Visual Abstract
TO THE EDITOR:
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is a life-threatening microangiopathy (TMA);1 however, death from iTTP is uncommon in the current era. Modern estimates of mortality are lacking, particularly in the United States, where, until recently, there were no multi-institutional registries on which to base their estimates. There are also few data reports on how mortality from iTTP has improved over time as a result of advances in the earlier recognition, diagnosis, and management of iTTP, including the development of clinical diagnostic scores,2-4 clinical guidelines,5-7 and education programs.8
The United States Thrombotic Microangiopathies (USTMA) iTTP registry contains data on 770 patients with iTTP from 15 high-volume centers across the United States between 1985 and 2019. We used the registry to estimate the overall risk of mortality from acute iTTP episodes and describe trends in iTTP-related mortality in the United States over time. The participants in the USTMA registry have been well described by Chaturvedi et al.1 We only included patients for whom presenting data were available. For participants diagnosed before ADAMTS13 testing was available (pre-2006), the iTTP diagnosis was based on the clinical course and absence of alternative causes. Not all variables needed to calculate PLASMIC scores were not included in the data set, so we compared the baseline characteristics of those with and without ADAMT13 confirmation. We selected the variables that had the strongest influence on mortality in our separate mortality-prediction study.2 All participating institutions were institutional review board approved. The study was conducted in accordance with the Declaration of Helsinki.
Deaths within 30 days of an iTTP episode were included and categorized as those occurring during the first episode (initial diagnosis of iTTP, including any exacerbation) or during iTTP relapse.3 iTTP exacerbation was defined as iTTP recurrences occurring within 30 days or >30 days after therapeutic plasma exchange discontinuation and were categorized as exacerbations or relapses, respectively. Deaths during iTTP exacerbation were considered part of the index episode along with deaths occurring before achieving a clinical response.
The acute iTTP mortality rate was calculated as the number of deaths per year divided by the total number of iTTP diagnoses made that same year and then grouped into 5-year intervals because of the low number of deaths. The association between mortality and time was tested using a log-binomial regression, assuming a linear change over time. We focused our analyses on iTTP mortality during the first presentation because most deaths occurred during the initial iTTP episode. Time to death and cumulative survival are presented graphically using the Kaplan-Meier method.
The overall iTTP-related mortality rate was 6.6% (51/771). Data on the first iTTP episode were available for 420 patients. The remaining 351 had data available for iTTP relapses but not for the first episode (treated at another institution or predated electronic records) and were excluded from the analysis. Of the 420 patients with presenting data, 33 (7.8%) died from iTTP; 24 deaths (5.7% mortality rate) occurred during the first episode, and 9 (2.1%) during relapse.
The demographics and presenting laboratory values of the nonsurvivors are summarized in Table 1. All patients were treated with therapeutic plasma exchange; 81% received steroids, a minority received rituximab (28%) or other immunosuppression (10%), and none received caplacizumab, because our study predated its approval. Baseline variables between survivors and nonsurvivors were compared in a separate analysis to identify predictors of mortality, and the results were reported and discussed separately.2 A total of 58 of 420 patients (14%) were diagnosed before the availability of the ADAMTS13 assay. Because we could not calculate their PLASMIC scores, we instead compared their baseline characteristics (supplemental Table 1) and found no significant differences, except for higher age and hemoglobin level in the ADAMTS13-confirmed group (both variables of which correlated with higher mortality in our mortality-prediction study2). The mortality rate was 4.7% (7/58) in patients with ADAMTS13 confirmation and 12.1% (17/362) in those without ADAMTS13 confirmation (supplemental Table 2).
