Thorough characterization of humanized NSG and NBSGW strains including human HSC engraftment, mobilization, and in vivo transduction.
Human HSCs in the murine BM lack sufficient low-density lipoprotein receptor expression and exhibit low VSV-G-LV transduction efficiency.
Visual Abstract
In vivo hematopoietic stem cell (HSC) gene therapy is an emerging and promising area of focus in the gene therapy field. Humanized mouse models are frequently used to evaluate novel HSC gene therapy approaches. Here, we comprehensively evaluated 2 mouse strains, NSG and NBSGW. We studied human HSC engraftment in the bone marrow (BM), mobilization of BM-engrafted HSCs into circulation, in vivo transduction using vesicular stomatitis virus glycoprotein–pseudotyped lentiviral vectors (VSV-G LVs), and the expression levels of surface receptors needed for transduction of viral vectors. Our findings reveal that the NBSGW strain exhibits superior engraftment of human long-term HSCs compared with the NSG strain. However, neither model resulted in a significant increase in circulating human HSCs after mobilization. We show that time after humanization as well as human chimerism levels and platelet counts in the peripheral blood can be used as surrogates for human HSC engraftment in the BM. Furthermore, we observed low expression of the low-density lipoprotein receptor, a requirement for VSV-G LV transduction, in the human HSCs present in the murine BM. Our comprehensive characterization of humanized mouse models highlights the necessity of proper validation of the model and methods to study in vivo HSC gene therapy strategies.
Introduction
In vivo hematopoietic stem cell (HSC) gene therapy offers a promising strategy to eliminate the need for burdensome mobilization and collection of HSCs through leukapheresis, complex and expensive modification of HSCs ex vivo in cleanroom facilities, as well as cytotoxic myeloablation for the removal and replacement of unmodified endogenous HSCs. Although in vivo application of gene therapy agents would overcome all these bottlenecks, many basic questions regarding the efficiency, feasibility, and safety are still unanswered and need comprehensive testing before moving into clinical applications. To preclinically test novel therapeutic agents and strategies for in vivo HSC gene therapy, various mouse strains humanized with CD34+ hematopoietic stem and progenitor cells (HSPC) are in use.1-4 Such humanized mouse models are often used with the assumption that the engrafted human HSCs are phenotypically and functionally identical to patient cells and mimic the clinical standard without prior in-depth confirmation. To close this knowledge gap, we comprehensively evaluated the possibilities and limitations of humanized mouse models for the preclinical testing of in vivo HSC gene therapy approaches, specifically for the use of lentiviral vectors.
Three basic requirements, among others, must be satisfied to allow testing of in vivo HSC gene therapy in humanized mouse models. First, the model should demonstrate high human long-term HSC (LT-HSC) engraftment in the bone marrow (BM) without compromising the animal’s health and the phenotypic and functional properties of human HSCs. Second, engrafted human HSCs in the murine BM need to be made accessible to the therapeutic reagents commonly introduced IV using mobilization agents such as granulocyte-colony stimulating factor (G-CSF) and/or AMD3100 in humans.5-11 Lastly, human HSCs mobilized into the peripheral blood (PB) need to be permissive for the gene therapy agents applied in vivo, for example, express crucial cell surface antigens needed for the binding and fusion of viral vectors.
Here, we comprehensively evaluated the NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG)12 and NOD.Cg-KitW-41JTyr+PrkdcscidIl2rgtm1Wjl/ThomJ (NBSGW)13 mouse strains for in vivo HSC gene therapy. NSG and NBSGW mice were humanized as neonates for evaluation of human HSC engraftment, efficiency of human HSC mobilization, and the in vivo transduction of human HSCs with vesicular stomatitis virus glycoprotein–pseudotyped lentiviral vectors (VSV-G-LVs).14,15 Our work reveals limitations and possibilities of using humanized mouse models for in vivo gene therapy and, thus, demonstrates the importance of thorough evaluation of preclinical models.
Methods
Xenotransplantation of NSG and NBSGW mice
NSG and NBSGW neonates aged 1 to 3 days were intrahepatically injected with 30 000 to 50 000 human umbilical cord blood (UCB)-derived CD34+ HSPCs per animal in 30 μL of injection media (filtered RPMI 1640, 1 μM EDTA), as previously described.16,17 At least 2 hours before human cell injection, NSG neonates were preconditioned with a sublethal dose of 150 cGy total body irradiation (TBI) in a cesium irradiator. NBSGW neonates did not undergo TBI. Starting at the eighth week after injection, human chimerism was measured as the percentage of human CD45+ cells among the mouse and human CD45+ in the PB. Blood analysis was performed every other week until necropsy, at which BM, PB, and spleen were collected. Resulting analyses were conducted on data pooled from 2 to 3 iterations of each experiment. Each iteration used UCB-derived CD34+ cells from different donors.
