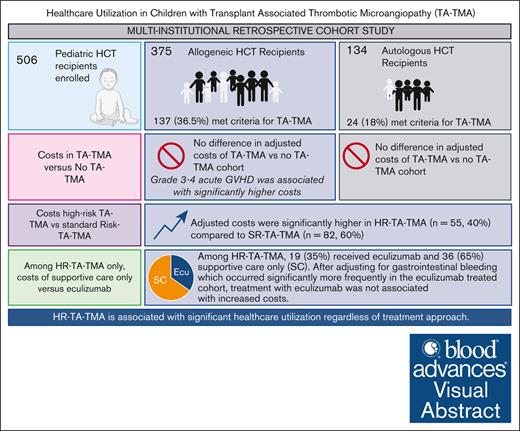
Among allo-HCT recipients, adjusted costs were significantly higher in HR TA-TMA (n = 55) vs standard-risk TA-TMA (n = 82).
Eculizumab was not associated with increased total costs after adjusting for gastrointestinal bleeding and GVHD in HR TA-TMA after allo-HCT.
Visual Abstract
The health care use (HCU) burden of transplant-associated thrombotic microangiopathy (TA-TMA) and its treatments are unknown. The objective of this study was to investigate inpatient costs associated with meeting criteria for TA-TMA in the first year after hematopoietic cell transplant (HCT). This institutional review board–approved retrospective multicenter study included serial children who underwent HCT from 1 January 2015 to 1 July 2019. A standardized unit cost (adjusted for geographic location, differences in cost of living, and inflation) for inpatient hospitalization was extracted from the Pediatric Health Information System data and linked to clinical data. Both total cost and cost per day from 15 days before stem cell infusion to 1-year after HCT were calculated. Among allogeneic (allo) transplant recipients, after adjusting for severe grade 3/4 acute graft-versus-host disease (GVHD), infections, and HLA mismatch, costs were not different in TA-TMA (n = 137) vs no TA-TMA (n = 238). Severe GVHD was significantly associated with increased costs. Among allo high-risk (HR) TMA-TMA, unadjusted costs were significantly higher in the eculizumab-treated cohort (n = 19) than in the supportive care group (n = 36). However, after adjusting for gastrointestinal bleeding that occurred disproportionately in the eculizumab (n = 6) vs supportive care (n = 0) cohort, eculizumab treatment was not associated with increased total costs. More studies are needed to determine the etiology of increased HCU costs in those with HR-TA-TMA and predict those more likely to benefit from eculizumab, reducing HCU and improving outcomes.
Introduction
Transplant-associated thrombotic microangiopathy (TA-TMA) is a complication of hematopoietic cell transplantation (HCT) that occurs in ∼20% to 30% of pediatric allogeneic HCT recipients and up to 25% of pediatric autologous HCT recipients with neuroblastoma.1-4 Although some patients have a self-resolving illness, 50% to 75% have a severe course with associated organ dysfunction.1 Mortality rates of severe TA-TMA exceed 50%.5 In addition to increased mortality, patients with TA-TMA have significantly longer lengths of hospital stay, more intensive care unit (ICU) admissions, and an increased risk of late organ dysfunction, particularly chronic kidney disease.1,6 Although there are no FDA-approved therapies for TA-TMA, eculizumab, a monoclonal antibody to complement C5, has resulted in improved overall survival (OS) in some studies,7,8 although not all.1,9,23
Eculizumab is one of the world's most expensive drugs,10 and costs and insurance coverage are challenges to using this off-label therapy for TA-TMA. One brief report suggested that the cost of eculizumab may exceed that of TA-TMA treatment with supportive care.11 However, this was a small study comparing the cost of treatment for adults with TA-TMA who responded to eculizumab with that for those who did not. The difference in health care use (HCU) between patients with TA-TMA and those without TA-TMA is unknown. Data for the impact of eculizumab vs supportive care on the HCU of children with TA-TMA are also scant.
The Pediatric Health Information Services (PHIS) database includes clinical and resource use data from 49 different free-standing Children’s Hospitals, capturing costs as a metric of inpatient HCU. Using the combined PHIS database and patient clinical data from 2 large pediatric hospitals, the objective of this study was to identify differences in HCU between children who met the criteria for TA-TMA vs those who did not. We also assessed the HCU implications of eculizumab treatment in children classified as having high-risk TA-TMA (HR TA-TMA).
Methods
Cohort
All patients who underwent an allogeneic or autologous HCT (excluding patients who received a genetically modified autologous HCT product) at Boston Children’s Hospital and Cincinnati Children’s Hospital Medical Center from 1 January 2015 to 1 July 2019 were included in this institutional review board–approved retrospective study. Detailed clinical data, including patient and transplant characteristics and complications and outcomes until the last follow-up, were extracted from the medical chart from each institution.
Definitions
Patients were classified as having met criteria for TA-TMA or not within the first 100 days after HCT via tissue diagnosis or if they met Jodele clinical diagnostic criteria; ≥4 of 7 of the following within a 2-week time frame: anemia or increased red blood cell transfusions, thrombocytopenia or increased platelet transfusions, elevated lactate dehydrogenase levels, hypertension ≥ 99th percentile for age or ≥140/90 mm Hg in patients aged ≥18 years, schistocytes, proteinuria (30 mg/dL), or elevated sC5b-9 levels (≥244 ng/mL).2 Patients who met the criteria for TA-TMA were then risk stratified: HR TA-TMA was defined as having both proteinuria and elevated sC5b-9 or proteinuria or elevated sC5b-9 with evidence of multiorgan dysfunction; otherwise, patients had standard-risk TA-TMA (SR TA-TMA). Previous definitions of multiorgan dysfunction were used (Table 1).12 Because screening and diagnostic practices differed based on the institution, both TA-TMA and risk stratification were captured in real time (ie, diagnosed by the provider), and criteria were retrospectively applied. Retrospective application ensured that data of all patients who met criteria for TA-TMA were captured and compared with data of those who did not meet the criteria for TA-TMA. Acute graft-versus-host disease (acute GVHD) was staged and graded using MAGIC criteria.13 CIBMTR definitions were used for conditioning regimen intensity.14 Infections were detected via culture, polymerase chain reaction, or, for fungal disease, positive fungal markers and supportive imaging findings.
Definitions of HR TA-TMA and multiorgan dysfunction
| HR TA-TMA . | |
|---|---|
| Proteinuria and elevated sC5-b9, or | |
| Proteinura or elevated sC5b-9 and any evidence of 2 organs or more in dysfunction. | |
| Cardiac |
|
| Pulmonary |
|
| Renal |
|
| Neurologic |
|
| Gastroenterology |
|
| HR TA-TMA . | |
|---|---|
| Proteinuria and elevated sC5-b9, or | |
| Proteinura or elevated sC5b-9 and any evidence of 2 organs or more in dysfunction. | |
| Cardiac |
|
| Pulmonary |
|
| Renal |
|
| Neurologic |
|
| Gastroenterology |
|
This definition was modified from prior publications.
ECHO, echocardiogram; HTN, hypertension; PRES, posterior reversible encephalopathy syndrome.
TA-TMA–directed therapy approach
TA-TMA–directed therapy was determined by the attending provider. Both institutions had changes in treatment approaches over time, and there was some variability in treatment approaches within each center. Thus, eculizumab was not administered to all high-risk patients.
