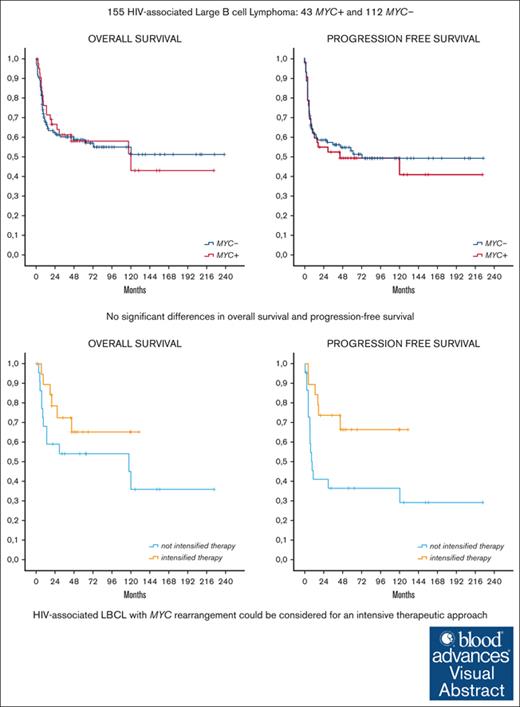
HIV-associated lymphomas with MYC rearrangement could be considered for an intensive therapeutic approach.
Standard (R)CHOP seems to give inferior complete remission rate and PFS in this subset of patients.
Visual Abstract
Large B-cell lymphoma (LBCL) carrying MYC rearrangement, alone or together with BCL2 and/or BCL6 translocations, have shown a poor prognosis when treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in the HIV population. Scanty data are available on the prevalence and prognostic impact of MYC rearrangements in HIV-associated LBCL. We conducted a retrospective study to evaluate the clinical effect of MYC rearrangement in HIV-associated LBCL. We evaluated clinical characteristics, treatment received, and outcome of LBCL in patients with HIV with MYC rearrangement (MYC+) and without MYC rearrangement (MYC–). A total of 155 patients with HIV who had received fluorescence in situ hybridization analysis for MYC were enrolled in 11 European centers: 43 with MYC+ and 112 MYC–. Among patients with MYC, 10 had double-/triple-hit lymphomas, and 33 had isolated MYC rearrangement (single-hit lymphoma). Patients with MYC+ had more frequently advanced stage, >2 extranodal site at presentation, and higher proliferative index. There were no significant differences in overall survival and progression-free survival (PFS) between the 2 groups. However, patients with MYC+ received more frequently intensive chemotherapy (iCT) (44%) than (R)CHOP alone (35%) or infusional treatment (DA-EPOCH-R and R-CDE) (19%). Among patients with MYC+, those who received iCT achieved a better outcome than patients who received nonintensive treatment (complete remission, 84% vs 52%; P = .028; 5-year PFS, 66% vs 36%; P = .021). Our retrospective results suggest that HIV-associated LBCL with MYC+ could be considered for an intensive therapeutic approach whenever possible, whereas (R)CHOP seems to give inferior results in this subset of patients in terms of complete remission and PFS.
Introduction
Diffuse large B-cell lymphomas (DLBCL) and high-grade B-cell lymphomas (HGBCL) are recognized as a group of heterogeneous diseases in the general HIV– population, and MYC rearrangement is present in a minority of cases (5%-15%).1 The presence of concomitant BCL2 and/or BCL6 translocation (“double-hit” or “triple-hit” lymphomas [DHL or THL]) is known to confer a worse prognosis to these aggressive B lymphomas when treated with standard therapy.1,2 Recent data have shown a distinct biology for MYC and BCL6 rearrangement compared with MYC and BCL2, and the International Consensus Classification of Mature Lymphoid Neoplasms has proposed a specific provisional entity.3 Some studies suggest that this subtype is even more aggressive and has worse overall survival (OS) than DHL with BCL2 translocation, whereas other studies indicate a similar or better prognosis. The conflicting conclusion may be the result of a limited sample population.4,5 Few data are available about the prognosis of MYC rearrangement alone (“single-hit” lymphomas [SHL]), and frequently, these lymphomas are analyzed together with DHL/THL. However, most authors have shown a poor prognosis for SHL, mainly in patients treated with standard rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).6-9
Patients without HIV with SHL and DHL/THL treated with R-CHOP show a 2-year OS and progression-free survival (PFS) ranging from 28% to 46% and from 15% to 46%, respectively.10-12 Some authors reported that the immunoglobulin gene as partner of MYC translocation confers the shortest survival time,13 and conflicting data have also been reported for the prognostic role of an increased number of MYC copy genes even without its rearrangement.14,15
Given the unfavorable prognosis with standard therapies, patients without HIV with DHL/THL are potential candidates for intensive chemotherapy (iCT), and some studies, mainly retrospective, have shown better results with regimens designed to treat Burkitt lymphoma (such as Hyper-CVAD, DA-EPOCH-R, and R-CODOX-M/IVAC) with 2-year OS of 44% to 82% and PFS of 41% to 65%.12,13,16,17 Consolidative autologous stem cell transplantation (ASCT) may represent a way to pursue, even though in retrospective studies, it was associated with improved OS only in patients who received R-CHOP, and not iCT, as first-line treatment.12,18,19 However, no prospective controlled studies in SHL and DHL/THL have been reported and a standard treatment has not yet been defined.
In consideration of the peculiar pathogenesis and tumor biology of lymphomas associated with HIV infection, the biological and prognostic features of the HIV– population cannot be directly translated into patients infected with HIV. Indeed, several studies demonstrated different molecular characteristics in DLBCL occurring in patients infected with HIV and in the general HIV– population.20,21 In a recent study, in HIV-associated DLBCL, treated with EPOCH and vorinostat, Myc protein expression was associated with significantly inferior outcomes.22 However, data regarding the prevalence and prognostic impact of MYC, BCL2, and BCL6 rearrangements, and the overexpression of the respective proteins in patients with HIV infection with lymphoma are few and inconclusive.23-25 Many questions are still unanswered. Do MYC rearrangements matter in HIV-associated large B-cell lymphomas (LBCLs) with or without BCL2/BCL6 translocations? Do SHL and DHL/THL have the same aggressive characteristics and worse outcome compared with LBCL without MYC rearrangements as it happens for HIV– population? Do we need “ad hoc” treatment for HIV-associated LBCL with MYC rearrangements?