Baseline demographics and laboratory variables of all patients with iTTP and fatal outcomes
| Variable . | Number of patients, N (%), median (interquartile range; n = 33) . |
|---|---|
| Demographic | |
| Age (y) | 51 (28-60) |
| Sex | Female: 18 (54.5) Male: 15 (45.5) |
| Race | White/Caucasian: 13 (39.4) Black: 18 (54.5) |
| Presenting symptoms | |
| Any neurologic symptom | 22 (66.7) |
| Confusion | 10 (30.3) |
| Cerebrovascular accident | 9 (27.3) |
| Seizure | 3 (9.1) |
| Memory deficit | 1 (3) |
| Stupor/coma | 4 (12.1) |
| Headache | 7 (21.2) |
| Fever | 9 (27.3) |
| Chest pain | 0 (0) |
| Abdominal pain | 11 (33.3) |
| Fatigue | 11 (33.3) |
| Petechiae or easy bruising | 6 (18.2) |
| Dark urine | 4 (12.1) |
| Presence of any symptoms | 32 (97) |
| Presenting laboratory findings | |
| Platelet count (×103/μL) | 16.0 (10.2-19.8) |
| Lactate dehydrogenase (U/L) | 1574.5 (1260.5-2373.8) |
| Serum creatinine (mg/dL) | 1.6 (1.2-2.5) |
| Hemoglobin (g/dL) | 9.4 (7.8-10.5) |
| Peak troponin (ng/mL) | 0.9 (0.3-3.4) |
| ADAMTS13 confirmation available (yes/no) | 23 (69.7) |
| Variable . | Number of patients, N (%), median (interquartile range; n = 33) . |
|---|---|
| Demographic | |
| Age (y) | 51 (28-60) |
| Sex | Female: 18 (54.5) Male: 15 (45.5) |
| Race | White/Caucasian: 13 (39.4) Black: 18 (54.5) |
| Presenting symptoms | |
| Any neurologic symptom | 22 (66.7) |
| Confusion | 10 (30.3) |
| Cerebrovascular accident | 9 (27.3) |
| Seizure | 3 (9.1) |
| Memory deficit | 1 (3) |
| Stupor/coma | 4 (12.1) |
| Headache | 7 (21.2) |
| Fever | 9 (27.3) |
| Chest pain | 0 (0) |
| Abdominal pain | 11 (33.3) |
| Fatigue | 11 (33.3) |
| Petechiae or easy bruising | 6 (18.2) |
| Dark urine | 4 (12.1) |
| Presence of any symptoms | 32 (97) |
| Presenting laboratory findings | |
| Platelet count (×103/μL) | 16.0 (10.2-19.8) |
| Lactate dehydrogenase (U/L) | 1574.5 (1260.5-2373.8) |
| Serum creatinine (mg/dL) | 1.6 (1.2-2.5) |
| Hemoglobin (g/dL) | 9.4 (7.8-10.5) |
| Peak troponin (ng/mL) | 0.9 (0.3-3.4) |
| ADAMTS13 confirmation available (yes/no) | 23 (69.7) |
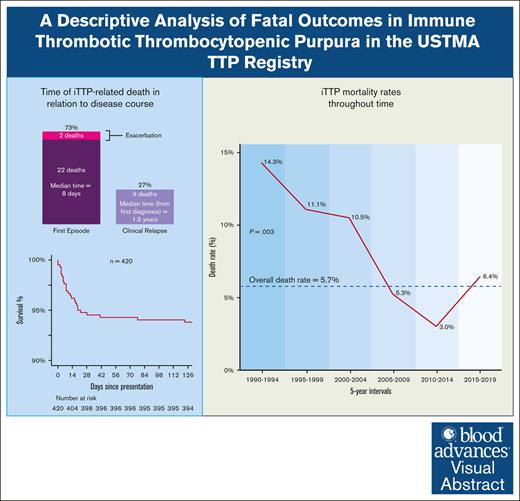
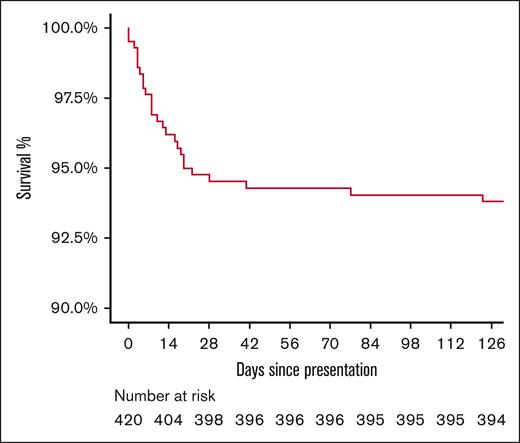
Of the 33 deaths in the cohort with presenting data available, 24 (72.7%) occurred during the first iTTP presentation. Of these, 22 occurred before achieving clinical response (66.7% of all deaths), with a median time to death of 8 days (interquartile range, 3-16), and 2 occurred during exacerbations (Figure 1). The remaining 9 deaths (27.3%) occurred during subsequent iTTP relapses. The median time to death due to iTTP relapse (n = 9) was 1.5 years (interquartile range, 0.7-5.3) after the first diagnosis and ranged between 1 and 5 days after relapse episode onset. The Kaplan-Meier survival curve is shown in Figure 1.
Kaplan-Meier survival curve of patients with iTTP since the time of first diagnosis.
Kaplan-Meier survival curve of patients with iTTP since the time of first diagnosis.
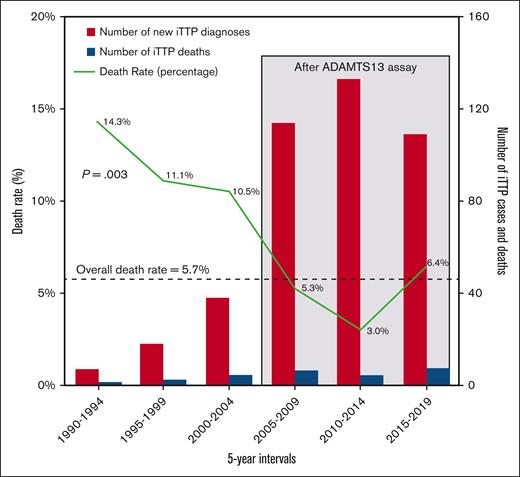
Over the past 3 decades, the mortality rate during the first episode of iTTP decreased over time, with an estimated risk reduction of 6.8% per year (95% CI, 2.1%-10.8%; P = .003) based on log-binomial regression, assuming a linear relationship between time and mortality rate. The mortality rate decreased from 14.3% between 1990 and 1994 to 6.4% in the final 5 years of the (2015-2019; Figure 2). Within the same cohort, the number of new iTTP diagnoses increased over time, most notably in the last decade.