Cost data
PHIS data were linked to clinical data via children’s hospital medical record numbers. Patient charges were captured only at their primary hospital of Boston Children’s Hospital and Cincinnati Children’s Hospital Medical Center. Thus, if a patient received care at a different hospital, these costs were not captured. However, the initial transplant admission only occurred at the primary hospital. PHIS data quality and reliability are assured through a joint effort of the Children’s Hospital Association and participating hospitals and are subject to a number of reliability and validity checks. Patient-specific charges were obtained from PHIS for transplant day −15 to 1 year after HCT. PHIS charges are reported using both billed and estimated costs. This study used standardized unit costs to account for the high variability in hospital cost accounting. The methods for obtaining this cost have been described.15 Briefly, the median of hospital costs was calculated for all billed items, adjusted for geographic location, differences in cost of living, and inflation (to 2019 US dollars). For each hospital stay, the inpatient length of stay was calculated as (discharge date) − (admit date) + 1; these were summed across all hospital stays for the total length of stays. The total cost was obtained by summing the costs across all stays for each patient. In addition to the total cost, cost per day (total cost/total length of stay) was analyzed from day −15 to 1 year after HCT. Subcategories of cost data were extracted in 2021 (to 2021 US dollars); given the differences in costs due to inflation between 2019 and 2021, subcategories of costs are reported as percentages of total standardized costs.
Analysis
Patient and transplant characteristics are reported descriptively (proportions, median, and range); subgroups were compared using Fisher exact or Wilcoxon rank sum test or χ2 test for trend in the case of >2 subgroup categories. Of note, although cost data described earlier were captured only throughout 1 year after first HCT, outcomes including death and relapse were captured until the last day of follow-up. The cost per day and total cost in the first year after HCT distributions were summarized using means, standard deviations, and quartiles; subgroups were compared using Wilcoxon rank sum tests because cost data were not normally distributed. The t test was used for subgroup comparisons of other continuous factors. Within allogeneic HCT recipients, a multivariable analysis of variance model tested for the effect of TA-TMA on cost, with adjustment for HLA mismatch, grade 3/4 acute GVHD, and infection (bacterial, viral, or fungal); these factors were selected a priori based on prior literature.
OS was defined as the time from HCT until death from any cause or censored on the date of last contact. Nonrelapse mortality (NRM) was defined as the time from HCT to any death unrelated to cancer relapse, censored on the date of last contact. OS curves were generated using the methods of Kaplan and Meier, with point estimates presented ± standard error (per Greenwood); subgroups were compared using a 2-sided log-rank test. When comparing survival within TA-TMA stratified based on treatment type, eculizumab vs supportive care, survival estimates started from the day of TA-TMA diagnosis. Cumulative incidence curves of NRM were generated using relapse and relapse-related death as competing risks. Subgroups were compared using Gray Test. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Overall cohort
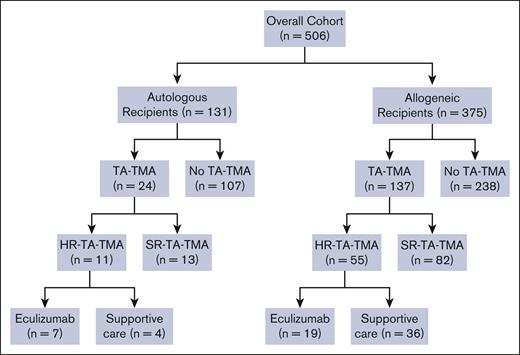
Of 506 serially eligible patients, 161 (31.8%) met the criteria for TA-TMA at a median of 26 days after HCT (range, 0-97). Compared with patients with no TA-TMA, the TA-TMA cohort was significantly more likely to have undergone an allogeneic transplant and have a diagnosis of veno-occlusive disease, severe grade 3 to 4 acute GVHD, infections, and engraftment failure. There were no differences in median age, sex, race, ethnicity, allogeneic donor type (related/unrelated), cell source, or HLA mismatch between the TA-TMA and the no–TA-TMA cohort (Table 2). The TA-TMA cohort (including both autologous and allogeneic HCT recipients) had significantly longer inpatient hospitalizations, with a median of 87 days (range, 22-411) vs 58 days (range, 16-489) for no TA-TMA (P < .0001). Of 161 children with TA-TMA, 66 (41%) had HR TA-TMA and 95 (59%) had SR TA-TMA (Figure 1).
Patient and HCT characteristics of patients with TA-TMA vs no TA-TMA (n = 506)
| . | TA-TMA (n = 161) . | No TA-TMA (n = 345) . | P value . |
|---|---|---|---|
| Age (y), mean (range) . | 8.3 (0.3-25.9) . | 8.0 (0.1-28.6) . | .776∗ . |
| . | n (%) . | n (%) . | . |
| Sex | |||
| Male | 103 (64) | 201 (58) | .222† |
| Female | 58 (36) | 144 (42) | |
| Race | .525† | ||
| Caucasian | 107 (80) | 255 (81) | |
| Black | 2 (1) | 11 (4) | |
| Asian | 5 (4) | 8 (3) | |
| Other | 20 (15) | 39 (12) | |
| Unknown | 27 | 32 | |
| Ethnicity | .239† | ||
| Hispanic | 14 (19) | 27 (13) | |
| Non-Hispanic | 59 (81) | 174 (87) | |
| Unknown | 88 | 144 | |
| Transplant indication | ND | ||
| Benign hematologic condition | 27 (17) | 76 (22) | |
| Hematologic malignancy | 49 (30) | 92 (27) | |
| Immune deficiency | 40 (25) | 48 (14) | |
| Solid tumor | 21 (13) | 86 (25) | |
| Other | 24 (15) | 43 (12) | |
| HCT type | <.001‡ | ||
| Allogeneic | 137 (85) | 238 (69) | |
| Autologous | 24 (15) | 107 (31) | |
| Donor | .057§ | ||
| Related | 39 (24) | 91 (26) | |
| Unrelated | 98 (61) | 147 (43) | |
| Not applicable (ie, autologous transplant) | 24 (15) | 107 (31) | |
| Cell source | <.001|| | ||
| BM | 100 (62) | 187 (55) | |
| Cord | 19 (12) | 15 (4) | |
| PBSC | 40 (25) | 136 (41) | |
| Unknown | 2 | 7 | |
| HLA mismatch | .072¶ | ||
| Mismatch | 56 (40) | 85 (31) | |
| No mismatch | 85 (60) | 190 (69) | |
| NA (autologous) | 20 | 70 | |
| Conditioning intensity | ND | ||
| Myeloablative | 88 (57) | 211 (70) | |
| Reduced intensity | 58 (37) | 84 (28) | |
| Nonmyeloablative | 9 (6) | 7 (2) | |
| None | 0 (0) | 1 (0.003) | |
| Unknown | 6 | 42 | |
| HCT number | .002† | ||
| 1 | 94 (84) | 172 (94) | |
| 2 | 18 (16) | 9 (5) | |
| 3 | 0 (0) | 2 (1) | |
| Unknown | 49 | 162 | |
| Engraftment failure | 8 (5) | 0 (0) | <.001† |
| VOD | 11 (7) | 8 (2) | .007† |
| Maximum acute GVHD | <.001† | ||
| Grade 0 | 120 (75) | 305 (89) | |
| Grade 1-2 | 14 (9) | 28 (8) | |
| Grade 3-4 | 27 (17) | 12 (3) | |