In vivo mobilization of human HSCs in NSG and NBSGW mice
Animals receiving G-CSF were subcutaneously injected once every 24 hours for 5 days at a dose of 5 μg/kg per day. The last injection was performed 3 hours before necropsy. Animals receiving AMD3100 were subcutaneously injected once, 3 hours before necropsy at a dose of 5 mg/kg per day. G-CSF and AMD3100 were delivered in filtered phosphate-buffered saline (PBS) using a 28-gauge insulin syringe.
Intraosseous (IO) injection of lentivirus
Local IO injections of VSV-G-LVs expressing green fluorescent protein (GFP; vector sequence provided in supplemental Data) were conducted on humanized NBSGW mice. The animals were anesthetized via aerosolized isoflurane and injected at the knee of 1 femur using a 28-gauge insulin syringe containing 15 μL of the viral vector (5.3 × 107 IU/mL), which was equivalent to 8 × 105 IU total per injection. During vector injection, the BM and the viral vector were mixed by drawing the mix into the syringe and unloading the mixture back into the BM. The other noninjected limb was processed at necropsy in parallel as a control. Control animals were given an IO injection of PBS into 1 femur.
More details are provided in supplemental Data.
Results
The NBSGW strain demonstrates enhanced human LT-HSC engraftment in the BM
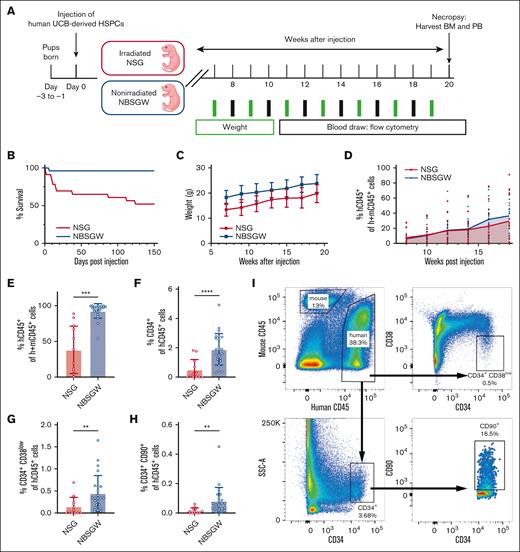
For a comprehensive comparison of the NSG and NBSGW strains as models for testing in vivo HSC gene therapy strategies, cohorts of the 2 strains were humanized and tracked for 20 weeks to assess survival, weight, human chimerism, multilineage output, and HSC composition (Figure 1A). Overall, NBSGW mice showed better survival and higher body weight than the NSG mice, allowing for more reliable blood sampling, easier administration of agents, and providing enhanced reliability for the long-term follow-up (Figure 1B-C). As reported previously,13 irradiated NSG and nonirradiated NBSGW strains showed similar human chimerism in the PB (Figure 1D) but different kinetics of lineage outputs, specifically for B- and T-cell lineages (supplemental Figure 1A-J). Phenotypic assessment of the BM by flow-cytometry revealed greater human chimerism (CD45+) and higher frequencies of human CD34+ HSPCs in NBSGW than in NSG mice (Figure 1E-F). Most importantly, the fractions of CD34+CD38low and CD34+CD90+ HSCs were significantly higher in the NBSGW strain (Figure 1G-I). To confirm that these phenotypic HSCs are functionally primitive, CD34+ and CD34+CD90+ cells were fluorescence-activated cell sorter purified and plated for colony-forming cell assays. The overall frequency of colonies and the types of colonies were comparable between the human HSCs isolated from the 2 strains (supplemental Figure 1K-L). From this, we concluded that NBSGW mice better support BM engraftment of LT-HSCs characterized by surface marker expression and, therefore, is a better model to evaluate the effect of human HSC mobilization and in vivo HSC targeting.
The NBSGW strain demonstrates enhanced human LT-HSC engraftment in the BM. (A) Schematic of generating humanized mouse models using UCB-derived human HSPCs in NSG and NBSGW strains. (B) Survival of mice over 150 days after humanization. Day 0 (NSG: n = 23; NBSGW: n = 25), day 150 (NSG: n = 12; NBSGW: n = 24). (C) Weights of mice. Error bars indicate standard deviation (SD). (D) Percent human CD45+ cells in the PB of mice. The curve represents the mean values of each time point. Week 8 (NSG: n = 15; NBSGW: n = 24). Week 18 (NSG: n = 12; NBSGW: n = 24). (E) Percent human CD45+ cells in the murine BM. (F) Percent human CD34+ cells of human CD45+ cells. (G) Percent human CD34+CD38low cells of human CD45+ cells. (H) Percent human CD34+CD90+ cells of human CD45+ cells. (NSG: n = 12; NBSGW: n = 24). Error bars indicate SD. Welch t test, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. (I) Representative plots showing the gating strategy used to characterize human cells isolated from murine BM.
