, activated factor X; FXIa, activated factor XI; FXIIa, activated factor XII; T:AT, thrombin in complex with antithrombin.
Statistically significant.
Baseline coagulation markers and their association with clinical and routine laboratory findings
The Spearman rank correlation coefficients between different parameters and either T:AT or D-dimers are presented in supplemental Table 2. Age positively correlated with T:AT (r = 0.33; P = .006) and D-dimers (r = 0.36; P = .003). T:AT levels positively correlated with PT (r = 0.28; P = .018), leukocytes (r = 0.26; P = .033), and proteinuria (r = 0.29; P = .029). D-dimers positively correlated with BVAS (r = 0.35; P = .003), PT (r = 0.37; P = .002), serum creatinine (r = 0.30; P = .014), C-reactive protein (r = 0.35; P = .003), ANCA levels (r = 0.27; P=.028), and proteinuria (r = 0.40; P = .002). There was a negative correlation between D-dimers and hemoglobin (r = −0.24; P=.044) and estimated glomerular filtration rate (r = −0.36; P = .003). Taken together, our findings suggest an intricate link among hypercoagulability, inflammation, and disease activity in patients with AAV.
Thromboembolic events
The observed VTEs were symptomatic and occurred in 5 out of 71 (7%) patients (deep venous thrombosis, n = 3; PE, n = 2) after a median of 1 month (IQR, 1-5) since presentation (Table 4). None of the patients had a prior VTE or associated risk factors (ie, intensive care unit admission, central venous–line catheter, end-stage kidney disease or malignancy) during study period. No between-group differences were found regarding clinical parameters and outcomes measures, that is, relapse rate and survival. T:AT levels were higher in patients with VTE as compared with those with not-documented VTE (8.8 μg/L [IQR, 3.3-14.7] vs 2.8 μg/L [IQR, 1.7-5.6]; P = .044). FXa:AT levels, although within the normal range, were higher in patients with not-documented VTE (146 pM [IQR, 121-171] vs 180 pM [164-209]; P = .019). None of the patients with VTE presented with a SARS-CoV-2 infection or other infections at presentation.
Baseline characteristics of patients with and without VTE
| . | Normal range . | Thrombosis . | No thrombosis . | Overall P value . |
|---|---|---|---|---|
| n = 5 | n = 66 | |||
| Male, (%) | 3 (60) | 40 (61) | .979 | |
| Age in y (IQR) | 71 (64-77) | 63 (55-71) | .092 | |
| BMI (IQR) | 18.5-25 | 27.1 (23.8-29.2) | 27.0 (24.1-29.7) | .954 |
| Diagnosis (%) | .651 | |||
| MPA | 2 (40) | 20 (30) | ||
| GPA | 3 (60) | 46 (70) | ||
| ANCA (%) | .053 | |||
| MPO | 3 (60) | 26 (39) | ||
| PR3 | 1 (20) | 39 (59) | ||
| Double positive | 1 (20) | 1 (2) | ||
| De novo presentation (%) | 4 (80) | 35 (53) | .243 | |
| BVAS (IQR) | 17 (13-21) | 14 (12-17) | .179 | |
| Organ involvement (%) | ||||
| General | 5 (100) | 37 (56) | .054 | |
| Cutaneous | 0 (0) | 6 (9) | .481 | |
| Mucous membranes | 0 (0) | 4 (6) | .571 | |