| Infections in the first 100 d | <.001#,† | ||
| Yes | 89 (55) | 80 (23) | |
| No | 72 (45) | 265 (77) |
| . | TA-TMA (n = 161) . | No TA-TMA (n = 345) . | P value . |
|---|---|---|---|
| Age (y), mean (range) . | 8.3 (0.3-25.9) . | 8.0 (0.1-28.6) . | .776∗ . |
| . | n (%) . | n (%) . | . |
| Sex | |||
| Male | 103 (64) | 201 (58) | .222† |
| Female | 58 (36) | 144 (42) | |
| Race | .525† | ||
| Caucasian | 107 (80) | 255 (81) | |
| Black | 2 (1) | 11 (4) | |
| Asian | 5 (4) | 8 (3) | |
| Other | 20 (15) | 39 (12) | |
| Unknown | 27 | 32 | |
| Ethnicity | .239† | ||
| Hispanic | 14 (19) | 27 (13) | |
| Non-Hispanic | 59 (81) | 174 (87) | |
| Unknown | 88 | 144 | |
| Transplant indication | ND | ||
| Benign hematologic condition | 27 (17) | 76 (22) | |
| Hematologic malignancy | 49 (30) | 92 (27) | |
| Immune deficiency | 40 (25) | 48 (14) | |
| Solid tumor | 21 (13) | 86 (25) | |
| Other | 24 (15) | 43 (12) | |
| HCT type | <.001‡ | ||
| Allogeneic | 137 (85) | 238 (69) | |
| Autologous | 24 (15) | 107 (31) | |
| Donor | .057§ | ||
| Related | 39 (24) | 91 (26) | |
| Unrelated | 98 (61) | 147 (43) | |
| Not applicable (ie, autologous transplant) | 24 (15) | 107 (31) | |
| Cell source | <.001|| | ||
| BM | 100 (62) | 187 (55) | |
| Cord | 19 (12) | 15 (4) | |
| PBSC | 40 (25) | 136 (41) | |
| Unknown | 2 | 7 | |
| HLA mismatch | .072¶ | ||
| Mismatch | 56 (40) | 85 (31) | |
| No mismatch | 85 (60) | 190 (69) | |
| NA (autologous) | 20 | 70 | |
| Conditioning intensity | ND | ||
| Myeloablative | 88 (57) | 211 (70) | |
| Reduced intensity | 58 (37) | 84 (28) | |
| Nonmyeloablative | 9 (6) | 7 (2) | |
| None | 0 (0) | 1 (0.003) | |
| Unknown | 6 | 42 | |
| HCT number | .002† | ||
| 1 | 94 (84) | 172 (94) | |
| 2 | 18 (16) | 9 (5) | |
| 3 | 0 (0) | 2 (1) | |
| Unknown | 49 | 162 | |
| Engraftment failure | 8 (5) | 0 (0) | <.001† |
| VOD | 11 (7) | 8 (2) | .007† |
| Maximum acute GVHD | <.001† | ||
| Grade 0 | 120 (75) | 305 (89) | |
| Grade 1-2 | 14 (9) | 28 (8) | |
| Grade 3-4 | 27 (17) | 12 (3) | |
| Infections in the first 100 d | <.001#,† | ||
| Yes | 89 (55) | 80 (23) | |
| No | 72 (45) | 265 (77) |
| Day 15 to day 365 after HCT . | Median (range) . | Median (range) . | . |
|---|---|---|---|
| Inpatient cost per day ($) | 4 369 (2 201-31 108) | 4 228 (2 275-27 012) | .490∗∗ |
| Total cost ($) | 470 493 (71 099-7 217 095) | 285 009 (21 531-6 212 835) | <.001∗∗ |
| Number of inpatient days | 87 (22-411) | 58 (6-489) | <.001∗∗ |
| Number of post-HCT days from TA-TMA | 26 (0-97) | ND | ND |
| ICU | <.001† | ||
| Admitted, n (%) | 58 (36) | 35 (10) | |
| Not admitted, n (%) | 103 (64) | 310 (90) | |
| ICU admission days | 18 (0-162) | 12 (0-90) | .135∗∗ |
| Day 15 to day 365 after HCT . | Median (range) . | Median (range) . | . |
|---|---|---|---|
| Inpatient cost per day ($) | 4 369 (2 201-31 108) | 4 228 (2 275-27 012) | .490∗∗ |
| Total cost ($) | 470 493 (71 099-7 217 095) | 285 009 (21 531-6 212 835) | <.001∗∗ |
| Number of inpatient days | 87 (22-411) | 58 (6-489) | <.001∗∗ |
| Number of post-HCT days from TA-TMA | 26 (0-97) | ND | ND |
| ICU | <.001† | ||
| Admitted, n (%) | 58 (36) | 35 (10) | |
| Not admitted, n (%) | 103 (64) | 310 (90) | |
| ICU admission days | 18 (0-162) | 12 (0-90) | .135∗∗ |
| . | OS ± SE (%) . | OS ± SE (%) . | P value . |
|---|---|---|---|
| OS | <.001†† | ||
| 100-d | 90.6 ± 2.3 | 98.2 ± 0.7 | |
| 180-d | 83.6 ± 2.9 | 97.3 ± 0.8 | |
| 1-y | 74.9 ± 3.5 | 92.6 ± 1.5 |
| . | OS ± SE (%) . | OS ± SE (%) . | P value . |
|---|---|---|---|
| OS | <.001†† | ||
| 100-d | 90.6 ± 2.3 | 98.2 ± 0.7 | |
| 180-d | 83.6 ± 2.9 | 97.3 ± 0.8 | |
| 1-y | 74.9 ± 3.5 | 92.6 ± 1.5 |
BM, bone marrow; BMF, bone marrow failure; ND, not done; PBSC, peripheral blood stem cells; VOD, veno-occlusive disease.
t test.
Fisher exact test.
Fisher exact test: comparison of allogeneic vs autologous.
Fisher exact test: comparison of related vs unrelated.
χ2 test: comparison of BM, cord, and PBSC as cell sources.
Fisher exact test: comparison of mismatch vs no mismatch.
Some patients had more than 1 infection.
Mann-Whitney test.
Log-rank test.
CONSORT diagram. A total of 506 patients underwent HCT during the study period. Allogeneic recipients were analyzed separately from autologous HCT recipients, and gene therapy recipients were excluded from the analysis. All patients who met criteria for TA-TMA were risk stratified into HR- TA-TMA or SR- TA-TMA. Patients were stratified as being at high risk if they had sC5b-9 level > the upper limit of normal and proteinuria > 2 mg/mg or either elevated sC5b-9 or proteinuria and multiorgan dysfunction. Among patients with HR-TA-TMA, patients were treated with either eculizumab or supportive care only.
CONSORT diagram. A total of 506 patients underwent HCT during the study period. Allogeneic recipients were analyzed separately from autologous HCT recipients, and gene therapy recipients were excluded from the analysis. All patients who met criteria for TA-TMA were risk stratified into HR- TA-TMA or SR- TA-TMA. Patients were stratified as being at high risk if they had sC5b-9 level > the upper limit of normal and proteinuria > 2 mg/mg or either elevated sC5b-9 or proteinuria and multiorgan dysfunction. Among patients with HR-TA-TMA, patients were treated with either eculizumab or supportive care only.
Autologous HCT characteristics and outcomes
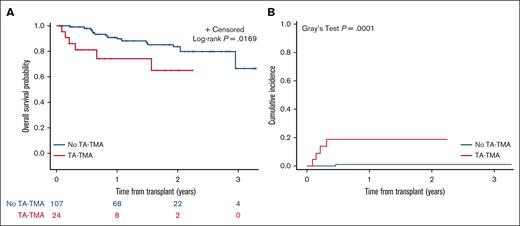
One hundred thirty-one patients received an autologous HCT for indications including neuroblastoma (n = 60), medulloblastoma (n = 12), Hodgkin lymphoma (n = 24), gene therapy (n = 2), solid tumor (n = 15), or other transplant indication (n = 18; supplemental Table 1). Twenty-four (18%) met the criteria for TA-TMA; significantly more children with neuroblastoma had TA-TMA (17 of 60 [28%]) vs any other autologous HCT indication (7 of 71 [9.9%]; P = .01; supplemental Table 2). Autologous HCT recipients with TA-TMA had a median of 62 (range, 22-411) hospital admission days vs 50 days (range, 6-313) for those without TA-TMA (P = .401; supplemental Table 3). OS from the first HCT in autologous HCT recipients was statistically significantly lower in patients with TA-TMA (1-year OS, 74.3% ± 10.2%) than in those with no TA-TMA (1-year OS, 90.9% ± 3.1%; P = .0169; Figure 2A). NRM was significantly higher in patients with TA-TMA (n = 24; 1-year NRM, 18.9% ± 8.8%) vs those with no TA-TMA (n = 107; 1-year NRM, 1.1% ± 1.1%; P < .0001; Figure 2B).