The NBSGW strain demonstrates enhanced human LT-HSC engraftment in the BM. (A) Schematic of generating humanized mouse models using UCB-derived human HSPCs in NSG and NBSGW strains. (B) Survival of mice over 150 days after humanization. Day 0 (NSG: n = 23; NBSGW: n = 25), day 150 (NSG: n = 12; NBSGW: n = 24). (C) Weights of mice. Error bars indicate standard deviation (SD). (D) Percent human CD45+ cells in the PB of mice. The curve represents the mean values of each time point. Week 8 (NSG: n = 15; NBSGW: n = 24). Week 18 (NSG: n = 12; NBSGW: n = 24). (E) Percent human CD45+ cells in the murine BM. (F) Percent human CD34+ cells of human CD45+ cells. (G) Percent human CD34+CD38low cells of human CD45+ cells. (H) Percent human CD34+CD90+ cells of human CD45+ cells. (NSG: n = 12; NBSGW: n = 24). Error bars indicate SD. Welch t test, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. (I) Representative plots showing the gating strategy used to characterize human cells isolated from murine BM.
G-CSF and AMD3100 treatment yields insufficient mobilization of human HSCs into the PB
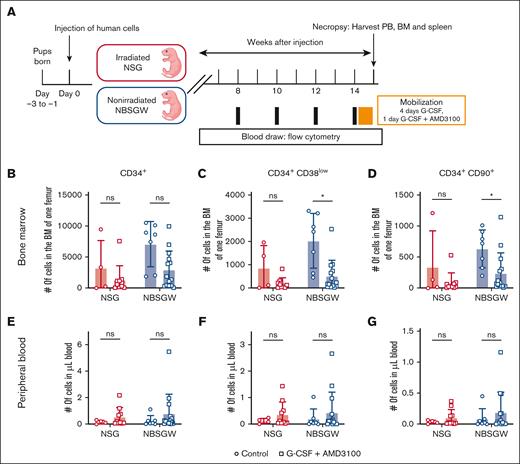
To directly expose BM-resident human HSCs to the gene therapy reagents commonly introduced IV, we compared the mobilization efficiency of human HSCs into the PB in humanized NSG and NBSGW mice. We treated mice with G-CSF and AMD3100, 2 of the most frequently used reagents for stem cell mobilization in the clinical setting (Figure 2A; supplemental Figure 2A-B).5-11 In NSG mice, no significant change in the number of HSCs in the BM was seen, whereas in NBSGW mice we observed a mild to significant decrease in the number of HSCs in the BM compared with the control group (Figure 2B-D). However, in both strains we did not see a significant increase in the number of HSCs in the PB after the mobilization (Figure 2E-G). The estimated total number of HSCs found in the PB did not account for the calculated number of cells mobilized from the BM (supplemental Figure 2C; see supplemental Methods for calculation). Moreover, in 1 of the cohorts, we found more HSCs in the PB of the control group, indicating that the mobilization was not effective nor reproducible across cohorts (supplemental Figure 2C, cohort 3). Interestingly, we noticed a slight increase in the fraction of HSCs within the spleen of mobilized animals (supplemental Figure 2D-F). Finally, we performed colony-forming cell assay to assess the quality of the HSCs characterized by surface marker expression and observed a slight decrease in the colony-forming potential of the HSPCs found in the PB of the mobilized animals in comparison with BM-isolated HSPCs (supplemental Figure 2G-H).
G-CSF and AMD3100 treatment yields insufficient mobilization of human HSCs into the PB. (A) Schematic of generating humanized mouse models and G-CSF + AMD3100 treatment for human HSC mobilization. (B-D) Numbers of human CD34+, CD34+CD38low, CD34+CD90+ cells in the BM of 1 femur. (E-F) Numbers of human CD34+, CD34+CD38low, CD34+CD90+ cells per μL of blood. (NSG: Control, n = 4; G-CSF + AMD3100, n = 10; NBSGW: Control, n = 7; G-CSF + AMD3100, n = 14.) Error bars indicate SD. Welch t test, ∗P < .05; ns, not significant.
G-CSF and AMD3100 treatment yields insufficient mobilization of human HSCs into the PB. (A) Schematic of generating humanized mouse models and G-CSF + AMD3100 treatment for human HSC mobilization. (B-D) Numbers of human CD34+, CD34+CD38low, CD34+CD90+ cells in the BM of 1 femur. (E-F) Numbers of human CD34+, CD34+CD38low, CD34+CD90+ cells per μL of blood. (NSG: Control, n = 4; G-CSF + AMD3100, n = 10; NBSGW: Control, n = 7; G-CSF + AMD3100, n = 14.) Error bars indicate SD. Welch t test, ∗P < .05; ns, not significant.