| ENT | 2 (40) | 36 (55) | .530 | |
| Chest | 2 (40) | 32 (49) | .691 | |
| Cardiovascular | 0 (0) | 2 (3) | .693 | |
| Abdominal | 0 (0) | 0 (0) | - | |
| Renal | 4 (80) | 42 (64) | .460 | |
| Nervous system | 2 (40) | 6 (9) | .037∗ | |
| Remission-induction scheme (%) | .323 | |||
| RTX | 3 (60) | 33 (50) | ||
| RTX/CYC | 2 (40) | 14 (21) | ||
| CYC | 0 (0) | 19 (29) | ||
| Time to ≤5 mg prednisone in days (IQR) | 75 (60-95) | 106 (79-140) | .091 | |
| Previous thrombotic events (%) | 0 (0) | 4 (6) | .565 | |
| Laboratory parameters | ||||
| Hemoglobin (IQR) | 8.2-11.0†/7.3-9.7‡ mmol/L | 7.9 (6.9-8.9) | 7.2 (6.2-8.0) | .164 |
| Platelet (IQR) | 130-350 ×109/L | 283 (237-441) | 332 (264-398) | .374 |
| Leukocytes (IQR) | 3.5-11.0 ×109/L | 10.3 (7.0-12.7) | 8.5 (6.7-11.8) | .547 |
| Serum creatinine (IQR) | 60-115 μmol/L | 95 (81-225) | 152 (95-234) | .398 |
| CRP (IQR) | <10 mg/L | 11 (3-90) | 23 (8-44) | .917 |
| IgG (IQR) | 7.0-16.0 g/L | 9.7 (8.6-14.4) | 11.9 (9.1-13.4) | .521 |
| ANCA level (IQR) | MPO ≤5.0 IU/mL PR3 ≤3.0 IU/mL | 64 (34-81) | 72 (30-138) | .667 |
| Coagulation parameters | ||||
| D-dimers (IQR) | <5.0§ μg/mL | 3.3 (1.2-15.7) | 1.1 (0.6-2.5) | .055 |
| Fibrinogen (IQR) | 1.7-4.0 g/L | 5.8 (3.2-7.4) | 6.5 (5.2-8.7) | .176 |
| PT (IQR) | 10-12 sec | 8.6 (8.1-10.5) | 8.7 (8.3-9.3) | .711 |
| APTT (IQR) | 28-32 sec | 29 (23-35) | 27 (25-29) | .685 |
| FVIIa:AT (IQR) | ≤2641 pM | 641 (433-824) | 770 (480-916) | .392 |
| FXIIa:AT (IQR) | ≤118 pM | 63 (35-97) | 59 (36-80) | .855 |
| FXIIa:C1Inh (IQR) | ≤2166 pM | 3784 (3659-4617) | 3904 (3585-4289) | .811 |
| FXIa:AT (IQR) | ≤43 pM | 30 (19-67) | 48 (25-98) | .354 |
| FXIa:α1AT (IQR) | ≤29 pM | 40 (25-68) | 25 (15-37) | .165 |
| FXIa:C1Inh (IQR) | ≤514 pM | 71 (71-199) | 71 (71-266) | .967 |
| FIXa:AT (IQR) | ≤472 pM | 296 (232-304) | 257 (197-383) | .908 |
| FXa:AT (IQR) | ≤279 pM | 146 (121-171) | 180 (164-209) | .019∗ |
| T:AT (IQR) | ≤4.7 μg/L | 8.8 (3.3-14.7) | 2.8 (1.7-5.6) | .044∗ |
| Outcome measures | ||||
| Relapse during follow-up | 2 (40) | 15 (23) | .383 | |
| Mortality during follow-up | 0 (0) | 7 (11) | .443 |
| . | Normal range . | Thrombosis . | No thrombosis . | Overall P value . |
|---|---|---|---|---|
| n = 5 | n = 66 | |||
| Male, (%) | 3 (60) | 40 (61) | .979 | |
| Age in y (IQR) | 71 (64-77) | 63 (55-71) | .092 | |
| BMI (IQR) | 18.5-25 | 27.1 (23.8-29.2) | 27.0 (24.1-29.7) | .954 |
| Diagnosis (%) | .651 | |||
| MPA | 2 (40) | 20 (30) | ||
| GPA | 3 (60) | 46 (70) | ||
| ANCA (%) | .053 | |||
| MPO | 3 (60) | 26 (39) | ||
| PR3 | 1 (20) | 39 (59) | ||
| Double positive | 1 (20) | 1 (2) | ||
| De novo presentation (%) | 4 (80) | 35 (53) | .243 | |
| BVAS (IQR) | 17 (13-21) | 14 (12-17) | .179 | |
| Organ involvement (%) | ||||
| General | 5 (100) | 37 (56) | .054 | |
| Cutaneous | 0 (0) | 6 (9) | .481 | |
| Mucous membranes | 0 (0) | 4 (6) | .571 | |
| ENT | 2 (40) | 36 (55) | .530 | |