Overall surival (OS) (A); and cumulative incidence of NRM (B) in autologous HCT recipients with TA-TMA (n = 24) vs those without- TA-TMA (n = 107). (A) OS was significantly lower for children with TA-TMA (n = 24; 1-year OS: 74.3% ± 10.2%) vs those with no TA-TMA (n = 107; 1-year OS: 90.9% ± 3.1%). (B) NRM was significantly higher for TA-TMA patients (n = 24; 1-year NRM: 18.9% ± 8.8%) vs those with no TA-TMA (n = 107; 1-year NRM: 1.1% ± 1.1%).
Overall surival (OS) (A); and cumulative incidence of NRM (B) in autologous HCT recipients with TA-TMA (n = 24) vs those without- TA-TMA (n = 107). (A) OS was significantly lower for children with TA-TMA (n = 24; 1-year OS: 74.3% ± 10.2%) vs those with no TA-TMA (n = 107; 1-year OS: 90.9% ± 3.1%). (B) NRM was significantly higher for TA-TMA patients (n = 24; 1-year NRM: 18.9% ± 8.8%) vs those with no TA-TMA (n = 107; 1-year NRM: 1.1% ± 1.1%).
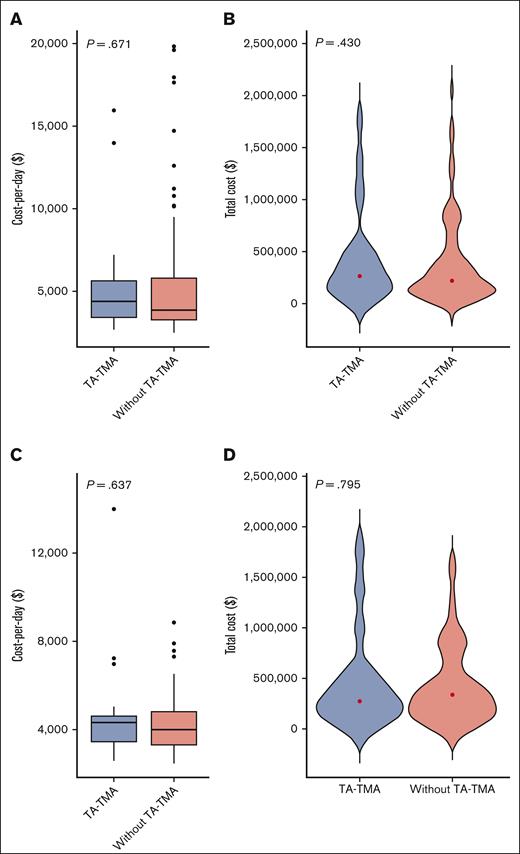
Unadjusted costs: TA-TMA vs no TA-TMA in autologous HCT
Among all autologous HCT recipients, there was no difference in cost per day (Figure 3A) or total cost for patients with TA-TMA (n = 24) vs no TA-TMA (n = 107); the median cost was $264 903 (range $71 098-$1 770 491) vs $220 680 (range $21 531-$2 050 522), respectively (P = .430; supplemental Table 3; Figure 3B). Given the significant differences in incidence based on HCT indication, we also compared the costs of TA-TMA (n = 17) vs no TA-TMA (n = 43) in patients with neuroblastoma only. We found no evidence of differences in cost per day or total cost for TA-TMA vs no TA-TMA among patients with neuroblastoma in the year after first HCT (P = .637 and P = .795, respectively; Figure 3C-D).
Distribution of inpatient cost per day and total cost for patients who received autologous HCT (A-B) (n = 131) and patients with neuroblastoma (C-D) (n = 60) with TA-TMA vs no-TA-TMA. (A) With TA-TMA (n = 24) vs those without TA-TMA (n = 107), cost per day from day 15 to day 365 after HCT (P = .671). (B) With TA-TMA (n = 24) vs those without TA-TMA (n = 107), total cost from day 15 to day 365 after HCT (P = .430). (C) With TA-TMA (n = 17) vs those without TA-TMA (n = 43): cost per day from day 15 to day 365 after HCT (P = .637). (D) With TA-TMA (n = 17) vs those without TA-TMA (n = 43): total cost (P = .795).
Distribution of inpatient cost per day and total cost for patients who received autologous HCT (A-B) (n = 131) and patients with neuroblastoma (C-D) (n = 60) with TA-TMA vs no-TA-TMA. (A) With TA-TMA (n = 24) vs those without TA-TMA (n = 107), cost per day from day 15 to day 365 after HCT (P = .671). (B) With TA-TMA (n = 24) vs those without TA-TMA (n = 107), total cost from day 15 to day 365 after HCT (P = .430). (C) With TA-TMA (n = 17) vs those without TA-TMA (n = 43): cost per day from day 15 to day 365 after HCT (P = .637). (D) With TA-TMA (n = 17) vs those without TA-TMA (n = 43): total cost (P = .795).
Unadjusted costs: HR TA-TMA and eculizumab vs supportive care in autologous HCT
Among the 24 children who received an autologous HCT and developed TA-TMA, 11 were stratified as having HR TA-TMA; 7 of 11 were treated with eculizumab, and 4 of 11 received supportive care only. Patients treated with eculizumab had significantly longer inpatient admission days (median, 89 vs 62.5; range, 41-127 vs 37-104). The cost per day was similar in the eculizumab and supportive care cohorts; $4534 vs $4313, respectively. However, the total costs appeared higher in the eculizumab-treated cohort ($446 961 [range, $229 056- $1 385 328] vs $229 295 [range, $150 776-$523 172]), likely due to longer hospitalizations (supplemental Table 4).
Allogeneic HCT characteristics and outcomes
Among the 375 patients who received an allogeneic HCT, 137 (36.5%) met criteria for TA-TMA, whereas 238 (63.5%) did not. The TA-TMA cohort had significantly longer inpatient days, with a median of 104 days (range, 28-483) vs the no–TA-TMA cohort with 66 days (range, 26-489; P < .001; supplemental Table 5). OS in allogeneic recipients was significantly lower in those with TA-TMA vs those without (P < .0001); 1-year OS was 75.0% ± 3.8% vs 93.2% ± 1.7%, respectively (Figure 4A). NRM was significantly higher in the TA-TMA cohort than in the no–TA-TMA cohort (P < .0001); 1-year NRM was 21.2% ± 3.6% vs 3.6% ± 1.3%, respectively (Figure 4B).
Overall survival (OS) (A) and cumulative incidence of NRM (B) of allogeneic HCT recipients with TA-TMA (n = 137) vs those with no TA-TMA (n = 238). (A) OS was significantly lower for children with TA-TMA (n = 137; 1-year OS: 75.0% ± 3.8%) vs those with no TA-TMA (n = 238; 1-year OS: 93.2% ± 1.7%) among allogeneic HCT recipients. (B) NRM was significantly higher for patients with TA-TMA (n = 137; 1-year NRM: 21.2% ± 3.6%) vs those with no TA-TMA (n = 238; 1-year NRM: 3.6% ± 1.3%) among allogeneic HCT recipients.
Overall survival (OS) (A) and cumulative incidence of NRM (B) of allogeneic HCT recipients with TA-TMA (n = 137) vs those with no TA-TMA (n = 238). (A) OS was significantly lower for children with TA-TMA (n = 137; 1-year OS: 75.0% ± 3.8%) vs those with no TA-TMA (n = 238; 1-year OS: 93.2% ± 1.7%) among allogeneic HCT recipients. (B) NRM was significantly higher for patients with TA-TMA (n = 137; 1-year NRM: 21.2% ± 3.6%) vs those with no TA-TMA (n = 238; 1-year NRM: 3.6% ± 1.3%) among allogeneic HCT recipients.