Herein, we report the experience of 11 centers from 4 countries on a series of 155 consecutive cases of HIV-associated LBCL assessed for MYC rearrangement, and BCL2 and BCL6 translocations. The role, prognostic value, and effect on treatment efficacy of these genetic abnormalities were addressed in this setting, and patients’ characteristics, treatment received, and outcome were analyzed and compared with those of patients not carrying MYC rearrangement.
Materials and methods
Study design
We conducted a multicentric retrospective study involving 11 centers from 4 countries to evaluate the impact of MYC rearrangements (isolated or with BCL2 and/or BCL6 translocations) on clinical features and outcome of HIV-associated LBCL. Eligibility included age >17 years and a confirmed histological diagnosis of HGBCL NOS, DLBCL NOS, and HGBCL DHL/THL according to the 2017 World Health Organization classification, with fluorescence in situ hybridization (FISH) data available for MYC, BCL2, and BCL6.
This study was approved by the institutional review boards of participating centers and conducted in accordance with the Declaration of Helsinki.
The collected clinical data included: sex, age, Ann Arbor stage, LDH, number and type of involved extranodal sites, performance status according to the Eastern Cooperative Oncology Group (ECOG) at baseline, International prognostic index (IPI), histological subtype, FISH analysis, EBV-encoded RNA expression, immunohistochemistry results for Myc, Bcl2, and ki67 percentage, cell of origin (COO) according to Hans algorithm,26 baseline CD4 count and HIV viral load, date of HIV diagnosis, history of AIDS defining events before lymphoma, antiretroviral therapy (cART) use, hepatitis B virus and hepatitis C virus infections, type of CT used as first-line treatment, date of last follow-up (or date of death), status of disease at last follow-up, survival status, cause of death, relapse status and type and date of relapse if any, and salvage treatment received and response.
Double expressor lymphoma (DEL) was defined as overexpression by immunohistochemical staining of Myc and Bcl2 proteins, using cutoffs of 40% and 50%, respectively.1
All histological samples were analyzed by expert pathologists and reviewed to be classified according to the 2017 World Health Organization classification, whenever necessary.1
The main objectives of the study were to evaluate the baseline clinical characteristics, treatment received, and outcome of patients with LBCL and MYC rearrangement, evaluated by FISH analysis, comparing these results with those of patients with LBCL without MYC alteration. We also evaluated separately the outcome of the DHL/THL subgroup.
Outcome assessment and statistics
Disease response was defined by the revised response criteria for malignant lymphoma.27
PFS was defined as the interval between diagnosis and disease progression, last follow-up in remission, or death from any cause; and OS was defined as the interval between diagnosis and death or last follow-up while alive. Baseline characteristics are described as median and range for quantitative variables and as numbers and percentage for categorical variables. Categorical data were analyzed by Fisher exact test and χ2 test and continuous variables by the Wilcoxon–Mann-Whitney test. Survival curves were plotted in accordance with the Kaplan-Meier method and were compared using the log-rank test. The Cox proportional hazard model was used for univariable and multivariable survival analyses. Statistical significance was set at P <.05 (2-sided). Statistical analyses were performed using the SPSS statistical package (version 22.0 for Windows; IBM).
Results
Clinical characteristics
A total of 155 consecutive patients were included. They were diagnosed between May 2000 and January 2020. Forty-three patients (28%) had MYC rearrangements (MYC+), and 112 patients (72%) were negative for MYC rearrangements (MYC–). Seven patients had MYC increased copy number/amplification in the absence of rearrangement and were included in MYC– group. One hundred and twenty-nine patients had DLBCL NOS; 16 HGBCL NOS; and 10 HGBCL DHL/THL. Table 1 shows the clinical characteristics of patients at diagnosis. There were no significant differences between the 2 groups (MYC+ and MYC–) with respect to most demographic and clinical characteristics, including CD4 count (median, 200; range, 8-1170 cells/mcL for the entire series), presence of detectable HIV viremia, PS, LDH, and IPI risk group. Forty-four patients had a diagnosis of HIV and lymphoma concomitantly. A previous diagnosis of AIDS was more frequent in the MYC– group (27% vs 12%; P = .054). Patients with MYC+ had more frequently advanced stage (93% vs 80%; P = .05) and the involvement of >2 extranodal sites (50% vs 32%; P = .05) and of central nervous system (CNS) at presentation (14% vs 3%; P = .052). Patients with DHL/THL showed similar clinical characteristics compared with those with SHL (data not shown), other than more frequent AIDS diagnosis before lymphoma onset (33% vs 6%; P = .028).