The number of new iTTP diagnoses and the death rate at first presentation between 1990 and 2019 per 5-year intervals.
The number of new iTTP diagnoses and the death rate at first presentation between 1990 and 2019 per 5-year intervals.
The overall acute iTTP-related mortality rate of 6.6% in our cohort was similar to other recent reports.4-11 The overwhelming majority (73%) of deaths occurred during the first episode, two-thirds of which occurred before achieving a clinical response. This is consistent with previous findings that suggest the highest risk of mortality at the first presentation.10 A quarter of all death events were due to disease relapse, occurring more than a year after the initial diagnosis. This highlights the importance of vigilant laboratory and clinical monitoring after achieving the first remission and during long-term follow-up, and considerations such as pre-emptive immunosuppression, as needed.12-14
The mortality rate of iTTP at first presentation decreased with time, from 14% to 6% over the past 3 decades, by an annual rate of 6.8% of the previous year, based on log-binomial regression that met statistical significance. During the same period, new diagnoses of iTTP increased. This is likely attributable to several developments, such as the availability of the ADAMTS13 assay, as well as clinical diagnostic scores, guidelines, and education efforts. We could not analyze whether the introduction of rituximab in iTTP management had an impact. We observed a small uptick in mortality in the last interval (6.4%), which we attributed to the fluctuating rates of iTTP mortality that may potentially vary with baseline risk factors, especially because the sample size of fatal outcomes was small. With the advancement of novel iTTP therapies, death rates may drop further.15
The limitations of our study include the low number of fatal outcomes, which may have impacted the time-to-death analyses and mortality rate over time. However, the correlation between mortality rate and time met statistical significance. Furthermore, the inclusion of patients diagnosed with iTTP before the development of the ADAMTS13 assay might have also led to other TMAs being incorrectly diagnosed as iTTP, although the proportion of those cases was relatively small, and we remain confident in their diagnosis. We attribute the difference in mortality between both groups to the significant improvements in the recognition and management of iTTP in more recent years compared with the era before the availability of the ADAMTS13 assay, as the only notable difference between both groups was time.
In conclusion, iTTP-related mortality appeared to decrease over time, suggesting the likely beneficial effects of advances in iTTP diagnosis and treatment. Although most deaths occurred during the first iTTP episode, nearly a quarter of the deaths occurred during the iTTP relapse. Ongoing clinical and laboratory monitoring, along with pre-emptive immunosuppression, as needed, can potentially reduce iTTP recurrence and related mortality.
Acknowledgments: The authors acknowledge the late J. Evan Sadler, for his contributions to the USTMA. The visual abstract was created with BioRender.com.
This investigation was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002538.
Contribution: C.Z. and A.P.P. performed the statistical analysis and model creation; M.Y.A-I., C.Z., A.P.P., M.M., and M.Y.L. designed the study and wrote the main manuscript; and all other authors contributed to the database and reviewed and edited the manuscript.
Conflict-of-interest disclosure: M.Y.L. has received an honorarium for participation in advisory boards from Takeda, Dova Pharmaceuticals, and Forma Therapeutics. M.Y.A-I. has participated on advisory boards with Sanofi. X.L.Z. services as a member of the advisory boards for Takeda, Sanofi, and Alexion. X.L.Z. is a cofounder of Clotsolution and is supported in part from National Heart, Lung, and Blood Institute (R01 HL126724 and HL144552). G.d.R. serves the speaker bureaus for Sanofi and advisory boards for Mallinckrodt. R.G. has received research funding from the National Heart, Lung, and Blood Institute (K08 HL159290). R.G. served as a consultant and participated on the advisory boards for Sanofi, Takeda, and Alexion. The remaining authors declare no competing financial interests.
A complete list of the members of the United States Thrombotic Microangiopathies Consortium appears in supplemental Table 3.
Correspondence: Yazan Abou-Ismail, Division of Hematology and Hematologic Malignancies, University of Utah, 2000 Circle of Hope Dr, Salt Lake City, UT 84112; email: yazan.abou-ismail@hsc.utah.edu.
References
Author notes
∗M.Y.L. and M.M. are joint senior authors.
Deidentified individual participant data are available on formal request from the corresponding author, Mouhamed Yazan Abou-Ismail (yazan.abou-ismail@hsc.utah.edu).
The request for data should include a proposed analysis, the approval of which will be granted after review by the participating members of the USTMA Consortium, full consensus on the terms of use (including review of the analysis and final manuscript as well as authorship arrangements), and signed data access agreement with the University of Minnesota.
The full-text version of this article contains a data supplement.