Unadjusted costs: TA-TMA vs no TA-TMA in allogeneic HCT
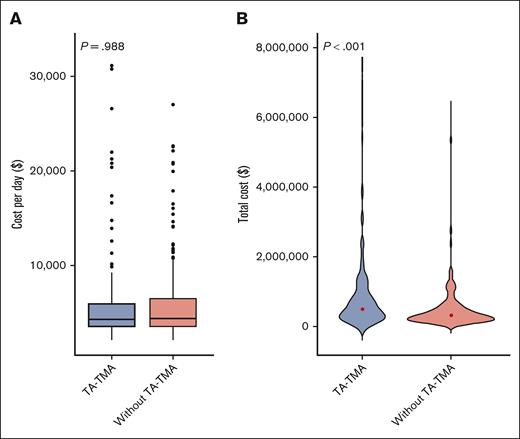
There was no significant difference in the median cost per day in patients with TA-TMA vs no TA-TMA; $4369 (range, $2201-$31 108) and $4379 (range, $2274-$27 012), respectively (P = .988; supplemental Table 5; Figure 5A). The total cost was significantly higher in patients with TA-TMA, at $501 594 (range, $120 614-$7 217 094) vs $319 285 ($82 996-$6 212 834) for no TA-TMA (P < .001; supplemental Table 5; Figure 5B).
Distribution of inpatient cost per day (A) and total cost (B) for patients who received an allogeneic transplant (n = 375), with TA-TMA vs without TA-TMA. (A) With TA-TMA (n = 137) vs those without TA-TMA (n = 238), cost per day from day 15 to day 365 after HCT (P = .988). (B) With TA-TMA (n = 137) vs those without TA-TMA (n = 238): total cost (P < .001).
Distribution of inpatient cost per day (A) and total cost (B) for patients who received an allogeneic transplant (n = 375), with TA-TMA vs without TA-TMA. (A) With TA-TMA (n = 137) vs those without TA-TMA (n = 238), cost per day from day 15 to day 365 after HCT (P = .988). (B) With TA-TMA (n = 137) vs those without TA-TMA (n = 238): total cost (P < .001).
Adjusted costs: TA-TMA vs no TA-TMA in allogeneic HCT
Among allogeneic HCT recipients, after adjusting for infections (bacterial, fungal, or viral), severe grade 3 to 4 acute GVHD, and HLA mismatch in the multivariable model, costs were not significantly different in the TA-TMA vs no–TA-TMA cohort. In this model, severe acute GVHD was associated with a significant difference in the cost per day (P = .014) and total cost (P < .001; Table 3).
Analysis of variance of the effect of TA-TMA on cost per day and total cost for patients who received allogeneic transplant
| . | Univariate model (n = 375) . | Multivariable model (n = 375) . | ||
|---|---|---|---|---|
| Factor . | P value . | Factors . | P value . | |
| Cost per day | TA-TMA | .434 | TA-TMA | .617 |
| HLA mismatch | .890 | |||
| Infection | .246 | |||
| Grade 3/4 GVHD | .014 | |||
| Total cost | TA-TMA | .002 | TA-TMA | .084 |
| HLA mismatch | .814 | |||
| Infection | .452 | |||
| Grade 3/4 GVHD | <.001 | |||
| . | Univariate model (n = 375) . | Multivariable model (n = 375) . | ||
|---|---|---|---|---|
| Factor . | P value . | Factors . | P value . | |
| Cost per day | TA-TMA | .434 | TA-TMA | .617 |
| HLA mismatch | .890 | |||
| Infection | .246 | |||
| Grade 3/4 GVHD | .014 | |||
| Total cost | TA-TMA | .002 | TA-TMA | .084 |
| HLA mismatch | .814 | |||
| Infection | .452 | |||
| Grade 3/4 GVHD | <.001 | |||
The bolded values are statistically significant.
HR TA-TMA vs SR TA-TMA in allogeneic recipients
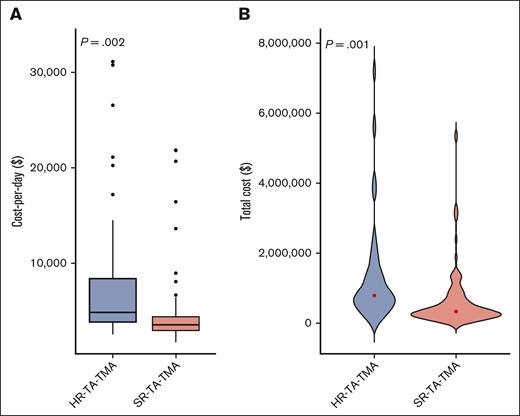
Of the 137 pediatric patients with TA-TMA, 55 (40%) were risk stratified as having HR-TA-TMA, and the remaining 82 (60%), as having SR-TA-TMA. Patients with HR-TA-TMA had significantly higher cost per day and total cost than patients with SR-TA-TMA (Figure 6A-B). The median total inpatient costs of the first year post HCT for patients with HR-TA-TMA (n = 55) was $791 767 (range, $156 379-$7 217 094) vs $338 829 (range, $120 614-$5 358 829) for SR-TA-TMA (n = 82; P = .001; supplemental Table 6). After adjusting for severe grade 3 to 4 acute GVHD, infections, and HLA mismatch among allogeneic recipients, both cost per day and total cost remained significantly higher in the HR-TA-TMA cohort vs SR-TA-TMA (Table 4).
Distribution of inpatient cost per day (A) and total cost (B) for allogeneic HCT recipients with TA-TMA (n = 137) according to risk stratification. (A) HR-TA-TMA (n = 55) vs SR-TA-TMA (n = 82): cost per day from day 15 to day 365 after HCT (P = .002). Patients were stratified as having high risk if they had sC5b-9 level > the upper limit of normal and proteinuria > 2 mg/mg or either elevated sC5b-9 level or proteinuria and multiorgan dysfunction. (B) HR-TA-TMA (n = 55) vs SR-TA-TMA (n = 82): total cost (P = .001).
Distribution of inpatient cost per day (A) and total cost (B) for allogeneic HCT recipients with TA-TMA (n = 137) according to risk stratification. (A) HR-TA-TMA (n = 55) vs SR-TA-TMA (n = 82): cost per day from day 15 to day 365 after HCT (P = .002). Patients were stratified as having high risk if they had sC5b-9 level > the upper limit of normal and proteinuria > 2 mg/mg or either elevated sC5b-9 level or proteinuria and multiorgan dysfunction. (B) HR-TA-TMA (n = 55) vs SR-TA-TMA (n = 82): total cost (P = .001).
Analysis of variance of the effect of HR TA-TMA on cost per day and total cost compared with that of SR TA-TMA
| . | Univariate model (n = 137) . | Multivariable model (n = 137) . | ||
|---|---|---|---|---|
| Factor . | P value . | Factors . | P value . | |
| Cost per day | HR-TA-TMA | .002 | HR-TA-TMA | .003 |
| HLA mismatch | .260 | |||
| Infection | .304 | |||
| Grade 3/4 GVHD | .045 | |||
| Total cost | HR-TA-TMA | .001 | HR-TA-TMA | .004 |
| HLA mismatch | .408 | |||
| Infection | .871 | |||
| Grade 3/4 GVHD | <.001 | |||
| . | Univariate model (n = 137) . | Multivariable model (n = 137) . | ||
|---|---|---|---|---|
| Factor . | P value . | Factors . | P value . | |
| Cost per day | HR-TA-TMA | .002 | HR-TA-TMA | .003 |
| HLA mismatch | .260 | |||
| Infection | .304 | |||
| Grade 3/4 GVHD | .045 | |||
| Total cost | HR-TA-TMA | .001 | HR-TA-TMA | .004 |
| HLA mismatch | .408 | |||
| Infection | .871 | |||
| Grade 3/4 GVHD | <.001 | |||
Patients were stratified as having HR TA-TMA if they had sCb-9 level > the upper limit of normal and proteinuria > 2 mg/mg or either elevated sC5b-9 level or proteinuria and multiorgan dysfunction.