Clinical characteristics of 2 groups of HIV-associated aggressive B lymphomas with or without MYC rearrangement
| . | Patients with MYC+, n = 43 . | Patients with MYC–, n = 112 . | Total, N = 155 . | P value . |
|---|---|---|---|---|
| Median age (range), y | 46 (26-74) | 48 (23-83) | 47 (23-83) | ns |
| Age >60 y | 7 (16%) | 14 (12%) | 21 (14%) | ns |
| Male sex | 37 (86%) | 88 (79%) | 125 (81%) | ns |
| Stage III-IV | 40/43 (93%) | 88/110∗ (80%) | 128/155 (82%) | .05 |
| B symptoms | 22/43 (51%) | 55/108∗ (51%) | 77/151∗ (51%) | ns |
| Increased LDH | 30/39∗ (77%) | 70/104∗ (67%) | 100/143∗ (70%) | ns |
| Extranodal sites | .05 | |||
| ≤ 2 | 19/38∗ (50%) | 68/100∗ (68%) | 87/138∗ (63%) | |
| > 2 | 19/38∗ (50%) | 32/100∗ (32%) | 51/138∗ (37%) | |
| CNS involvement | 4/29∗ (14%) | 2/64∗ (3%) | 6/93∗ (6%) | .052 |
| Kidney/adrenal gland involvement | 8/29∗ (27%) | 8/64∗ (12%) | 16/93∗ (17%) | .074 |
| ECOG performance status ≥2 | 19/34∗ (56%) | 42/87∗ (48%) | 61/121∗ (50%) | ns |
| IPI intermediate high-high | 23/39∗ (64%) | 49/92∗ (53%) | 72/131∗ (55%) | ns |
| HCV seropositivity | 11/38∗ (29%) | 34/93∗ (36%) | 45/131∗ (34%) | ns |
| Positive HBsAg | 3/36∗ (8%) | 8/89∗ (9%) | 11/125∗ (9%) | ns |
| Median CD4+ cell baseline (range), n/mmc | 215 (32-1170) | 198 (8-990) | 198 (8-1170) | ns |
| CD4+ cell <200/mmc | 19/38∗ (50%) | 51/100∗ (51%) | 70/138∗ (51%) | ns |
| Detectable HIV load | 24/40∗ (60%) | 65/107∗ (61%) | 89/147∗ (60%) | ns |
| AIDS before lymphoma diagnosis | 5/41∗ (12%) | 30/111∗ (27%) | 35/152∗ (23%) | .054 |
| cART before lymphoma diagnosis | 20/42∗ (48%) | 62/110∗ (56%) | 32/152∗ (54%) | ns |
| . | Patients with MYC+, n = 43 . | Patients with MYC–, n = 112 . | Total, N = 155 . | P value . |
|---|---|---|---|---|
| Median age (range), y | 46 (26-74) | 48 (23-83) | 47 (23-83) | ns |
| Age >60 y | 7 (16%) | 14 (12%) | 21 (14%) | ns |
| Male sex | 37 (86%) | 88 (79%) | 125 (81%) | ns |
| Stage III-IV | 40/43 (93%) | 88/110∗ (80%) | 128/155 (82%) | .05 |
| B symptoms | 22/43 (51%) | 55/108∗ (51%) | 77/151∗ (51%) | ns |
| Increased LDH | 30/39∗ (77%) | 70/104∗ (67%) | 100/143∗ (70%) | ns |
| Extranodal sites | .05 | |||
| ≤ 2 | 19/38∗ (50%) | 68/100∗ (68%) | 87/138∗ (63%) | |
| > 2 | 19/38∗ (50%) | 32/100∗ (32%) | 51/138∗ (37%) | |
| CNS involvement | 4/29∗ (14%) | 2/64∗ (3%) | 6/93∗ (6%) | .052 |
| Kidney/adrenal gland involvement | 8/29∗ (27%) | 8/64∗ (12%) | 16/93∗ (17%) | .074 |
| ECOG performance status ≥2 | 19/34∗ (56%) | 42/87∗ (48%) | 61/121∗ (50%) | ns |
| IPI intermediate high-high | 23/39∗ (64%) | 49/92∗ (53%) | 72/131∗ (55%) | ns |
| HCV seropositivity | 11/38∗ (29%) | 34/93∗ (36%) | 45/131∗ (34%) | ns |
| Positive HBsAg | 3/36∗ (8%) | 8/89∗ (9%) | 11/125∗ (9%) | ns |
| Median CD4+ cell baseline (range), n/mmc | 215 (32-1170) | 198 (8-990) | 198 (8-1170) | ns |
| CD4+ cell <200/mmc | 19/38∗ (50%) | 51/100∗ (51%) | 70/138∗ (51%) | ns |
| Detectable HIV load | 24/40∗ (60%) | 65/107∗ (61%) | 89/147∗ (60%) | ns |
| AIDS before lymphoma diagnosis | 5/41∗ (12%) | 30/111∗ (27%) | 35/152∗ (23%) | .054 |
| cART before lymphoma diagnosis | 20/42∗ (48%) | 62/110∗ (56%) | 32/152∗ (54%) | ns |
HCV, Hepatitis C Virus; HBsAg, Hepatitis B surface antigen; ns, not significant.
Denominators regard assessed patients.
Histopathological characteristics
The main histological features of the MYC+ and MYC– lymphomas are shown in Table 2. Patients with MYC+ had more frequent translocation of BCL2 (14% vs 3%; P = .019), germinal center B phenotype according to Hans algorithm (87% vs 49%; P = .0001), and a higher median proliferative index (median Ki-67, 91% vs 85%; P = .012). There were no significant differences between the 2 groups with respect to the number of DEL and incidence of EBV-encoded RNA positivity. Among patients with MYC+, 10 (23%) had HGBCL DHL/THL, and 33 (77%) had SHL (25 within the DLBCL NOS and 8 within the HGBCL NOS). Of note, SHL had a significantly higher proliferative index than DHL/THL (median ki67, 95% [range, 60%-100%] vs 80% [range, 60%-100%]; P = .003).
Histopathological characteristics of 2 groups of HIV-associated aggressive B lymphomas with or without MYC rearrangement
| . | Patients with MYC+ n = 43 . | Patients with MYC– n = 112 . | Total N = 155 . | P value . |
|---|---|---|---|---|
| Histology | ||||
| DLBCL NOS | 25 (58%) | 104 (93%) | 129 (83%) | .0001 |
| HGBCL NOS | 8 (19%) | 8 (7%) | 16 (10%) | .036 |
| HGBCL DH-TH | 10 (23%) | 0 | 10 (7%) | - |
| Median Ki67% (range) | 91 (40-100) | 85 (40-100) | 90 (40-100) | .012 |
| Ki67 ≥90% | 25/38∗ (66%) | 38/83∗ (46%) | 63/121∗ (52%) | .041 |
| DEL† | 15/39∗ (38%) | 27/95∗ (28%) | 42/134∗ (31%) | ns |
| BCL2 translocation | 6/43 (14%) | 3/93∗ (3%) | 9/136∗ (7%) | .019 |
| BCL6 translocation | 7/40∗,‡ (17%) | 25/91∗ (27%) | 32/131∗ (24%) | ns |
| EBV–encoded RNA positive | 3/14∗ (21%) | 17/52∗ (33%) | 20/66∗ (30%) | ns |
| COO GCB | 25/32∗ (87%) | 45/91∗ (49%) | 73/123∗ (59%) | .0001 |
| COO non-GCB | 5/32∗ (16%) | 46/91∗ (51%) | 51/123∗ (41%) |
| . | Patients with MYC+ n = 43 . | Patients with MYC– n = 112 . | Total N = 155 . | P value . |
|---|---|---|---|---|
| Histology | ||||
| DLBCL NOS | 25 (58%) | 104 (93%) | 129 (83%) | .0001 |
| HGBCL NOS | 8 (19%) | 8 (7%) | 16 (10%) | .036 |
| HGBCL DH-TH | 10 (23%) | 0 | 10 (7%) | - |
| Median Ki67% (range) | 91 (40-100) | 85 (40-100) | 90 (40-100) | .012 |
| Ki67 ≥90% | 25/38∗ (66%) | 38/83∗ (46%) | 63/121∗ (52%) | .041 |
| DEL† | 15/39∗ (38%) | 27/95∗ (28%) | 42/134∗ (31%) | ns |
| BCL2 translocation | 6/43 (14%) | 3/93∗ (3%) | 9/136∗ (7%) | .019 |
| BCL6 translocation | 7/40∗,‡ (17%) | 25/91∗ (27%) | 32/131∗ (24%) | ns |
| EBV–encoded RNA positive | 3/14∗ (21%) | 17/52∗ (33%) | 20/66∗ (30%) | ns |
| COO GCB | 25/32∗ (87%) | 45/91∗ (49%) | 73/123∗ (59%) | .0001 |
| COO non-GCB | 5/32∗ (16%) | 46/91∗ (51%) | 51/123∗ (41%) |
EBV, Epstein-Barr virus; GCB, germinal center B; HGBCL DH-TH, high-grade B-cell lymphoma “double or triple hit”; ns, not significant.