To test whether the combinatorial use of G-CSF and AMD3100 led to differentiation and loss of HSCs in humanized mice, we compared treatments with G-CSF alone and AMD3100 alone, to G-CSF in combination with AMD3100 in humanized NBSGW mice (supplemental Figure 3A-D). We observed a reproducible decrease in the fraction of human HSCs in the BM of mice treated with either G-CSF alone or G-CSF in combination with AMD3100 compared with the control group (supplemental Figure 3E-G). However, we did not see any significant increase of human HSCs in the PB (supplemental Figure 3H-J). AMD3100 treatment alone did not lead to any significant changes in human HSC level in either the BM or the PB (supplemental Figure 3E-J).
In summary, mobilization regimens using G-CSF and AMD3100 successfully released human HSCs from the BM in the NBSGW strain but most of the released HSCs were not detected in the PB, demonstrating an important limitation of this model for the evaluation of in vivo gene therapy approaches, at least with the mobilization agents and method tested here.
The combination of human chimerism in the PB, platelet (PLT) count, and time after transplantation can predict human chimerism in the BM
Because the mobilization regimens we tested did not yield sufficient human HSCs in circulation, we hypothesized that injecting therapeutic agents directly into the BM would allow targeting of human HSCs. For this approach, it is essential to identify healthy mice with robust human HSC engraftment in the BM for an efficient use of agents, mouse husbandry resources, and time. This is especially important if the models are needed for long-term follow-up studies, in which injection of gene therapy agents at the earliest time possible and maintenance of the animals before the onset of graft-versus-host disease (GVHD) are crucial. To this end, we investigated different parameters that could be used to predict human chimerism in the BM without having to perform BM aspiration.
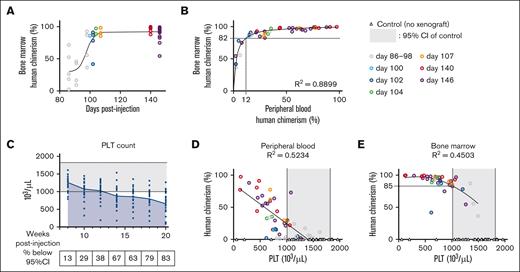
We first looked at human chimerism in the BM over time from different cohorts euthanized at different time points (Figure 3A; supplemental Figure 4A-C). At ∼12 weeks after human HSPC injection, some animals started to exhibit close to 90% human chimerism in the BM (Figure 3A). Overall, human chimerism increased until ∼110 days after human HSPC injection and plateaued thereafter (Figure 3A). Interestingly, the fractions of human HSCs also increased in the first 110 days, but the variability within cohorts became larger at the later time points (supplemental Figure 4A-C). Next, we plotted the correlation between PB and BM human chimerism, and observed that >12% human chimerism in the PB reflected >82% human chimerism in the BM (Figure 3B). The correlation was robust enough to allow identification of animals with low BM human chimerism, independent of their time after transplantation (Figure 3B). Therefore, the 2 parameters, days after human HSPC injection and PB human chimerism, allowed reliable identification of animals with high BM human chimerism.
The combination of human chimerism in the PB, PLT count, and time after transplantation can predict human chimerism in the BM. (A) Human chimerism in the murine BM over time. A sigmoidal curve was fitted to the data points. (B) Human chimerism (human CD45+ cells of human and mouse CD45+ cells) in the murine BM at necropsy. A hyperbolic curve was fitted to the data points. (C) PLT count from the PB over time. The curve represents the mean values of each time point; n = 24. (D) Relationship between human chimerism in the PB and the PLT count at necropsy. Linear regression (r = –0.72, P < .0001). (E) Relationship between human chimerism in the BM and the PLT count at necropsy. A second order polynomial curve was fitted to the data points. The legend shows days after injection. The gray shades in panels C through E show the 95% confidence interval (CI) of the PLT counts of 37 control animals (mean, 1408.2 ± 406.3).
The combination of human chimerism in the PB, PLT count, and time after transplantation can predict human chimerism in the BM. (A) Human chimerism in the murine BM over time. A sigmoidal curve was fitted to the data points. (B) Human chimerism (human CD45+ cells of human and mouse CD45+ cells) in the murine BM at necropsy. A hyperbolic curve was fitted to the data points. (C) PLT count from the PB over time. The curve represents the mean values of each time point; n = 24. (D) Relationship between human chimerism in the PB and the PLT count at necropsy. Linear regression (r = –0.72, P < .0001). (E) Relationship between human chimerism in the BM and the PLT count at necropsy. A second order polynomial curve was fitted to the data points. The legend shows days after injection. The gray shades in panels C through E show the 95% confidence interval (CI) of the PLT counts of 37 control animals (mean, 1408.2 ± 406.3).