The bolded values are statistically significant.
HR TA-TMA, eculizumab vs supportive care, and allogeneic recipients
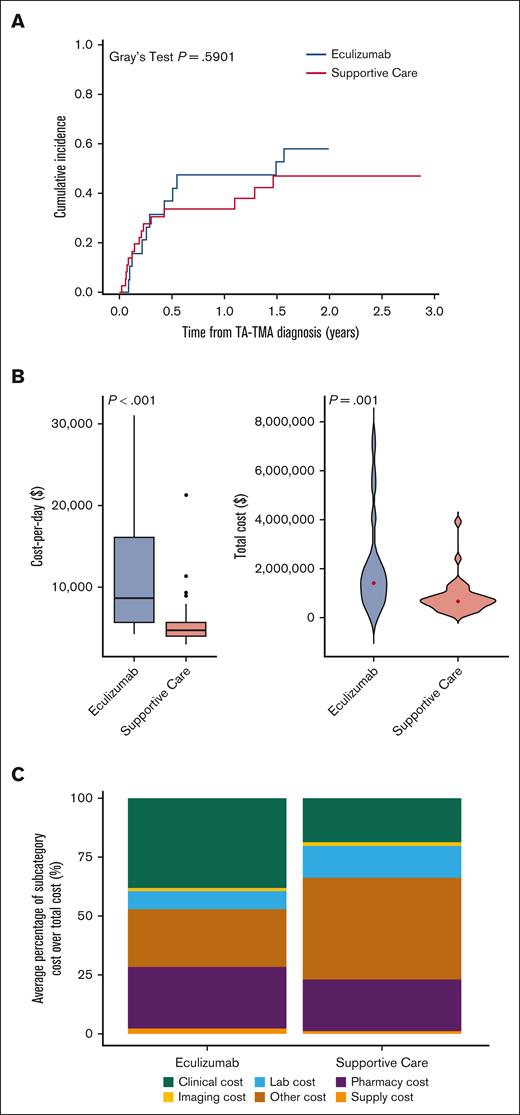
Of the patients stratified as HR-TA-TMA, 19 (35%) received eculizumab and 36 (65%) received supportive care. There was significantly more gastrointestinal bleeding (GI bleeding) in those treated with eculizumab than in those in the supportive care–only cohort. However, there were no differences in respiratory support needs, renal replacement, or grade 3 to 4 acute GVHD (Table 5). From the time of TA-TMA diagnosis, NRM of those treated with eculizumab was similar to that of those who received supportive care (P = .5901; Table 5; Figure 7A). From the time of HCT, the NRM of those treated with eculizumab was similar to that of those who received supportive care (P = .4778; supplemental Figure 1). Unadjusted cost per day (median, $8593; range, $4148-$31 108 vs median, $4609; range, $2939-$21 258; P < .001) and total costs (median, $1 419 823; range, $286 175-$7 217 095 vs median, $672 578; range, $156 379-$3 911 402; P = .001) were higher in the eculizumab-treated cohort than for those who received supportive care only (Table 5; Figure 7B). After adjusting for GI bleeding and grade 3/4 acute GVHD, costs per day in the eculizumab-treated cohort were higher (Figure 7C). However, the adjusted total cost was not associated with eculizumab and, instead, was associated with GI bleeding (Table 6). By subcategory, clinical, pharmacy, and supply costs were significantly higher in the eculizumab cohort than in the supportive care cohort (Figure 7D).
Demographic and HCT characteristics of recipients of allogeneic HCT with HR TA-TMA, treated with eculizumab vs supportive care only
| . | Eculizumab (N = 19) . | Supportive care only (N = 36) . | P . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Organ dysfunction∗ | |||
| Pericardial effusion | 2 (11) | 6 (17) | .700 |
| Pulmonary HTN | 2 (11) | 2 (6) | .602 |
| DAH | 0 (0) | 1 (3) | >.999 |
| Respiratory support | 10 (53) | 21 (58) | .685 |
| ECMO | 0 (0) | 1 (3) | >.999 |
| Double of serum Cr | 14 (74) | 27 (75) | >.999 |
| Renal replacement | 3 (16) | 9 (25) | .511 |
| HTN requiring ggt | 10 (53) | 14 (39) | .328 |
| AMS or PRES | 2 (11) | 7 (19) | .473 |
| GI bleeding | 6 (32) | 0 (0) | <.001 |
| Concurrent morbidities∗ | |||
| Severe grade 3/4 acute GVHD | 5 (26) | 9 (25) | >.999 |
| VOD | 2 (11) | 4 (11) | >.999 |
| Infection (bacterial or viral) | 10 (53) | 25 (69) | .218 |
| . | Eculizumab (N = 19) . | Supportive care only (N = 36) . | P . |
|---|---|---|---|
| n (%) . | n (%) . | ||
| Organ dysfunction∗ | |||
| Pericardial effusion | 2 (11) | 6 (17) | .700 |
| Pulmonary HTN | 2 (11) | 2 (6) | .602 |
| DAH | 0 (0) | 1 (3) | >.999 |
| Respiratory support | 10 (53) | 21 (58) | .685 |
| ECMO | 0 (0) | 1 (3) | >.999 |
| Double of serum Cr | 14 (74) | 27 (75) | >.999 |
| Renal replacement | 3 (16) | 9 (25) | .511 |
| HTN requiring ggt | 10 (53) | 14 (39) | .328 |
| AMS or PRES | 2 (11) | 7 (19) | .473 |
| GI bleeding | 6 (32) | 0 (0) | <.001 |
| Concurrent morbidities∗ | |||
| Severe grade 3/4 acute GVHD | 5 (26) | 9 (25) | >.999 |
| VOD | 2 (11) | 4 (11) | >.999 |
| Infection (bacterial or viral) | 10 (53) | 25 (69) | .218 |
| . | Estimate ± SE (%) . | Estimate ± SE (%) . | P value . |
|---|---|---|---|
| OS | .613 | ||
| 100-d | 84.2 ± 8.4 | 80.6 ± 6.6 | |
| 180-d | 73.7 ± 10.1 | 69.4 ± 7.7 | |
| 1-y | 52.6 ± 11.4 | 66.6 ± 7.9 | |
| NRM | .590 | ||
| 100-d | 26.3 ± 10.4 | 27.8 ± 7.6 | |
| 180-d | 36.8 ± 11.4 | 33.6 ± 8.0 | |
| 1-y | 47.4 ± 11.9 | 33.6 ± 8.0 |
| . | Estimate ± SE (%) . | Estimate ± SE (%) . | P value . |
|---|---|---|---|
| OS | .613 | ||
| 100-d | 84.2 ± 8.4 | 80.6 ± 6.6 | |
| 180-d | 73.7 ± 10.1 | 69.4 ± 7.7 | |
| 1-y | 52.6 ± 11.4 | 66.6 ± 7.9 | |
| NRM | .590 | ||
| 100-d | 26.3 ± 10.4 | 27.8 ± 7.6 | |
| 180-d | 36.8 ± 11.4 | 33.6 ± 8.0 | |
| 1-y | 47.4 ± 11.9 | 33.6 ± 8.0 |
| . | Median (range) . | Median (range) . | P value . |
|---|---|---|---|
| Time from transplant until patient met TA-TMA criteria (d) | 14 (0-85) | 29 (6-97) | .021 |
| Number of inpatient days | 156 (29-267) | 132.5 (47-331) | .405 |
| Duration of ICU stay (d) | 17 (0-61)† | 20 (1-162)† | .405 |
| Day 15 to day 365 after HCT | |||
| Inpatient cost per day ($) | 8 593 (4 148-31 108) | 4 609 (2 939-21 258) | <.001 |
| Total cost ($) | 1 419 823 (286 175-7 217 095) | 672 578 (156 379-3 911 402) | .001 |
| . | Median (range) . | Median (range) . | P value . |
|---|---|---|---|
| Time from transplant until patient met TA-TMA criteria (d) | 14 (0-85) | 29 (6-97) | .021 |
| Number of inpatient days | 156 (29-267) | 132.5 (47-331) | .405 |
| Duration of ICU stay (d) | 17 (0-61)† | 20 (1-162)† | .405 |
| Day 15 to day 365 after HCT | |||
| Inpatient cost per day ($) | 8 593 (4 148-31 108) | 4 609 (2 939-21 258) | <.001 |
| Total cost ($) | 1 419 823 (286 175-7 217 095) | 672 578 (156 379-3 911 402) | .001 |
Fisher exact test was used to calculate P values for binary data.