Denominators regard assessed patients.
The double expression was considered in the entire series (DLBCL NOS; HGBCL NOS; and HGBCL DH-TH).
In 3 patients with HGBCL DH-TH, BCL6 translocation was not available.
Among the 104 patients with MYC– DLBCL NOS, only 3% had BCL2 rearrangement, whereas 27% had BCL6 rearrangement.
Treatment
The treatment received by the 2 groups (MYC+ and MYC–) is reported in Table 3. In the MYC+ group, 44% of patients received iCT (GMALL, CODOX IVAC, CARMEN regimen,28 standard CT followed by ASCT as consolidation, and others), 35% received (R)CHOP or (R)CHOP-like regimens, 19% infusional therapy (R-DA-EPOCH, R-CDE), and 2% supportive care. In the MYC– group, 68% received (R)CHOP or (R)CHOP-like regimens, 20% iCT, 8% infusional therapy, and 3% supportive care. Therefore, patients with MYC+ received more frequently iCT (P = .003) and less often (R)CHOP regimen (P = .0001) than the MYC– group. In all series, patients with ki67 percentage >90% received more frequently iCT than patients with ki67 <90% (25/63 [39%] vs 9/58 [15%]; P = .003).
Treatment received in 2 groups of HIV–associated aggressive B lymphomas with or without MYC rearrangement
| . | Patients with MYC+ n = 43 . | Patients with MYC– n = 112 . | Total N = 155 . | P value . |
|---|---|---|---|---|
| Rituximab | 41 (95%) | 99 (88%) | 140 (90%) | ns |
| CHOP/CHOP-like | 15 (35%) | 76 (68%) | 91 (59%) | .0001 |
| Infusional therapy | 8 (19%) | 9 (8%) | 17 (11%) | .063 |
| DA-EPOCH | 6 | 6 | 12 | |
| CDE | 2 | 3 | 5 | |
| iCT | 19 (44%) | 23 (20%) | 42 (27%) | .003 |
| CARMEN Regimen28 | 9 | 1 | 10 | |
| GMALL | 3 | 2 | 5 | |
| CODOX IVAC | 2 | 1 | 3 | |
| CT∗ + ASCT consolidation | 3 | 15 | 18 | |
| Other† | 2 | 4 | 6 | |
| Palliative care | 1 (2%) | 4 (3%) | 5 (3%) | ns |
| CNS prophylaxis | 32/37‡ (86%) | 62/94‡ (66%) | 94/131‡ (72%) | .011 |
| IT (MTX ± ARA-C) | 24 | 55 | 79 | |
| Iv MTX ± IT | 8 | 7 | 15 | |
| Radiotherapy | 2 (5%) | 9 (8%) | 11 (7%) | ns |
| . | Patients with MYC+ n = 43 . | Patients with MYC– n = 112 . | Total N = 155 . | P value . |
|---|---|---|---|---|
| Rituximab | 41 (95%) | 99 (88%) | 140 (90%) | ns |
| CHOP/CHOP-like | 15 (35%) | 76 (68%) | 91 (59%) | .0001 |
| Infusional therapy | 8 (19%) | 9 (8%) | 17 (11%) | .063 |
| DA-EPOCH | 6 | 6 | 12 | |
| CDE | 2 | 3 | 5 | |
| iCT | 19 (44%) | 23 (20%) | 42 (27%) | .003 |
| CARMEN Regimen28 | 9 | 1 | 10 | |
| GMALL | 3 | 2 | 5 | |
| CODOX IVAC | 2 | 1 | 3 | |
| CT∗ + ASCT consolidation | 3 | 15 | 18 | |
| Other† | 2 | 4 | 6 | |
| Palliative care | 1 (2%) | 4 (3%) | 5 (3%) | ns |
| CNS prophylaxis | 32/37‡ (86%) | 62/94‡ (66%) | 94/131‡ (72%) | .011 |
| IT (MTX ± ARA-C) | 24 | 55 | 79 | |
| Iv MTX ± IT | 8 | 7 | 15 | |
| Radiotherapy | 2 (5%) | 9 (8%) | 11 (7%) | ns |
IT, intrathecal; ns, not significant.
Mainly CHOP regimen.
Other terapies included HD MTX plus HD ARAC, as well as stanford regimen for Burkitt.
Denominators regard assessed patients.
Rituximab was administered in 95%, 100%, and 88% of patients treated with (R)CHOP or (R)CHOP-like regimens, infusional therapy, and iCT, respectively.
Of the 10 patients with DHL/THL, 30% received (R)CHOP or (R)CHOP-like regimens, 30% received infusional therapy, 30% iCT, and 10% palliative care. Of the 33 patients with SHL, 36% received (R)CHOP ore (R)CHOP-like, 15% infusional therapy, and 49% iCT. All patients received cART during lymphoma treatment.