| Chest | 2 (40) | 32 (49) | .691 | |
| Cardiovascular | 0 (0) | 2 (3) | .693 | |
| Abdominal | 0 (0) | 0 (0) | - | |
| Renal | 4 (80) | 42 (64) | .460 | |
| Nervous system | 2 (40) | 6 (9) | .037∗ | |
| Remission-induction scheme (%) | .323 | |||
| RTX | 3 (60) | 33 (50) | ||
| RTX/CYC | 2 (40) | 14 (21) | ||
| CYC | 0 (0) | 19 (29) | ||
| Time to ≤5 mg prednisone in days (IQR) | 75 (60-95) | 106 (79-140) | .091 | |
| Previous thrombotic events (%) | 0 (0) | 4 (6) | .565 | |
| Laboratory parameters | ||||
| Hemoglobin (IQR) | 8.2-11.0†/7.3-9.7‡ mmol/L | 7.9 (6.9-8.9) | 7.2 (6.2-8.0) | .164 |
| Platelet (IQR) | 130-350 ×109/L | 283 (237-441) | 332 (264-398) | .374 |
| Leukocytes (IQR) | 3.5-11.0 ×109/L | 10.3 (7.0-12.7) | 8.5 (6.7-11.8) | .547 |
| Serum creatinine (IQR) | 60-115 μmol/L | 95 (81-225) | 152 (95-234) | .398 |
| CRP (IQR) | <10 mg/L | 11 (3-90) | 23 (8-44) | .917 |
| IgG (IQR) | 7.0-16.0 g/L | 9.7 (8.6-14.4) | 11.9 (9.1-13.4) | .521 |
| ANCA level (IQR) | MPO ≤5.0 IU/mL PR3 ≤3.0 IU/mL | 64 (34-81) | 72 (30-138) | .667 |
| Coagulation parameters | ||||
| D-dimers (IQR) | <5.0§ μg/mL | 3.3 (1.2-15.7) | 1.1 (0.6-2.5) | .055 |
| Fibrinogen (IQR) | 1.7-4.0 g/L | 5.8 (3.2-7.4) | 6.5 (5.2-8.7) | .176 |
| PT (IQR) | 10-12 sec | 8.6 (8.1-10.5) | 8.7 (8.3-9.3) | .711 |
| APTT (IQR) | 28-32 sec | 29 (23-35) | 27 (25-29) | .685 |
| FVIIa:AT (IQR) | ≤2641 pM | 641 (433-824) | 770 (480-916) | .392 |
| FXIIa:AT (IQR) | ≤118 pM | 63 (35-97) | 59 (36-80) | .855 |
| FXIIa:C1Inh (IQR) | ≤2166 pM | 3784 (3659-4617) | 3904 (3585-4289) | .811 |
| FXIa:AT (IQR) | ≤43 pM | 30 (19-67) | 48 (25-98) | .354 |
| FXIa:α1AT (IQR) | ≤29 pM | 40 (25-68) | 25 (15-37) | .165 |
| FXIa:C1Inh (IQR) | ≤514 pM | 71 (71-199) | 71 (71-266) | .967 |
| FIXa:AT (IQR) | ≤472 pM | 296 (232-304) | 257 (197-383) | .908 |
| FXa:AT (IQR) | ≤279 pM | 146 (121-171) | 180 (164-209) | .019∗ |
| T:AT (IQR) | ≤4.7 μg/L | 8.8 (3.3-14.7) | 2.8 (1.7-5.6) | .044∗ |
| Outcome measures | ||||
| Relapse during follow-up | 2 (40) | 15 (23) | .383 | |
| Mortality during follow-up | 0 (0) | 7 (11) | .443 |
The Mann-Whitney U test was used for continuous variables and the chi-square test or Fisher exact test was used for categorical variables, when appropriate.
α1AT, α1-antitrypsin; ANCA, antineutrophil cytoplasmic antibody; aPTT, activated partial thromboplastin time; AT, antithrombin; BMI, body mass index; C1Inh, C1 esterase inhibitor; CRP, C-reactive protein; CYC, cyclophosphamide; ENT, ear, nose, and throat; GPA, granulomatosis with polyangiitis; IgG, immunoglobulin G; IQR, interquartile range; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3; PT, Prothrombin time; RTX, rituximab; FIXa, activated factor IX; FVIIa, activated FVII; FXa, activated factor X; FXIa, activated factor XI; FXIIa, activated factor XII; SD, standard deviation; T:AT, thrombin in complex with antithrombin.
Statistically significant.