Mann-Whitney test was used to calculate P values for continuous data.
Log-rank test was used to calculate P values for time-to-event data.
Patients were stratified as being at high risk if they had sCb-9 level > the upper limit of normal and proteinuria > 2 mg/mg or either elevated sC5b-9 level or proteinuria and multiorgan dysfunction.
Treatment of eculizumab vs supportive care was at the discretion of the attending provider.
AMS, altered mental status; Cr, creatinine; DAH, diffuse alveolar hemorrhage; ECMO, extra corporal membrane oxygenation; ggt, continuous medication; HTN, hypertension; PRES, posterior reversible encephalopathy syndrome; VOD, veno-occlusive disease.
The bolded values are statistically significant.
Not mutually exclusive. A given patient could have more than 1 type of organ dysfunction or concurrent morbidity.
n = 17 eculizumab-treated and n = 26 supportive care patients were in the ICU.
Cumulative incidence of NRM of recipients of allogeneic HCT with HR TA-TMA: eculizumab (n = 19) vs supportive care (n = 36) (A), distribution of inpatient cost per day (B), total cost (C), and subcategories of cost in recipients of allogeneic HCT with HR TA-TMA (n = 55) based on treatment approach (D). (A) There was no significant difference in the rate of NRM for allogeneic HCT recipients who were stratified as having HR TA-TMA and treated with eculizumab vs those who received supportive care only (P = .48). One-year NRM of patients with TA-TMA diagnosis treated with eculizumab vs supportive care only was 47.4% ± 11.9% and 33.6% ± 8.0%, respectively. (B) Eculizumab (n = 19) vs supportive care (n = 36): cost per day from day 15 to day 365 after HCT (P < .001). (C) Eculizumab (n = 19) vs supportive care (n = 36): total cost (P = .001). (D) Total costs broken down according to cost subcategory in the allogeneic HCT HR TA-TMA cohort, depicted as cost percentage (%) among those treated with eculizumab and supportive care.
Cumulative incidence of NRM of recipients of allogeneic HCT with HR TA-TMA: eculizumab (n = 19) vs supportive care (n = 36) (A), distribution of inpatient cost per day (B), total cost (C), and subcategories of cost in recipients of allogeneic HCT with HR TA-TMA (n = 55) based on treatment approach (D). (A) There was no significant difference in the rate of NRM for allogeneic HCT recipients who were stratified as having HR TA-TMA and treated with eculizumab vs those who received supportive care only (P = .48). One-year NRM of patients with TA-TMA diagnosis treated with eculizumab vs supportive care only was 47.4% ± 11.9% and 33.6% ± 8.0%, respectively. (B) Eculizumab (n = 19) vs supportive care (n = 36): cost per day from day 15 to day 365 after HCT (P < .001). (C) Eculizumab (n = 19) vs supportive care (n = 36): total cost (P = .001). (D) Total costs broken down according to cost subcategory in the allogeneic HCT HR TA-TMA cohort, depicted as cost percentage (%) among those treated with eculizumab and supportive care.
Analysis of variance of the effect of eculizumab on cost per day and total cost for recipients of allogeneic HCT with HR-TA-TMA (n = 55)
| . | Univariate model (n = 55) . | Multivariable model (n = 55) . | ||
|---|---|---|---|---|
| Factor . | P value . | Factors . | P value . | |
| Cost per day | Eculizumab | <.001 | Eculizumab | .0185 |
| GI bleeding | .0176 | |||
| Grade 3/4 GVHD | .952 | |||
| Total cost | Eculizumab | .002 | Eculizumab | .219 |
| GI bleeding | <.001 | |||
| Grade 3/4 GVHD | .464 | |||
| . | Univariate model (n = 55) . | Multivariable model (n = 55) . | ||
|---|---|---|---|---|
| Factor . | P value . | Factors . | P value . | |
| Cost per day | Eculizumab | <.001 | Eculizumab | .0185 |
| GI bleeding | .0176 | |||
| Grade 3/4 GVHD | .952 | |||
| Total cost | Eculizumab | .002 | Eculizumab | .219 |
| GI bleeding | <.001 | |||
| Grade 3/4 GVHD | .464 | |||
The bolded values are statistically significant.
Discussion
HCT offers potentially curative therapy for malignant and nonmalignant diseases but is a costly procedure associated with significant HCU.16,17 Previously described drivers of HCU in children after HCT include acute GVHD, engraftment failure, and HLA mismatch.18,19 However, TA-TMA was not captured in these published studies. Consistent with prior literature, the TA-TMA cohort in this study had significantly more severe acute GVHD, infections, and HLA mismatch, all confounding drivers of cost. To appropriately adjust costs, we separated the cohorts by autologous and allogeneic recipients as acute GVHD and HLA mismatch would not affect the analysis of autologous transplant recipients.
Meeting criteria for TA-TMA was not associated with increased cost for autologous recipients in the year after the first HCT, including the subset of patients who underwent autologous HCT for neuroblastoma. It is possible that costs in the first year after HCT in the autologous setting are driven more by post-HCT treatments (ie, antibody therapy) than the early post-HCT period. It is also possible that other differences between the groups that were not captured might have masked higher costs in the TA-TMA cohort (eg, more relapses in the no–TA-TMA cohort), although these data are not available. In contrast, allogeneic HCT recipients with TA-TMA had significantly higher total costs and costs per day. As expected from prior data among allogeneic recipients, acute GVHD was also a major driver of increased cost.18,20 After adjusting for severe acute GVHD, TA-TMA was not associated with cost. More frequent infections, graft failure, and SOS were observed in the univariate analysis of the allogeneic cohort and might have contributed to the increased HCU, though multivariate analyses did not bear this out for these individual variables. SR TA-TMA is not associated with significant morbidity and mortality, so the HCU in the HR- TA-TMA allogeneic HCT recipient group were examined further. The patients with HR-TA-TMA had significantly higher total costs and cost per day than patients with SR-TA-TMA, even after adjusting for severe grade 3/4 acute GVHD, infections, and HLA mismatch, reflecting the severity of illness of patients with HR-TA-TMA.
A challenge of studying HCU among patients with TA-TMA is that they have significantly lower OS and, thus, may have lower total costs because of early death. To address this, we examined cost per day, which incorporated the time alive in total costs over 1 year. Among allogeneic HCT recipients, unadjusted costs per day were not significantly different, though total costs over 1 year were significantly higher in the TA-TMA cohort. These disparate results suggest that significantly longer inpatient hospital and ICU stays in the TA-TMA vs no–TA-TMA cohort are driving differences in HCU regardless of survival status.