Clinical outcome
In the MYC+ group, 42 of 43 patients were evaluable for response (1 patient received palliative care). Twenty-eight patients (66%) achieved a complete remission (CR), 2 (5%) a partial remission (PR), and 12 (28%) no response or progressive disease. CR rate was 53% in patients who received (R)CHOP or (R)CHOP-like regimens, 50% in patients who received infusional therapy, and 84% in patients who received iCT (iCT vs [R]CHOP; P = .05; iCT vs other treatments; P = .028). In the MYC– group, 102 of 112 patients were evaluable for response (10 patients had an early death or received palliative care). Seventy-one patients (70%) achieved a CR, 10 (10%) a PR, and 21 (20%) no response or progressive disease. CR was 69%, 78%, and 65% in patients treated with (R)CHOP or (R)CHOP-like regimens, infusional therapy, and iCT, respectively (P = not significant).
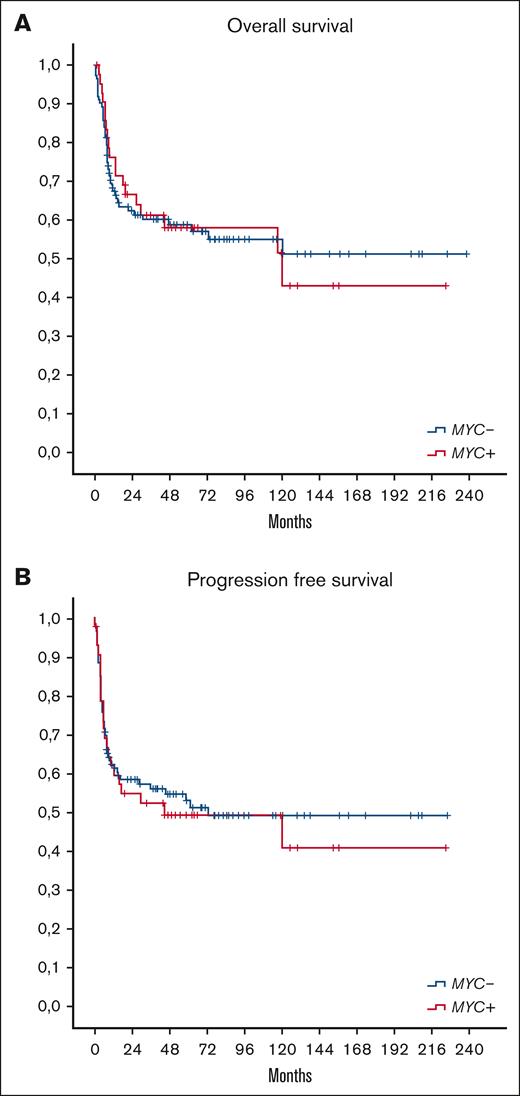
After a median follow-up of 57 months (range, 1-238) in all populations, including 58 months in the MYC+ group and 53 months in the MYC– group, there were no significant differences in OS and PFS between patients with MYC+ and patients with MYC–, respectively (5-year OS and PFS, 58% and 49% in patients with MYC+, respectively, and 59% and 53% in patients with MYC–; as shown in Figure 1). In MYC+ group, 19 of 43 patients (44%) died (15 patients [35%] due to lymphoma progression, 1 [2%] to HIV complications, 2 [4%] to other cancers, and 1 [2%] from unknown cause). In MYC– group, 47 of 112 patients (42%) died (29 patients [26%] due to lymphoma progression, 6 [5%] to treatment toxicities [all patients had received [R]CHOP or [R]CHOP-like regimens], 3 [3%] to HIV complications, 1 [1%] to a second malignancy, and 8 [7%] from other causes). The main cause of death was progressive disease for both groups. Of note, no patient treated with iCT died due to treatment toxicity. Patients with relapsed/refractory disease had a little chance to be salvaged with second-line therapy. Of 53 patients with documented disease progression, 12 and 26 patients received salvage treatment in the MYC+ and MYC– groups, followed by ASCT consolidation in 4 and 8 patients, respectively. Only 3 and 6 patients achieved a second CR in MYC+ and MYC– groups, respectively.
OS and PFS according to MYC status. OS (A) and PFS (B) of patients with MYC+ and those with MYC– (P not significant).
OS and PFS according to MYC status. OS (A) and PFS (B) of patients with MYC+ and those with MYC– (P not significant).
No difference in OS was seen between SHL and DHL/THL (5-year OS, 61% vs 50%; P = .145), whereas PFS was better in SHL vs DHL/THL (55% vs 30%; P = .045) (Figure 2).
OS and PFS in SHL and DHL-THL lymphoma. OS (A) and PFS (B) of “single-hit” and “double-triple hit” lymphomas (P = .145 for OS; and P = .045 for PFS between 2 groups).
OS and PFS in SHL and DHL-THL lymphoma. OS (A) and PFS (B) of “single-hit” and “double-triple hit” lymphomas (P = .145 for OS; and P = .045 for PFS between 2 groups).
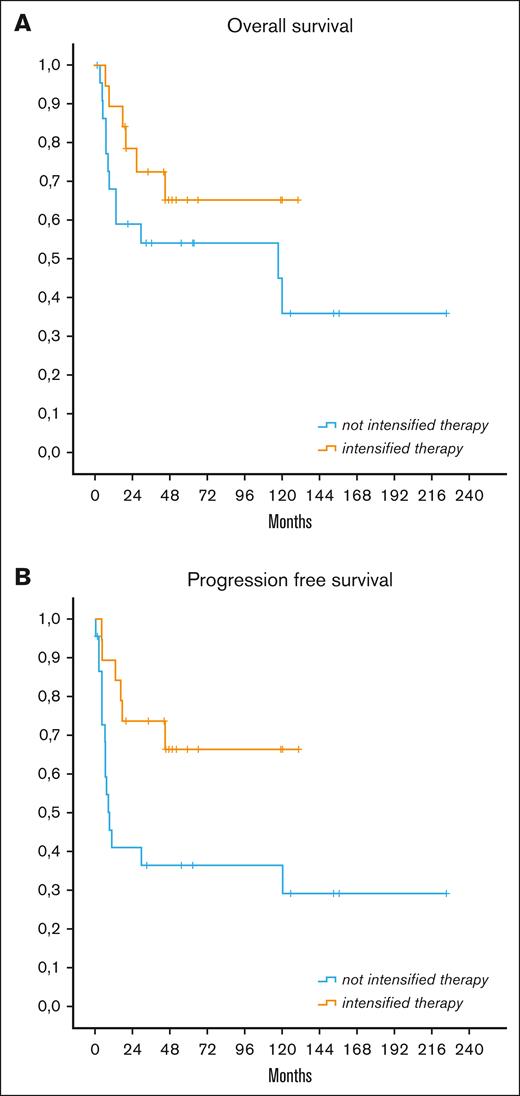
In the MYC+ group, 5-year PFS and OS were 65% and 66% with iCT, 37% and 47% with infusional therapy, and 37% and 57% with (R)CHOP, respectively. The use of iCT correlated with significantly better PFS than with other treatments (P = .021) and with a trend toward better OS (P = .19) (Figure 3). Although iCT maintained a statistically significant advantage in PFS vs (R)CHOP treatment (P = .025), no statistically significant difference in survival was registered between patients receiving iCT and the low number of patients receiving infusional therapy.