With the reliable parameters to exclude mice with low human chimerism, we wanted to find other parameters that take the health of the mouse into account. As NBSGW mice are anemic because of their severe combined immunodeficiency background,18 we performed complete blood counts to determine whether xenotransplantation leads to more severe anemia as human cells replace mouse hematopoiesis and whether the values correlate with the extent of human chimerism. Among the complete blood count parameters, we tracked PLT and red blood cell (RBC) counts, which exclusively reflect the status of mouse hematopoiesis as human RBC and PLT development is very limited in NSG and NBSGW mice and mostly restricted to the BM.19-22 Over time, murine PLT and RBC counts decreased as expected (Figure 3C; supplemental Figure 4D). At 14 weeks after transplantation, >67% of animals exhibited PLT counts below the lower limit of normal range (Figure 3C). When plotted against human chimerism, PLT counts showed a negative correlation (linear regression: r = −0.72, P < .0001) with PB human chimerism (Figure 3D). As for the BM human chimerism, most of the animals with PLT counts of <1000 × 103/μL (Figure 3E; the lower limit of the 95% confidence interval) had >85% human chimerism (Figure 3E), demonstrating that PLT counts can serve as another surrogate of BM human chimerism in combination with time and PB chimerism. In contrast, RBC count did not distinguish animals with high human chimerism in either the PB or BM because RBC values in the PB remained in the normal range for most mice during the study (supplemental Figure 4D-F).
VSV-G-LV transduces mature blood cells and progenitors in vivo
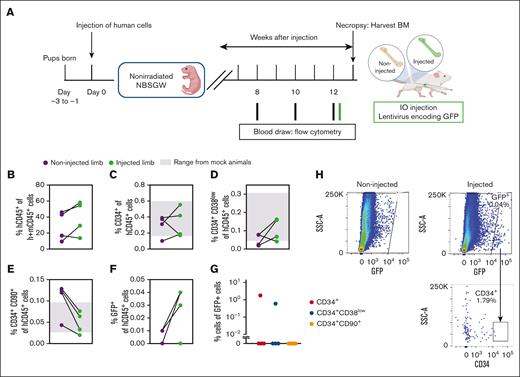
Because many current gene therapy applications use VSV-G LVs, we evaluated the in vivo transduction efficiency of VSV-G LVs in humanized NBSGW mice. With animals that had received transplantation with human HSCs for 12 weeks, we tested whether IO injection of VSV-G-LV could efficiently target human HSCs in vivo. One femur of each animal (treated) was injected with the vector, whereas the other femur was not injected and used as a noninjected control (Figure 4A). Human CD45+ and HSC chimerism in the BM were comparable between the 2 limbs of each animal, which ruled out any effect on the cellularity and composition caused by injection (Figure 4B-E; supplemental Figure 5A-B). The frequency of human chimerism and phenotypic HSCs within the injected animals were close to those of control animals that were injected with PBS in 1 of the limbs (gray shadow in Figure 4C-E), indicating that the vector injection did not affect the frequency of HSCs (Figure 4C-D). The colony-forming potential of the HSCs isolated from the injected and noninjected limbs were also comparable (supplemental Figure 5C-D). One day after vector injection, GFP+ human cells were found in the injected limbs of 3 out of 4 treated animals (Figure 4F,H). In only 1 of the animals, 1.79% and 0.6% of the detected GFP+ cells were CD34+ cells and CD34+CD38low cells, respectively (Figure 4G). GFP+ CD34+CD90+ human HSCs were undetectable (Figure 4G).
VSV-G-LV transduces mature blood cells and progenitors in vivo. (A) Schematic of generating a humanized NBSGW mouse model and injecting a lentivirus encoding GFP in the BM. (B) Percent human CD45+ cells in the murine BM. (C-E) Percent human CD34+, CD34+CD38low, and CD34+CD90+ cells in the BM of noninjected and injected limbs. Shaded in gray are the range of values from control (mock) animals. (F) Percent GFP+ cells of human CD45+ cells. (B-F) Treated (injected/noninjected), n = 4; and control, n = 3. (G) Percent human CD34+, CD34+CD38low, and CD34+CD90+ cells of GFP+ cells (n = 4). (H) Representative plots showing the gating strategy used to characterize the GFP+ population.
VSV-G-LV transduces mature blood cells and progenitors in vivo. (A) Schematic of generating a humanized NBSGW mouse model and injecting a lentivirus encoding GFP in the BM. (B) Percent human CD45+ cells in the murine BM. (C-E) Percent human CD34+, CD34+CD38low, and CD34+CD90+ cells in the BM of noninjected and injected limbs. Shaded in gray are the range of values from control (mock) animals. (F) Percent GFP+ cells of human CD45+ cells. (B-F) Treated (injected/noninjected), n = 4; and control, n = 3. (G) Percent human CD34+, CD34+CD38low, and CD34+CD90+ cells of GFP+ cells (n = 4). (H) Representative plots showing the gating strategy used to characterize the GFP+ population.