We hypothesized that children with HR-TA-TMA treated with eculizumab would have fewer ICU days and less organ failure than those who received supportive care only; thus, costs would be lower or equivalent to the supportive care cohort only. Although unadjusted costs were significantly higher for eculizumab-treated allogeneic HCT recipients with HR TA-TMA than for those who received supportive care alone, total costs after adjusting for GI bleeding were not associated with increased costs. In the adjusted models, GI bleeding was associated with significant increases in HCU, not eculizumab therapy. The patients who were most ill with lower-GI bleeding had significant HCU and costs that may have obscured lower costs for those who were less ill with HR-TA-TMA.
Although adjusted costs per day remain higher in the HCT recipients with eculizumab-treated HR- TA-TMA, these differences are not explained by the cost of eculizumab. The cost of eculizumab will vary based on patient weight and length of therapy. The median age of the TA-TMA cohort was 7.4 years, with an approximate expected median weight of 22 kg. A vial of 300 mg of eculizumab costs $6523.21 For a 22-kg child requiring 11 doses of eculizumab, the median number reported in the literature,12 one could expect the course of eculizumab to cost $143 506. The median cost for patients with HR-TA-TMA treated with eculizumab was $1 229 773; thus, the course of eculizumab would represent 11.6% of inpatient costs. The median cost per day for patients with eculizumab-treated HR-TA-TMA was nearly double, or 100% higher than for those who received supportive care only ($8953 vs $4609), far more than the difference of the expected cost of eculizumab. Both adjusted costs and differences in the subcost clinical and supply categories suggest that patients treated with eculizumab were more ill in ways our data did not capture. We posit that increased HCU for those with HR-TA-TMA is driven by costs of supportive care needed for children who are critically ill and that eculizumab is a small fraction of the overall cost of caring for patients with HR-TA-TMA. Thus, drug cost alone should not be a contraindication to using this targeted therapy. The discrepancy between the cost per day and total costs in the eculizumab-treated vs supportive care HR-TA-TMA may be due to earlier death in eculizumab-treated patients with very severe illness.
We also hypothesized that the outcomes would be better for patients with HR-TA-TMA treated with eculizumab. However, the NRM was not different for those who received eculizumab compared with for those who received supportive care only. After evaluating for organ failure and other potential concurrent diagnoses, the only identified factor driving severity of illness in patients with HR-TA-TMA who received eculizumab vs supportive care was GI bleeding which occured significantly more in the eculizumab-treated cohort vs the supportive care cohort (n = 6 vs 0 respectively; P < .001). Patients with GI bleeding and TA-TMA have been shown to have poorer response to eculizumab12 and increased mortality.22 It is likely that GI TA-TMA may represent a more difficult organ to treat or that it is linked to other life-threatening diagnoses that cannot be captured, such as occult infections that lead to vessel destruction. Furthermore, there is overlap in the clinical manifestations and histologic findings of steroid refractory grade 3 to 4 acute GVHD and intestinal TA-TMA (even when biopsy samples of the intestines are obtained, it is not always possible to distinguish between the two. It is possible that some patients diagnosed with severe grade 3 to 4 lower gut GVHD also had intestinal TA-TMA that was missed. Although there were no differences in the proportion of severe grade 3/4 acute GVHD in the cohort who received eculizumab vs supportive care only, biomarker stratification is now known to be a more accurate predictor of severity and mortality,13 and these data were not available for this cohort.
This study's strengths include a large sample size, data from 2 institutions, and detailed clinical information paired with PHIS-cost data. Limitations include retrospective study design and challenges capturing TA-TMA. TA-TMA is a clinical diagnosis that relies on a combination of nonspecific clinical and laboratory findings. There is no “gold standard” test; although a renal biopsy is diagnostic when there is renal involvement, it is typically not obtained for children after HCT. Furthermore, TA-TMA can be a multisystemic disease sparing the kidneys but involving other organs, for which there are no universally accepted histologic diagnostic criteria. As with all clinical diagnoses, there is a risk of misclassification- labeling a patient as having TA-TMA who in fact does not have TA-TMA and vice-versa. These challenges exist whether the diagnosis is made prospectively or retrospectively. To overcome this limitation, decrease bias, and ensure the cohort was classified similarly, Jodele criteria, which are now recommended by expert consensus,24 were retrospectively applied. Another limitation of the study is potential institutional effect. Criteria for eculizumab administration, dosing frequency, and laboratory monitoring differed slightly among the 2 institutions. Differences in the practice of TA-TMA–directed therapy between the institutions and among providers at the same institution may result in biases in the patients who received TA-TMA–directed therapy with eculizumab. Although different treatment approaches allowed for comparison of costs in patients with HR-TA-TMA based on the treatment approach, biases also limit comparisons of both outcomes (OS and NRM) and costs in treatment groups. Furthermore, costs in this study are a marker of HCU but do not reflect total costs; neither outpatient costs, out-of-pocket costs, nor specific cellular therapy (ie, acquisition of an umbilical cord cell source) were accounted for in this study. In addition, no patient-related quality-of-life data were available in this study, which has often been included with HCU data to further quantify the effect of interventions.
In summary, we show that HR-TA-TMA in children after allogeneic HCT, regardless of treatment approach, is associated with significantly increased HCU. Given both HCU and mortality rates, strategies to prevent and pre-empt HR-TA-TMA are an urgent unmet need. Although treatment with eculizumab was associated with unadjusted higher HCU, adjusted total costs were not associated with eculizumab use: costs were higher in multiple subcategories of cost, and cost differences could not be attributed to cost of eculizumab for all (all suggesting that increased HCU in this population is multifactorial or children who are severely ill). GI bleeding was a critical confounder of HCU in the HR-TA-TMA cohort treated with eculizumab and may, in part, represent a higher HCU for patients with lower-GI GVHD, a known driver of cost, and a population less likely to respond to eculizumab. Future studies should investigate the source of the increase in HCU in TA-TMA and the implications of HCU, outcomes, and quality of life of TA-TMA–directed treatments. Randomized clinical trials are needed to understand the impact of TA-TMA–directed therapy and can be leveraged to further understand differences in HCU for patients who receive treatment vs supportive care only. Lastly, although differences in institutional treatment approaches to TA-TMA limit the interpretation of both HCU and NRM in the HR-TA-TMA group, these results, in combination with emerging data of varying response rates to eculizumab, highlight the need to identify which patients are most likely to benefit from eculizumab or other complement-inhibition approaches to mitigate costs and improve outcomes.
Acknowledgments
The authors are grateful for the funding that supported this work ([M.L.S.] National Institutes of Health LRP and National Institutes of Health K12 National Cancer Institute).
Authorship
Contribution: M.L.S. designed the study and wrote the manuscript with the oversight of C. Duncan and C. Dandoy; P.-C.K., N.C., and W.B.L. performed the statistical analysis; and all authors edited the manuscript.
Conflict-of-interest disclosure: M.L.S. reports honorarium and consulting to Omeros and Alexion. C.D. reports honorarium from Omeros and Alexion. W.B.L. reports consulting with Merck Sharp & Dohme Corp and Jubilant DraxImage, Inc; and scientific advisory board participation for Y-mAbs Therapeutics, Inc. S.J. reports honoraria from Omeros, SOBI, and Alexion, Patent US 10 815 296 B2 and PI for trial, with the drug provided by Alexion. The remaining authors declare no competing financial interests.
Correspondence: Michelle L. Schoettler, Department of Pediatrics, Division Blood and Marrow Transplantation, Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University, 1405 Clifton Rd NE, Atlanta, GA 30322; email: michelle.schoettler@emory.edu.
References
Author notes
C. Duncan and C. Dandoy are joint senior authors.
Deidentified data are available upon request from the corresponding author, Michelle L. Schoettler (michelle.schoettler@emory.edu).
The full-text version of this article contains a data supplement.