OS and PFS in patients with MYC+ according to treatment received. OS (A) and PFS (B) according to treatment received in MYC+ group (P = .021 for PFS between intensified and not intensified therapy; P was not significant for OS).
OS and PFS in patients with MYC+ according to treatment received. OS (A) and PFS (B) according to treatment received in MYC+ group (P = .021 for PFS between intensified and not intensified therapy; P was not significant for OS).
In patients with MYC–, there were no significant differences in OS and PFS according to the different treatments received.
Analyzing the homogeneous group of patients with DLBCL treated with nonintensive regimens ([R]CHOP or [R]CHOP-like regimens) a nonstatistically significant better outcome was reported in patients with MYC– than those with MYC+, with a 5-year PFS of 52% and 36% (P = .3), respectively.
Considering only DEL, there were no significant differences in OS and PFS in patients who received (R)CHOP or (R)CHOP-like therapy or iCT.
Prognostic factors
In the univariate analysis of the whole population (n = 155), IPI ≥3, ECOG performance status ≥2, and increased LDH were related to worse OS and PFS, whereas BCL2 translocation and DHL/THL were correlated with shorter PFS and ki67 >90% with better PFS. Ann Arbor stage III-IV predicted a worse OS and PFS, even if not statistically significant; ki67>90% predicted a better OS, B symptoms a worse OS, and BCL6 translocation a worse PFS. Major prognostic factors, including those with significant or borderline impact on survival and those with a major clinical impact are reported in Table 4. In the multivariate analysis, ECOG performance status ≥2 and elevated LDH maintained their negative prognostic impact on OS and PFS, ki67 >90% was related to a better OS and PFS, and BCL6 translocation to a worse PFS (Table 5). COO did not have a prognostic impact on survival, as did the double expression of Myc and Bcl2.
Univariate analysis for OS and PFS of the entire population of 155 patients
| Variable . | n . | OS . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median, mo . | IC 95% . | % 5y . | P . | Median, mo . | IC 95% . | % 5y . | P value . | ||
| Age, y | |||||||||
| ≤50 | 102 | 120 | 42-198 | 55 | .26 | 46 | 0-124 | 47 | .115 |
| >50 | 53 | NR | - | 65 | NR | - | 61 | ||
| Stage | |||||||||
| III-IV | 128 | 120 | 42-192 | 54 | .054 | 46 | 1-90 | 48 | .057 |
| Not III-IV | 27 | NR | - | 77 | NR | - | 70 | ||
| B symptoms | 77 | 120 | 0-265 | 52 | .095 | 36 | 0-114 | 47 | .173 |
| Not B symptoms | 78 | NR | - | 64 | NR | - | 57 | ||
| ECOG performance status not | |||||||||
| ≥2 | 94 | NR | - | 72 | .0001 | NR | - | 63 | .0001 |
| ≥2 | 61 | 13 | 4-23 | 38 | 8 | 3-13 | 34 | ||
| Not increased LDH | 55 | NR | - | 81 | .002 | NR | - | 70 | .002 |
| Increased LDH | 100 | 31 | - | 46 | 15 | 0-40 | 42 | ||
| IPI | |||||||||
| Not int. high-high | 83 | NR | - | 69 | .012 | NR | - | 59 | .027 |
| Int. high-high | 72 | 31 | 0-84 | 47 | 13 | 0-47 | 43 | ||
| CD4 <200/uL | 70 | 73 | 0-155 | 51 | .13 | 46 | 0-102 | 47 | .357 |
| Not CD4 <200/uL | 85 | NR | - | 64 | NR | - | 56 | ||
| Ki67 ≥90% | 63 | NR | - | 65 | .058 | NR | - | 64 | .007 |
| Not Ki67 ≥90% | 92 | 117 | 35-200 | 53 | 16 | 0-42 | 43 | ||
| MYC+ | 43 | 120 | 12-228 | 58 | .942 | 50 | 0-143 | 49 | .712 |
| MYC– | 112 | NR | - | 59 | NR | - | 53 | ||
| BCL2 rearranged | 9 | 18 | 0-45 | 44 | .37 | 9 | 5-13 | 0 | .016 |
| Not BCL2 rearranged | 146 | 120 | - | 59 | NR | - | 55 | ||
| BCL6 rearranged | 32 | 120 | 0-270 | 52 | .226 | 10 | 0-58 | 39 | .089 |
| Not BCL6 rearranged | 123 | NR | - | 60 | NR | - | 56 | ||
| DHL or THL | |||||||||
| Yes | 10 | 18 | 0-76 | 50 | .217 | 9 | 3-15 | 30 | .048 |
| No | 145 | NR | - | 59 | NR | - | 53 | ||
| iCT | |||||||||
| Yes | 42 | NR | - | 59 | .297 | NR | - | 57 | .181 |
| No | 113 | 120 | 62-178 | 58 | 59 | 0-135 | 50 | ||
| Variable . | n . | OS . | PFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median, mo . | IC 95% . | % 5y . | P . | Median, mo . | IC 95% . | % 5y . | P value . | ||
| Age, y | |||||||||
| ≤50 | 102 | 120 | 42-198 | 55 | .26 | 46 | 0-124 | 47 | .115 |
| >50 | 53 | NR | - | 65 | NR | - | 61 | ||
| Stage | |||||||||
| III-IV | 128 | 120 | 42-192 | 54 | .054 | 46 | 1-90 | 48 | .057 |
| Not III-IV | 27 | NR | - | 77 | NR | - | 70 | ||
| B symptoms | 77 | 120 | 0-265 | 52 | .095 | 36 | 0-114 | 47 | .173 |
| Not B symptoms | 78 | NR | - | 64 | NR | - | 57 | ||
| ECOG performance status not | |||||||||
| ≥2 | 94 | NR | - | 72 | .0001 | NR | - | 63 | .0001 |
| ≥2 | 61 | 13 | 4-23 | 38 | 8 | 3-13 | 34 | ||
| Not increased LDH | 55 | NR | - | 81 | .002 | NR | - | 70 | .002 |
| Increased LDH | 100 | 31 | - | 46 | 15 | 0-40 | 42 | ||
| IPI | |||||||||
| Not int. high-high | 83 | NR | - | 69 | .012 | NR | - | 59 | .027 |
| Int. high-high | 72 | 31 | 0-84 | 47 | 13 | 0-47 | 43 | ||
| CD4 <200/uL | 70 | 73 | 0-155 | 51 | .13 | 46 | 0-102 | 47 | .357 |
| Not CD4 <200/uL | 85 | NR | - | 64 | NR | - | 56 | ||
| Ki67 ≥90% | 63 | NR | - | 65 | .058 | NR | - | 64 | .007 |