Low-density lipoprotein receptor (LDL-R) expression is low on human HSCs in the BM of humanized mice
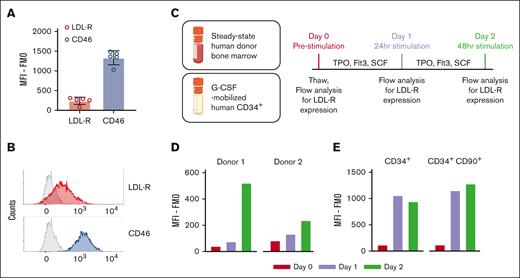
VSV-G-LV uses LDL-R as the major cellular receptor, whereas other viral vectors used for gene therapy such as adenoviral or measles vectors use CD46.23,24 To determine whether the lack of in vivo transduction with LVs is because of the absence of LDL-R on HSCs, we assessed the surface expression levels of LDL-R on steady-state human HSCs in humanized murine BM. As previously reported for freshly isolated, unstimulated human HSCs,25 we observed a low level of LDL-R on murine-engrafted human CD34+ cells (Figure 5A-B; supplemental Figure 6A-D). Expression of LDL-R on HSCs engrafted in humanized NBSGW and NSG mice closely matched the LDL-R-expression of CD34+ in the steady-state BM from healthy donors before in vitro stimulation (Figure 5D; day 0). In contrast, CD46 was abundantly expressed on the surface of the same cells from either steady-state BM or extracted from the humanized mice (Figure 5A-B; supplemental Figure 6A-D). To evaluate whether LDL-R expression is affected by G-CSF mobilization, we measured the surface expression levels of LDL-R in G-CSF–mobilized human CD34+ and CD34+CD90+ cells collected by leukapheresis from healthy donors (Figure 5E) and did not observe a noticeable difference in LDL-R expression between the steady-state (Figure 5D; day 0) and mobilized cells (Figure 5E; day 0). Because VSV-G-LVs are successfully used in vitro to transduce human HSCs, we stimulated steady-state BM and G-CSF-mobilized human CD34+ with stem cell factor, Flt-3 ligand, and thrombopoietin and confirmed the upregulation of LDL-R expression after 24 to 48 hours of in vitro stimulation in both the CD34+ and CD34+CD90+ populations (Figure 5C-E; supplemental Figure 6E).
LDL-R expression is low on human HSCs in the BM of humanized mice. (A) LDL-R and CD46 expressions on human CD34+ cells in steady-state BM of humanized NBSGW mice. Mean fluorescence intensity (MFI) corrected by the fluorescence-minus-1 (FMO) control is displayed; n = 5; error bars indicate SD. (B) Representative histogram of LDL-R, CD46, and FMO control (gray) signals detected from flow cytometry of the samples in panel A. (C) Schematic of in vitro culture, stimulation, and flow-cytometry analysis using steady-state human donor BM and G-CSF–mobilized human CD34+ cells. (D) LDL-R signals of steady-state human donor BM samples corrected by FMO signals. Data from 2 independent donors are displayed. (E) LDL-R signals of G-CSF–mobilized human CD34+ and CD34+CD90+ cells corrected by FMO signals.
LDL-R expression is low on human HSCs in the BM of humanized mice. (A) LDL-R and CD46 expressions on human CD34+ cells in steady-state BM of humanized NBSGW mice. Mean fluorescence intensity (MFI) corrected by the fluorescence-minus-1 (FMO) control is displayed; n = 5; error bars indicate SD. (B) Representative histogram of LDL-R, CD46, and FMO control (gray) signals detected from flow cytometry of the samples in panel A. (C) Schematic of in vitro culture, stimulation, and flow-cytometry analysis using steady-state human donor BM and G-CSF–mobilized human CD34+ cells. (D) LDL-R signals of steady-state human donor BM samples corrected by FMO signals. Data from 2 independent donors are displayed. (E) LDL-R signals of G-CSF–mobilized human CD34+ and CD34+CD90+ cells corrected by FMO signals.
Discussion
Here, we examined the capabilities and limitations of 2 widely used mouse strains, NSG and NBSGW, engrafted with human UCB-derived CD34+ HSPCs for the assessment of in vivo HSC gene therapy using lentiviral vectors. Superior survival as well as enhanced engraftment of phenotypically and functionally primitive human HSCs (CD34+CD38low and CD34+CD90+) were observed in the BM of NBSGW mice compared with the NSG strain. Robust mobilization of phenotypic HSCs out of the BM using G-CSF and AMD3100 could only be achieved in NBSGW mice; however, most mobilized HSCs were not detected in the PB and therefore were not accessible to gene therapy vectors commonly applied IV. Finally, modification of engrafted human HSCs directly in the BM by injecting viral vectors intraosseously was highly dependent on the presence of key proteins required for the binding and fusion of the virus. Discovery of these roadblocks highlights the importance of comprehensive assessment of animal models for the expected quality/quantity of target cells, the best route of administration, and the types of gene therapy agents.