Normal hemoglobin ranges for males.
Normal hemoglobin ranges for females.
D-dimers normal range for age 50 years or younger, with an incremental increase of 0.1 for each year above 50.
No significant difference was found among groups when using logistic regression analyses to assess the prognostic value of routine clinical parameters and activated coagulation factors in relation to VTE (supplemental Table 3).
Discussion
In this prospective study, we uniquely characterized hypercoagulability in detail in a well-characterized cohort of patients with AAV. We observed that activated coagulation markers of the intrinsic and extrinsic coagulation pathways are elevated in patients with active disease. These elevated levels persisted in a subgroup of patients after 6 months of follow-up. T:AT formation and increased D-dimers were predominantly associated with activation of the intrinsic pathway at the time of active AAV and linked to disease activity and inflammation.
Patients with AAV are at an increased risk of developing VTE because of a hypercoagulable state, especially during active disease.1,14 This is indicated by elevated levels of D-dimers and fibrinogen, as well as by increased levels of activated coagulation factors with their natural inhibitors in our study. Our findings extend previous studies that reported elevated D-dimers and fibrinolysis in patients with active AAV.14,15 After achieving remission, not all patients showed an improvement in their prothrombotic state as assessed by the levels of coagulation markers at 6 months. This is in line with findings of Hilhorst et al on sustained hypercoagulability in patients with AAV in remission, indicated by an increased endogenous thrombin potential, elevated factor VIII, and tissue factor (TF) pathway inhibitor levels.4 Ongoing low-grade inflammation and subsequent endothelial damage may contribute to hypercoagulability, although patients were assumed to be in remission as based on clinical and laboratory markers.
To understand the mechanism of hypercoagulability, we investigated the coagulation cascade. We primarily focused on the impact of the assessed coagulation markers on T:AT and D-dimers rather than studying the intricate in-between interactions of the coagulation markers because the interpretation of such associations can be challenging under pathological conditions. At the time of active disease, thrombin generation was associated with activation of the intrinsic rather than extrinsic pathway, as illustrated by increased levels of complexes involving FXIIa and FXIa. T:AT and D-dimers, indeed, were associated with coagulation markers of the intrinsic pathway. In contrast, FVIIa:AT, was found to be normal in the majority of patients with active disease, and its association with T:AT was limited. These findings point toward an important role of the intrinsic coagulation pathway in hypercoagulability in patients with active AAV.
Clinically, higher disease activity and inflammation, as reflected by higher BVAS, ANCA levels, and C-reactive protein at presentation, and more severe kidney disease, as reflected by increased serum creatinine and spot urinary protein excretion, were associated with higher D-dimers. Our data are in line with previous publications, indicating that elevated D-dimers correlate with inflammation, disease activity and renal impairment.14,15 It has been known that kidney disease is associated with increased coagulation markers16,17, implying that renal involvement is an independent risk factor of hypercoagulability in AVV.
A detailed understanding of the factors driving hypercoagulability in AAV is currently limited. However, the majority of VTEs are observed during active disease, pointing toward an interaction between inflammation, complement, platelets, and endothelial damage. Neutrophil extracellular traps (NETs) formation has been associated with hypercoagulability in other conditions, including but not limited to COVID-19.12,18 NETs, containing DNA fragments, activate FXII and platelets, leading to thrombin formation.19 In addition, TF has been associated with mediators released during NETs formation.20 In AAV, several studies implicated that NETs formation is abundant in patients with active disease.21-23 NETs colocalized with thrombi provide the in vivo counterpart of our findings.24 In this context, complement 5a (C5a) primed neutrophils in patients with active AAV released NETs expressing high levels of TF that induced thrombin formation.25,26 We believe that the intricate link between neutrophils and NETs, inflammation, and endothelial damage cause hypercoagulability via activation of the intrinsic and, to a lesser extent, extrinsic pathway. The aggregation and activation of platelets may cause an amplification loop. At the time of remission, inflammation and endothelial damage improves, attenuating coagulation.
In this study, 7% of the patients presented with a symptomatic VTE after a median of 1 month. Patients who developed VTE had increased T:AT levels at presentation, suggesting that hypercoagulability was present before the clinical significance of thrombosis was identified in these individuals. Because the FXa:AT levels are within the normal limits in both groups, the lower levels in the VTE group are most likely not clinically meaningful. Previous studies have reported higher BVAS, myeloperoxidase-ANCA positivity, skin or pulmonary involvement, and kidney disease as risk factors developing VTE in AAV.1,27 We were not able to confirm these data, which may be attributed to the small number of events.