| Not Ki67 ≥90% | 92 | 117 | 35-200 | 53 | 16 | 0-42 | 43 | ||
| MYC+ | 43 | 120 | 12-228 | 58 | .942 | 50 | 0-143 | 49 | .712 |
| MYC– | 112 | NR | - | 59 | NR | - | 53 | ||
| BCL2 rearranged | 9 | 18 | 0-45 | 44 | .37 | 9 | 5-13 | 0 | .016 |
| Not BCL2 rearranged | 146 | 120 | - | 59 | NR | - | 55 | ||
| BCL6 rearranged | 32 | 120 | 0-270 | 52 | .226 | 10 | 0-58 | 39 | .089 |
| Not BCL6 rearranged | 123 | NR | - | 60 | NR | - | 56 | ||
| DHL or THL | |||||||||
| Yes | 10 | 18 | 0-76 | 50 | .217 | 9 | 3-15 | 30 | .048 |
| No | 145 | NR | - | 59 | NR | - | 53 | ||
| iCT | |||||||||
| Yes | 42 | NR | - | 59 | .297 | NR | - | 57 | .181 |
| No | 113 | 120 | 62-178 | 58 | 59 | 0-135 | 50 | ||
Only those parameters that achieved statistical or borderline significance on at least 1 end point or parameters with clinical relevance are listed.
DHL or THL, double-hit or triple-hit lymphoma; NR, not reached.
Multivariate analysis for OS and PFS of the entire population of 155 patients
| OS . | |||
|---|---|---|---|
| . | Hazard ratio . | 95% confidence interval . | P value . |
| ECOG PS ≥2 | 2.8 | 1.4-5.6 | .003 |
| Increased LDH | 2.2 | 1.1-4.1 | .018 |
| Ki67>90% | 0.56 | 0.3-0.96 | .035 |
| PFS | |||
| ECOG PS ≥2 | 2.9 | 1.5-5.7 | .002 |
| Increased LDH | 2.4 | 1.3-4.4 | .006 |
| Ki67>90% | 0.5 | 0.3-0.9 | .014 |
| BCL6 rearranged | 2 | 1.1-3.6 | .018 |
| OS . | |||
|---|---|---|---|
| . | Hazard ratio . | 95% confidence interval . | P value . |
| ECOG PS ≥2 | 2.8 | 1.4-5.6 | .003 |
| Increased LDH | 2.2 | 1.1-4.1 | .018 |
| Ki67>90% | 0.56 | 0.3-0.96 | .035 |
| PFS | |||
| ECOG PS ≥2 | 2.9 | 1.5-5.7 | .002 |
| Increased LDH | 2.4 | 1.3-4.4 | .006 |
| Ki67>90% | 0.5 | 0.3-0.9 | .014 |
| BCL6 rearranged | 2 | 1.1-3.6 | .018 |
Discussion
MYC gene rearrangements are usually recorded in 5% to 15% of HIV– DLBCL,8,10 whereas old studies analyzing the pathogenesis of HIV-associated lymphoma in the pre-cART era reported this genetic abnormality in ∼20% of AIDS-related diffuse large cell lymphomas.29 However, no data on BCL2 and BCL6 translocations were reported in this subgroup of patients, and the potential prognostic value of MYC translocations was not investigated. In the more recent cART era, only few data are available on the incidence, clinical characteristics, and outcome of SHL and DHL/THL in the setting of HIV.22-25 Our study aims to analyze HIV-associated BCL, comparing those with or without MYC genetic alteration, associated or not to BCL2 and BCL6 translocations, from clinical presentation to treatment choice and survival outcome, to add knowledge in a field with really scanty information.
In our series, 28% of patients had MYC rearrangement. The only significant clinical differences between MYC+ and MYC– groups were a more frequent involvement of >2 extranodal sites and advanced stage in the MYC+ group and a more common previous diagnosis of AIDS in the MYC– group. Patients with MYC+ also tend to have more often CNS localization at onset. As already reported in the HIV-negative setting, patients with MYC+ had significantly more frequent germinal center B phenotype. Moreover, higher ki67 percentage and more frequent translocation of BCL2 were found in the patients with MYC+. As a result, we could not find clinicopathological factors able to adequately discriminate patients who deserve to investigate the presence of MYC translocation, which appears advisable in all patients with aggressive HIV+ LBCL.
Of note in DLBCL cases, the frequency of BCL6 rearrangement (21%) was similar to those described in patients without HIV infection, whereas BCL2 rearrangement was infrequent (2.6%), with an incidence substantially lower than that reported in immunocompetent patients, in line with the study of Baptista et al. 25
In our retrospective series, the clinical outcome of patients with MYC+ was not significantly different compared with patients with MYC–, but the different performed treatment among the 2 groups of patients could be a bias of final results. Patients with MYC+ more frequently received iCT (including intensive CT schedules and high dose CT with ASCT consolidation after standard (R)CHOP) than patients with MYC–. This choice was probably a consequence of the analogous translocation (MYC) that characterizes the Burkitt lymphoma and of the experience in the HIV-negative setting. Indeed, in our series, the use of iCT demonstrated to confer a better outcome than other treatments and standard (R)CHOP, particularly in the MYC+ group. On the other hand (R)CHOP seems to better perform in patients with MYC–. This appears in line with results of retrospective studies in patients without HIV12,13,18,19 and underlines the role of intensive first-line treatment in this subset of LBCL. Moreover, because patients with MYC rearrangements had a higher incidence of CNS involvement, diagnostic lumbar puncture and CNS prophylaxis is highly recommended in this group of patients. Notably, no patients treated with iCT died from toxicity in our study. The main cause of death remains lymphoma for both patients with MYC+ and those with MYC–.