Human HSCs have been successfully transplanted into naïve adult mice of various strains to assess the homing capability of human HSPCs into the BM stem cell compartment, multilineage differentiation potential, and long-term persistence of differentiation potential after ex vivo modification or culture.2,12,13,26-30 However, there are factors that limit the duration of long-term follow-up studies. First, xenotransplantation of mice as adults significantly shortens the study duration because animals are usually 8 to 12 weeks old at the time of transplantation and assessment of human chimerism is not feasible until animals are 4 to 5 months old. Second, mice are exposed to the risk of xenogeneic GVHD as human T cells start developing.31 Here, we show the use of neonatally immunocompromised mice to circumvent these limiting factors. In neonatally humanized mice, animals achieve high human chimerism already at 10 to 12 weeks after birth, shortening the waiting period until test reagent administration and extending the duration of long-term follow-up study. Moreover, xenotransplantation in mice as neonates allows human T cells to develop and mature in mice, reducing the risk of xenogeneic GVHD.16,30,32 Because of its high levels of engraftment of human cells and increased tolerance to human immune cell development, the method to humanize neonatal mice has been used for various applications such as autologous chimeric antigen receptor T-cell therapy32 and HIV treatment.33 The model also demonstrated superior engraftment of phenotypic human LT-HSCs,28,34 and has become an attractive model for testing in vivo HSC gene therapy approaches. As reported previously,13 and here, the NBSGW strain exhibits slower T-cell development kinetics than the NSG strain, which makes it a more suitable model for long-term follow-up when combined with neonatal injection. Furthermore, the use of NSG mice also poses the limitation to the study because of the frequent early death caused by anemia and thrombocytopenia, likely because of the required TBI process before xenotransplantation. Of note, our study focuses on the use of UCB-derived CD34+ cells and some findings may not extend to other cell sources such as fetal liver–derived CD34+ cells or G-CSF–mobilized PB CD34+ cells.29
Testing in vivo HSC gene therapy strategies in humanized mice requires animals with high levels of human chimerism and without health issues to allow for the reliable long-term follow-up of treated animals. It has been reported that the NBSGW strain displays a macrocytic anemia, thrombocytosis, and lymphopenia18,35 consistent with its severe combined immunodeficiency background, and the RBC count decreases further after humanization,19 in line with the observation made in this study. Here, we speculated that almost complete replacement of murine HSCs in the BM with human cells could exacerbate anemia and thrombocytopenia, because human RBC and PLT formation is limited in these mice.19-22 To establish a correlation between the health status of the animals and the levels of human chimerism in the PB and BM, we followed the murine RBC and PLT counts in the PB and concluded that the level of replacement in the BM could be deduced from murine PLT counts. In contrast, the RBC count did not reflect the level of human chimerism, likely because of the long life span of RBCs.36 Our finding provided an easy and reliable assessment of animals, in addition to traditional flow-cytometry–based measurement of the frequency of human blood cells in the PB. The combination of PLT count, human chimerism in the PB, and time after humanization allowed us to reliably determine the best time point for the enrollment of mice into in vivo HSC gene therapy studies, guaranteeing adequate engraftment of human HSCs in the BM and maximizing the possible duration of long-term follow-up.