Our study has several limitations. First, owing to the ongoing nature of our prospective observational study and the impact of the COVID-19 pandemic (ie, travel restrictions) on patient follow-up, we could not assess coagulation factors in all patients during follow-up; however, they remained included in the study. Given that most insights are derived from the baseline assessments, the impact of the missing follow-up data on the study’s conclusion is limited. Furthermore, to our knowledge, our study represents the first prospective detailed analysis of the coagulation cascade in a well-defined cohort of patients with AAV, with extensive assessments conducted over time. Second, the enrollment of patients partially occurred during the COVID-19 pandemic. It is worth noting that hypercoagulability has been reported to be present and persistent in patients with COVID-19.10,28 None of our patients, however, presented with a SARS-CoV-2 infection before sampling.
In conclusion, patients with active AAV have an increased hypercoagulable state, characterized by elevated levels of coagulation factors in complexes with their natural inhibitors, especially those linked to the intrinsic coagulation pathway and D-dimers. Although the precise mechanisms underlying hypercoagulability in AAV are not fully understood, it is most likely related to an interplay of higher disease activity, excessive vascular inflammation, and endothelial damage. Future studies are warranted to investigate the link between hyperinflammation, including NETs formation, and hypercoagulability in AAV. Furthermore, studies should focus on discriminative markers, such as T:AT or D-dimers, to identify and target patients at the highest risk of VTE. Potential targets for intervention in AAV include the intrinsic coagulation pathway, including novel FXIa inhibitors, and specific inhibition of neutrophil activation, such as through C5a (receptor) inhibition.
Acknowlegdments
H.t.C. and H.M.H.S. received grant support from the Netherlands Heart Foundation (CVON2014-09, Reappraisal of Atrial Fibrillation: Interaction between HyperCoagulability, Electrical Remodeling, and Vascular Destabilization in the Progression of Atrial Fibrillation (RACE V). H.t.C. was supported by a fellowship of the Gutenberg University Mainz. M.N. was supported by the Dutch Heart Foundation. S.A.M.E.G.T. received funding from the Dutch Kidney Foundation (ie, Nierstichting; 23OK1056).
Authorship
Contribution: M.H.B., R.Y., J.P.A., J.P., and P.v.P. enrolled the patients; M.H.B., R.Y., and J.P.A. collected and stored the samples and managed the data; M.H.B., S.A.M.E.G.T., H.M.H.S., H.t.C., C.P.R., M.N., and P.v.P. designed and performed the statistical analyses and interpreted the data; M.H.B. and M.N. performed the experiments; M.H.B. wrote the first draft of the manuscript; S.A.M.E.G.T., J.G.M.C.D., H.M.H.S., H.t.C., C.P.R., M.N., and P.v.P. supervised and cowrote the manuscript; and all authors critically reviewed and approved the final version of the manuscript and agreed to the published version.
Conflict-of-interest disclosure: H.t.C. and H.M.H.S. received funding for research from Bayer and Pfizer, and are stakeholders in Coagulation Profile. H.t.C. is a consultant for Alveron and has served on advisory boards for Bayer, Pfizer, Daiichi, Leo, Gilead, Novostia, Galapagos, Viatris and AstraZeneca. C.P.R. is a co-inventor of a patent describing use of low anticoagulant heparins in sepsis and owned by Maastricht University, and is a scientific consultant for Matisse Pharmaceuticals and Annexin Pharmaceuticals. S.A.M.E.G.T. participated in TMA expert meetings and received travel/speaker fees from Alexion Pharmaceuticals Inc (AstraZeneca). The remaining authors declare no competing financial interests.
Correspondence: Pieter van Paassen, Department Of Nephrology and Clinical Immunology, Maastricht University Medical Center, P. Debyelaan 25, 6229 HX, Maastricht, The Netherlands; email: p.vanpaassen@maastrichtuniversity.nl.
References
Author notes
Original data are available upon reasonable request from the corresponding author, Pieter van Paassen (p.vanpaassen@maastrichtuniversity.nl).
The full-text version of this article contains a data supplement.