Although DA-EPOCH-R is associated with durable remissions in HIV-negative MYC+ aggressive B-cell lymphomas and should be considered for the treatment of these diseases,16 we were not able to confirm its favorable outcome in the HIV-positive setting, maybe due to low number of patients treated with infusional therapy in our series, and no firm conclusion can be drawn.
Only 10 of our patients were diagnosed with DHL/THL; however, they showed a particularly poor outcome, despite most patients receiving iCT or infusional therapies, thus, confirming what is known from the experience in the HIV-negative setting.
In our series, in the MYC+ group, 2-year OS and PFS were 57% and 36% in patients treated with (R)CHOP/CHOP-like regimens, respectively, and 79% and 74% in patients treated with iCT.
These results compare favorably with those reported on SHL and DHL/THL in patients without HIV, in which 2-year OS and PFS ranged from 28% to 46% and 15% to 46%, respectively, for patients treated with (R)CHOP/CHOP-like regimens and 44% to 82% and 41% to 65% for patients treated with iCT.10-13,16,17
Some other information may come from our study. The frequency of DEL in the entire series was similar between the 2 groups (38% in MYC+ and 28% in MYC–) and did not confer different prognosis, in contrast to what has been reported in several studies on HIV-negative lymphoma.30 Although the role of COO has a recognized impact on the outcome of lymphomas of the general population, in our study there was no impact of COO on survival. However, conflicting results are reported in previous studies in the HIV setting.31,32 In our series, by multivariate analysis, higher PS and increased LDH correlated with worse survival, whereas ki67 >90% resulted to be a prognostic factor of better survival. The predictive positive role of elevated proliferation index had been demonstrated also in a previous study,31 suggesting an increased chemosensitivity in this subgroup.
This study has several limitations that underpowered the conclusions: its retrospective design, some missing data, low numbers for single subgroups, and the wide time period of patient enrollment. Considering the retrospective and multicentric design of this study, the patients were treated based on the choice of the treating physician, and likely, the more aggressive lymphomas received the more aggressive therapy, leading to better outcomes with iCT. The strength of this study relies in the high number of patients with FISH available for MYC abnormalities and the comparison with MYC– lymphomas, as well as the ability to describe a real-world series of non-Burkitt MYC rearranged HIV+ LBCL, in a setting in which data are very rare. Anyway, we are conscious that prospective studies are needed to draw definitive and more useful conclusions.
Based on our data, it could be reasonable to treat patients with HIV infection with MYC+ with iCT schedules, usually used for Burkitt lymphoma, or with ASCT consolidation after induction therapy, at least in fit and young patients with a good control of HIV infection from cART. Due to the encouraging results obtained in patients with MYC+ with DA-EPOCH-R in the HIV-negative setting, we think it remains however reasonable to apply the same strategy in patients with HIV infection.16 However, our data regarding this treatment do not help due to the low number of patients.
Because lymphoma progression remains the major cause of death in these patients and patients with relapsed and refractory have few chances to be cured even with ASCT, a better knowledge of the pathogenetic mechanisms of these diseases could help improving the therapeutic approach also in terms of target therapies.33 Nowadays, combination of targeted or immunomodulators drugs with standard CT are being evaluated in patients without HIV infection. If effective, they hopefully will be used also in patients with HIV infection who are usually excluded from experimental clinical trial.
Interestingly, recent studies, using gene expression profiling, developed signatures derived from genes differentially expressed between DHL and non-DHL DLBCL.34,35 Notably, this signature could identify most DHL even among patients with DLBCL who did not harbor gene rearrangements of MYC and BCL2, suggesting the existence of alternative genetic or epigenetic alterations causing a high-grade lymphoma phenotype.
In the meantime, a FISH analysis for MYC and, if positive, also for BCL2 and BCL6 is advisable for all aggressive BCL in patients with HIV infection. All patients with MYC+ BCLs might be considered for an intensive therapeutic approach whenever possible because (R)CHOP seems to give inferior results in these patients. A diagnostic intrathecal puncture and CNS prophylaxis are also highly recommended.
Acknowledgments
The authors appreciate the excellent technical assistance and collaboration of hematologists, pathologists, infectious disease specialists, research nurses, and data managers of the participating centers. The authors especially thank Lorenza Pecciarini, Giovanni Rindone, and Raida Ahmad for their help in the histological review.
P.S. is supported by the DFG-Emmy Noether Program (project number 495793173).
Authorship
Contribution: A. Re. designed the research study; C.P., A. Re., and C.R. designed and completed the database; C.P. conducted the statistical analysis; C.P., A. Re., and A.D.P. were involved in data analysis and interpretation; C.P., C.R., A.D.P., E.R., P.S., M.B-O., L.V., G.G., M.S., L.A., S.S., D.D., L.C., L.L., P.B., C.C., M.B., F.F., L.B., A. Rosenwald., A.J.M.F., G.R., A.T., and A. Re registered and treated patients, collected clinical data, and performed histological, molecular, and phenotypical analysis; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: M.S. reports honoraria from Gilead, Servier, Novartis, Incyte, BeiGene, and Istituto Gentili; and research funding from Menarini. L.A. reports consultancy fees from or advisory role in Roche, Janssen-Cilag, Verastem, Incyte, EUSA Pharma, Celgene/Bristol Myers Squibb, Kite/Gilead, and ADC Therapeutics; speakers’ bureau fees from EUSA Pharma and Novartis; and research funding from Gilead Sciences. D.D. reports travel grants from Roche, Gentili, Lilly, and Eisai; and other remuneration from Gentili and Daikii Sanchio. The remaining authors declare no competing financial interests.
Correspondence: Chiara Pagani, Department of Hematology, ASST Spedali Civili di Brescia, Piazzale Spedali Civili 1, 25123 Brescia, Italy; email: chiara.pagani@asst-spedalicivili.it.
References
Author notes
Original data are available upon reasonable request from the corresponding author, Chiara Pagani (chiara.pagani@asst-spedalicivili.it).