Most in vivo HSC gene therapy approaches currently aim for the administration of agents IV after mobilization of BM-residing HSCs into the PB.3,4 Because steady-state HSCs are exclusively restricted to the BM with supportive microenvironments,37-39 different mobilization mechanisms can be used to actively egress HSCs out of the BM. Among numerous mobilizing agents such as G-CSF, AMD3100, Gro-β, and interleukin-8 that have different modes of action,40 G-CSF with or without AMD3100 are the current gold standard in the field to induce mobilization of HSCs.5-11 Although AMD3100 is an antagonist of SDF-1 to the chemokine receptor CXC motif receptor 4 expressed on the surface of HSCs controlling integrin-mediated adhesion,41,42 G-CSF stimulates myeloid cells in the BM to secrete marrow matrix metalloproteinase 9 and soften the microenvironment,43 both resulting in the release of HSCs. Interestingly, G-CSF–mediated release of human HSCs is significantly improved in humanized NBSGW mice in comparison with NSG mice likely because of the improved maturation of human myeloid cell in the BM of NBSGW mice responding to the G-CSF stimulation. However, AMD3100 treatment in neither NSG nor NBSGW mice led to a noticeable mobilization of human HSCs when used alone, or enhanced release of cells from the BM when combined with G-CSF. Although human HSCs were successfully released from the BM in NBSGW mice, only a small fraction of these cells were found in circulation. The successful release of human HSCs from the BM mirrors the recent report from Omer-Javed et al44 using the humanized NSGW41 strain,45 a strain with a similar genetic background to NBSGW. However, less efficient preservation of human CD34+ cells was seen in this study presumably because of differences in the mobilization protocol. A potential reason for this discrepancy may be the phenotypic assessment and quantification of human HSPCs in the blood and BM of mice. Although CD34 is expressed on many mature progenitors as a continuum, we only quantified CD34high cells lacking CD38 and/or expressing CD90 in the blood and BM, a small subset within all human CD34+ cells in the mouse BM. This more stringent quantification excludes most downstream progenitors and focuses primarily on the mobilization of phenotypically and functionally primitive stem and progenitor cells, the main target for gene therapy agents. Inclusion of additional cell surface markers in our study revealed that committed progenitors may well be mobilized; however, the phenotypically primitive HSCs are less well mobilized and lost from the BM upon G-CSF stimulation in NBSGW mice. Interestingly, some of the mobilized human HSCs were found in the spleen of the animals, the primary site of T- and B-cell development for humanized mice.
Although VSV-G-LVs are widely used for gene transfer in vitro for their wide tropism and robust infectivity,46 it has been reported that unstimulated T, B, and CD34+ cells are not as permissive to them24 because of low expression of LDL-Rs. Based on this observation, we wanted to evaluate the expression levels of LDL-R on human HSCs in the BM of humanized mice and demonstrated a low level of LDL-R expression on human HSCs. This explained why the transduction efficiency of the VSV-G-LV injected directly into the murine BM was low in steady-state human HSCs. The LDL-R expression was also low on HSCs in the steady-state BM as well as mobilized by G-CSF from human donors. This indicated that the wild-type VSV-G-LVs are not likely to be highly efficient for in vivo gene therapy for targeting human HSCs both in the BM niche and after mobilization. In contrast, human HSCs expressed high levels of CD46 in vivo, indicating that other viral vectors such as measles or adenovirus that uses CD46 as a cellular receptor would be more efficient in targeting the cells in vivo. Another attractive alternative would be LDL-R–independent LVs with engineered moieties that recognize HSC markers. Although CD34-targeted vectors have not been successful in targeting human HSCs,47 other HSC markers, such as CD90, CD117, or CD133,48-50 could be used in combination with CD34 for LDL-R–independent transduction of human HSCs in vivo, increasing both specificity and efficiency for HSC targeting.47-50
Overall, our work suggests that the development and optimization of new in vivo gene therapy strategies require careful characterization and upfront testing of available animal models to determine the possibilities, identify the limitations, and adjust the expectations. In vivo models need to be selected based on the tropism of the gene therapy agent, the anticipated mode of injection, as well as the target cell distribution and development to allow for objective analysis and interpretation of data. Furthermore, comprehensive follow-up studies are needed to further test alternative mobilization agents or methods40,44 to transiently increase the LDL-R expression on human HSCs in the mouse xenograft model to allow intravenous testing of gene therapy agents after HSC mobilization as well as the use of naïve lentiviral vectors without surface modification.
Acknowledgments
The authors thank the Comparative Medicine department and Flow Cytometry Core Facility of the Fred Hutchinson Cancer Center for their research support. The authors thank Mike McCune at the Gates Foundation for helpful discussion on experimental designs and executions.
This work was supported by the Gates Foundation.
Authorship
Contribution: S.C. and S.R. designed and performed experiments, analyzed/interpreted data, and wrote the manuscript; C.B.W. performed mouse studies, compiled the raw data, and edited the manuscript; H.M.M. and M.J.E. helped with mouse studies; H.-P.K. and S.R. conceived and oversaw the study and interpreted data; and all authors reviewed the results and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.R. is a consultant or has ownership interests with 48 Bio, Inc, Proteios Technology Inc, and Ensoma Inc. H.-P.K. is, or was, a consultant to, and has, or had, ownership interests with, Rocket Pharmaceuticals, Homology Medicines, Vor Biopharma, and Ensoma Inc, and has been a consultant to CSL Behring and Magenta Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Stefan Radtke, Division of Translational Sciences and Therapeutics, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109-1024; email: sradtke@fredhutch.org; and Hans-Peter Kiem, Department of Medicine and Pathology, University of Washington School of Medicine, Division of Translational Sciences and Therapeutics, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109-1024; email: hkiem@fredhutch.org.
References
Author notes
Renewable materials, data sets, and protocols are available on request from the corresponding authors, Stefan Radtke (sradtke@fredhutch.org) and Hans-Peter Kiem (hkiem@fredhutch.org).
The full-text version of this article contains a data supplement.